Coordination of Genomic RNA Packaging with Viral Assembly in HIV-1
Abstract
:1. Introduction
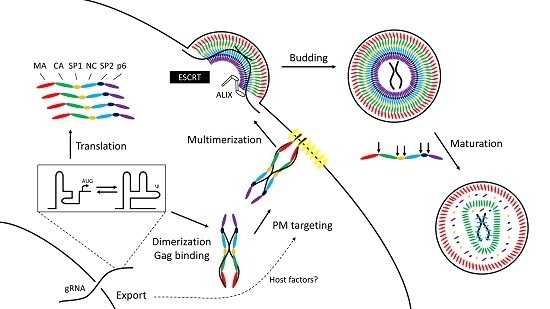
2. Overview
- Translation: In HIV-1 and feline immunodeficiency virus (FIV) gRNA control begins at the stage of translation to produce Gag and Gag-Pol. A conformational RNA switch in the 5’ untranslated region (UTR) of the RNA affects the balance between translation and packaging from the same template by altering exposure of the start codon and the elements required for genome dimerization and packaging [2,3,4,5,6]. Moloney murine leukemia virus (MoMuLV) similarly uses an RNA structural change to expose Gag binding sites upon dimerization [7,8].
- Genome capture: Viral RNA molecules act as scaffolds tethering adjacent Gag proteins through their nucleocapsid (NC) domains [9,10] allowing the newly-transcribed HIV-1 Gag to form oligomers in the cytoplasm [10,11] before trafficking to the plasma membrane where there is evidence that targeting to membrane lipids is linked to the process of gRNA nuclear export [12,13,14].
- Virion particle formation: Multimerization of Gag to form immature virus particles occurs at the plasma membrane; HIV-1 gRNA act as nucleation sites for the assembly of immature virions [15,16,17]. Alternative models exist for RNA viruses that transport their gRNA into a preformed capsid. A prototypic example is φ6 [18,19]. Despite differences in the mechanism of packaging, gRNA coordinates the process in both.
- Release from the cell: HIV-1 Gag engages with the host endosomal sorting complexes required for transport (ESCRT) machinery to bud from the cell [20,21]; subsequent morphological maturation of the virion occurs by proteolysis of Gag by the viral protease. These linked processes can be disturbed by mutations in the cis-acting packaging elements [22,23,24,25,26] suggesting that gRNA binding to Gag is important for virus maturation. The NC domain of Gag serves as a docking site for the ESCRT proteins TSG101 and ALIX [26,27] in addition to their better-studied binding sites in the p6 domain. In the case of ALIX, at least, RNA and membrane lipids appear necessary to stabilize the interaction with Gag [28].
3. RNA Structural Switches Affecting Translation and Packaging
4. Spatiotemporal Dynamics of the Interaction between Group-specific Antigen (Gag) and Genomic RNA (gRNA)
5. Targeting Gag to the Plasma Membrane
6. Assembly of Virions and Packaging
7. Viral Budding
8. Maturation of the Viral Core
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacks, T.; Power, M.D.; Masiarz, F.R.; Luciw, P.A.; Barr, P.J.; Varmus, H.E. Characterisation of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 1988, 336, 403–405. [Google Scholar]
- Huthoff, H.; Berkhout, B. Two alternating structures of the HIV-1 leader RNA. RNA 2001, 7, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Abbink, T.E.M.; Berkhout, B. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J. Biol. Chem. 2003, 278, 11601–11611. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Heng, X.; Garyu, L.; Monti, S.; Garcia, E.L.; Kharytonchyk, S.; Dorjsuren, B.; Kulandaivel, G.; Jones, S.; Hiremath, A.; et al. NMR detection of structures in the HIV-1 5’-leader RNA that regulate genome packaging. Science 2011, 334, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.C.; Prestwood, L.J.; Le Grice, S.F.J.; Lever, A.M.L. In-gel probing of individual RNA conformers within a mixed population reveals a dimerization structural switch in the HIV-1 leader. Nucleic Acids Res. 2013, 41, e174. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.C.; Tanner, S.J.; Legiewicz, M.; Phillip, P.S.; Rizvi, T.A.; Le Grice, S.F.J.; Lever, A.M.L. SHAPE analysis of the FIV Leader RNA reveals a structural switch potentially controlling viral packaging and genome dimerization. Nucleic Acids Res. 2011, 39, 6692–6704. [Google Scholar] [CrossRef] [PubMed]
- Mougel, M.; Tounekti, N.; Darlix, J.L.; Paoletti, J.; Ehresmann, B.; Ehresmann, C. Conformational analysis of the 5' leader and the gag initiation site of Mo-MuLV RNA and allosteric transitions induced by dimerization. Nucleic Acids Res. 1993, 21, 4677–4684. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Garcia, E.L.; King, S.R.; Iyalla, K.; Loeliger, K.; Starck, P.; Syed, S.; Telesnitsky, A.; Summers, M.F. An RNA structural switch regulates diploid genome packaging by Moloney murine leukemia virus. J. Mol. Biol. 2010, 396, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Mattei, S.; Flemming, A.; Anders-Össwein, M.; Kräusslich, H.-G.; Briggs, J.A.G.; Müller, B. RNA and Nucleocapsid are dispensable for mature HIV-1 capsid assembly. J. Virol. 2015, 89, 9739–9747. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R.; Ma, Y.M.; Vogt, V.M.; Webb, W.W. Direct measurement of Gag-Gag interaction during retrovirus assembly with FRET and fluorescence correlation spectroscopy. J. Cell Biol. 2003, 162, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- El Meshri, S.E.; Dujardin, D.; Godet, J.; Richert, L.; Boudier, C.; Darlix, J.L.; Didier, P.; Mély, Y.; De Rocquigny, H. Role of the nucleocapsid domain in HIV-1 gag oligomerization and trafficking to the plasma membrane: A fluorescence lifetime imaging microscopy investigation. J. Mol. Biol. 2015, 427, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.M.; Puffer, B.A.; Ahmad, K.M.; Doms, R.W.; Malim, M.H. Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J. 2004, 23, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Sherer, N.M.; Swanson, C.M.; Papaioannou, S.; Malim, M.H. Matrix mediates the functional link between human immunodeficiency virus type 1 RNA nuclear export elements and the assembly competency of Gag in murine cells. J. Virol. 2009, 83, 8525–8535. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sturgeon, T.; Weisz, O.A.; Mothes, W.; Montelaro, R.C. HIV-1 Matrix dependent membrane targeting is regulated by Gag mRNA trafficking. PLoS ONE 2009, 4, e6551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaitchik, O.A.; Dilley, K.A.; Fu, W.; Gorelick, R.J.; Tai, S.-H.S.; Soheilian, F.; Ptak, R.G.; Nagashima, K.; Pathak, V.K.; Hu, W.-S. Dimeric RNA recognition regulates HIV-1 genome packaging. PLoS Pathog. 2013, 9, e1003249. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rahman, S.A.; Nikolaitchik, O.A.; Grunwald, D.; Sardo, L.; Burdick, R.C.; Plisov, S.; Liang, E.; Tai, S.; Pathak, V.K.; et al. HIV-1 RNA genome dimerizes on the plasma membrane in the presence of Gag protein. Proc. Natl. Acad. Sci. USA 2016, 113, E201–E208. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, J.; Shioda, T.; Panganiban, A.T. Duplication of the primary encapsidation and dimer linkage region of human immunodeficiency virus type 1 RNA results in the appearance of monomeric RNA in virions. J. Virol. 2001, 75, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Casini, G.; Qiao, J.; Mindich, L. in vitro packaging of individual genomic segments of bacteriophage phi 6 RNA: Serial dependence relationships. J. Virol. 1995, 69, 2926–2931. [Google Scholar] [PubMed]
- Mindich, L. Packaging, replication and recombination of the segmented genomes of bacteriophage φ6 and its relatives. Virus Res. 2004, 101, 83–92. [Google Scholar] [CrossRef] [PubMed]
- VerPlank, L.; Bouamr, F.; LaGrassa, T.J.; Agresta, B.; Kikonyogo, A.; Leis, J.; Carter, C.A. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc. Natl. Acad. Sci. USA 2001, 98, 7724–7729. [Google Scholar] [CrossRef] [PubMed]
- Strack, B.; Calistri, A.; Craig, S.; Popova, E.; Göttlinger, H.G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 2003, 114, 689–699. [Google Scholar] [CrossRef]
- Liang, C.; Rong, L.; Cherry, E.; Kleiman, L.; Laughrea, M.; Wainberg, M.A. Deletion mutagenesis within the dimerization initiation site of human immunodeficiency virus type 1 results in delayed processing of the p2 peptide from precursor proteins. J. Virol. 1999, 73, 6147–6151. [Google Scholar] [PubMed]
- L’Hernault, A.; Greatorex, J.S.; Crowther, R.A.; Lever, A.M.L. Dimerisation of HIV-2 genomic RNA is linked to efficient RNA packaging, normal particle maturation and viral infectivity. Retrovirology 2007, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- L’Hernault, A.; Weiss, E.U.; Greatorex, J.S.; Lever, A.M. HIV-2 genome dimerization is required for the correct processing of Gag: A second-site reversion in matrix can restore both processes in dimerization-impaired mutant viruses. J. Virol. 2012, 86, 5867–5876. [Google Scholar] [CrossRef] [PubMed]
- Dussupt, V.; Sette, P.; Bello, N.F.; Javid, M.P.; Nagashima, K.; Bouamr, F. Basic residues in the Nucleocapsid domain of Gag are critical for late events of HIV-1 budding. J. Virol. 2011, 85, 2304–2315. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Popova, E.; Inoue, M.; Gottlinger, H.G. Human immunodeficiency virus type 1 Gag engages the Bro1 domain of ALIX/AIP1 through the Nucleocapsid. J. Virol. 2007, 82, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Chamontin, C.; Rassam, P.; Ferrer, M.; Racine, P.J.; Neyret, A.; Laine, S.; Milhiet, P.E.; Mougel, M. HIV-1 Nucleocapsid and ESCRT-component Tsg101 interplay prevents HIV from turning into a DNA-containing virus. Nucleic Acids Res. 2015, 43, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Sette, P.; O’Connor, S.K.; Yerramilli, V.S.; Dussupt, V.; Nagashima, K.; Chutiraka, K.; Lingappa, J.; Scarlata, S.; Bouamr, F. HIV-1 Nucleocapsid mimics the membrane adaptor Syntenin PDZ to gain access to ESCRTs and promote virus budding. Cell Host Microbe 2016, 19, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Houzet, L.; Paillart, J.C.; Smagulova, F.; Maurel, S.; Morichaud, Z.; Marquet, R.; Mougel, M. HIV controls the selective packaging of genomic, spliced viral and cellular RNAs into virions through different mechanisms. Nucleic Acids Res. 2007, 35, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, L.; Racine, P.J.; Houzet, L.; Chamontin, C.; Berkhout, B.; Mougel, M. Role of HIV-1 RNA and protein determinants for the selective packaging of spliced and unspliced viral RNA and host U6 and 7SL RNA in virus particles. Nucleic Acids Res. 2011, 39, 8915–8927. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.; Gottlinger, H.; Haseltine, W.; Sodroski, J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. 1989, 63, 4085–4087. [Google Scholar] [PubMed]
- Abd El-Wahab, E.W.; Smyth, R.P.; Mailler, E.; Bernacchi, S.; Vivet-Boudou, V.; Hijnen, M.; Jossinet, F.; Mak, J.; Paillart, J.-C.; Marquet, R. Specific recognition of the HIV-1 genomic RNA by the Gag precursor. Nat. Commun. 2014, 5, 4304. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.H.; Child, L.A.; Lever, A.M. Packaging of human immunodeficiency virus type 1 RNA requires cis- acting sequences outside the 5’ leader region. J. Virol. 1993, 67, 3997–4005. [Google Scholar] [PubMed]
- Kutluay, S.B.; Zang, T.; Blanco-Melo, D.; Powell, C.; Jannain, D.; Errando, M.; Bieniasz, P.D. Global changes in the RNA binding specificity of HIV-1 Gag regulate virion genesis. Cell 2014, 159, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Keane, S.C.; Heng, X.; Lu, K.; Kharytonchyk, S.; Ramakrishnan, V.; Carter, G.; Barton, S.; Hosic, A.; Florwick, A.; Santos, J.; et al. Structure of the HIV-1 RNA packaging signal. Science 2015, 348, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.D.; Li, H.; Kenyon, J.C.; Symmons, M.; Klenerman, D.; Lever, A.M.L. Three-dimensional RNA structure of the major HIV-1 packaging signal region. Structure 2013, 21, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.P.; Cantara, W.A.; Olson, E.D.; Musier-Forsyth, K. Small-angle X-ray scattering-derived structure of the HIV-1 5’ UTR reveals 3D tRNA mimicry. Proc. Natl. Acad. Sci. USA 2014, 111, 3395–3400. [Google Scholar] [CrossRef] [PubMed]
- Lanchy, J.M.; Rentz, C.A.; Ivanovitch, J.D.; Lodmell, J.S. Elements located upstream and downstream of the major splice donor site influence the ability of HIV-2 leader RNA to dimerize in vitro. Biochemistry 2003, 42, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.F.; Lever, A.M. Human immunodeficiency Virus types 1 and 2 differ in the predominant mechanism used for selection of genomic RNA for encapsidation. J. Virol. 1999, 73, 3023–3031. [Google Scholar] [PubMed]
- Aldovini, A.; Young, R.A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J. Virol. 1990, 64, 1920–1926. [Google Scholar] [PubMed]
- Schmalzbauer, E.; Strack, B.; Dannull, J.; Guehmann, S.; Moelling, K. Mutations of basic amino acids of NCp7 of human immunodeficiency virus type 1 affect RNA binding in vitro. J. Virol. 1996, 70, 771–777. [Google Scholar] [PubMed]
- Berkhout, B.; van Wamel, J.L. The leader of the HIV-1 RNA genome forms a compactly folded tertiary structure. RNA 2000, 6, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.P.S.; Rhodes, T.D.; Chen, J.; Fu, W.; Hu, W.-S. Identification of a major restriction in HIV-1 intersubtype recombination. Proc. Natl. Acad. Sci. USA 2005, 102, 9002–9007. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.C.; Lever, A.M.L. Human immunodeficiency virus type 1 Gag polyprotein modulates its own translation. J. Virol. 2006, 80, 10478–10486. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Spahr, P.F. Nucleotide sequence at the binding site for coat protein on RNA of bacteriophage R17. Proc. Natl. Acad. Sci. USA 1972, 69, 3033–3037. [Google Scholar] [CrossRef] [PubMed]
- Poot, R.; Tsareva, N.V.; Boni, I.V.; van Duin, J. RNA folding kinetics regulates translation of phage MS2 maturation gene. Proc. Natl. Acad. Sci. 1997, 94, 10110–10115. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, B.; Ooms, M.; Beerens, N.; Huthoff, H.; Southern, E.; Verhoef, K. in vitro evidence that the untranslated leader of the HIV-1 genome is an RNA checkpoint that regulates multiple functions through conformational changes. J. Biol. Chem. 2002, 277, 19967–19975. [Google Scholar] [CrossRef] [PubMed]
- Seif, E.; Niu, M.; Kleiman, L. Annealing to sequences within the primer binding site loop promotes an HIV-1 RNA conformation favoring RNA dimerization and packaging. RNA 2013, 19, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Gamarnik, A.V.; Andino, R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev 1998, 12, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, G.; Guo, R.; Shapiro, B.A.; Simon, A.E. A pseudoknot in a preactive form of a viral RNA is part of a structural switch activating minus-strand synthesis. J. Virol. 2006, 80, 9181–9191. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Simon, S.M.; Bieniasz, P.D. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc. Natl. Acad. Sci. USA 2009, 106, 19114–19119. [Google Scholar] [PubMed]
- Kemler, I.; Meehan, A.; Poeschla, E.M. Live-cell coimaging of the genomic RNAs and Gag proteins of two lentiviruses. J. Virol. 2010, 84, 6352–6366. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Lainé, S.; Pessel-Vivares, L.; Mougel, M. Cell biology of retroviral RNA packaging. RNA Biol. 2011, 8, 572–580. [Google Scholar] [PubMed]
- Baumgärtel, V.; Müller, B.; Lamb, D.C. Quantitative live-cell imaging of human immunodeficiency virus (HIV-1) assembly. Viruses 2012, 4, 777–799. [Google Scholar] [PubMed]
- Kutluay, S.B.; Bieniasz, P.D. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 2010, 6, e1001200. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Grunwald, D.; Sardo, L.; Galli, A.; Plisov, S.; Nikolaitchik, O.A.; Chen, D.; Lockett, S.; Larson, D.R.; Pathak, V.K.; Hu, W.-S. Cytoplasmic HIV-1 RNA is mainly transported by diffusion in the presence or absence of Gag protein. Proc. Natl. Acad. Sci. USA 2014, 111, E5205–E5213. [Google Scholar] [CrossRef] [PubMed]
- Göttlinger, H.G.; Sodroski, J.G.; Haseltine, W.A. Role of Capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1989, 86, 5781–5785. [Google Scholar] [PubMed]
- Saad, J.S.; Miller, J.; Tai, J.; Kim, A.; Ghanam, R.H.; Summers, M.F. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc. Natl. Acad. Sci. USA 2006, 103, 11364–11369. [Google Scholar] [PubMed]
- Chukkapalli, V.; Ono, A. Molecular determinants that regulate plasma membrane association of HIV-1 Gag. J. Mol. Biol. 2011, 410, 512–524. [Google Scholar] [PubMed]
- Bukrinsky, M.I.; Sharova, N.; McDonald, T.L.; Pushkarskaya, T.; Tarpley, W.G.; Stevenson, M. Association of Integrase, Matrix, and Reverse Transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. USA 1993, 90, 6125–6129. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.; Dupont, S.; Stevenson, M.; Green, M.R. Sequence-specific interaction between HIV-1 Matrix protein and viral genomic RNA revealed by in vitro genetic selection. RNA 2001, 7, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Lochrie, M.A.; Waugh, S.; Pratt, D.G.; Clever, J.; Parslow, T.G.; Polisky, B. In vitro selection of RNAs that bind to the human immunodeficiency virus type-1 Gag polyprotein. Nucleic Acids Res. 1997, 25, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Ott, D.E.; Coren, L.V.; Gagliardi, T.D. Redundant roles for Nucleocapsid and Matrix RNA-binding sequences in human immunodeficiency virus type 1 assembly. J. Virol. 2005, 79, 13839–13847. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, A.; Still, A.; Barklis, E. Analysis of human immunodeficiency virus type 1 Matrix binding to membranes and nucleic acids. J. Virol. 2009, 83, 12196–12203. [Google Scholar] [CrossRef] [PubMed]
- Parent, L.J.; Gudleski, N. Beyond plasma membrane targeting: Role of the MA domain of Gag in retroviral genome encapsidation. J. Mol. Biol. 2011, 410, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, A.; Barklis, E. The roles of lipids and nucleic acids in HIV-1 assembly. Front. Microbiol. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Katoh, I.; Kyushiki, H.; Sakamoto, Y.; Ikawa, Y.; Yoshinaka, Y. Bovine Leukemia Virus Matrix-associated protein MA(p15): Further processing and formation of a specific complex with the dimer of the 5’-terminal genomic RNA fragment. J. Virol. 1991, 65, 6845–6855. [Google Scholar] [PubMed]
- Leis, J.P.; McGinnis, J.; Green, R.W. Rous Sarcoma Virus p19 binds to specific double-stranded regions of viral RNA: Effect of p19 on cleavage of viral RNA by RNase III. Virology 1978, 84, 87–98. [Google Scholar] [CrossRef]
- Chukkapalli, V.; Oh, S.J.; Ono, A. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the Matrix domain. Proc. Natl. Acad. Sci. USA 2010, 107, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, A.; McNett, H.; Tsagli, S.; Bächinger, H.P.; Peyton, D.H.; Barklis, E. HIV-1 Matrix protein binding to RNA. J. Mol. Biol. 2011, 410, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.A.K.; Curtis, J.E.; Ratcliff, W.; Clark, P.K.; Crist, R.M.; Lebowitz, J.; Krueger, S.; Rein, A. Conformation of the HIV-1 Gag Protein in Solution. J. Mol. Biol. 2007, 365, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.A.K.; Heinrich, F.; Raghunandan, S.; Krueger, S.; Curtis, J.E.; Rein, A.; Nanda, H. HIV-1 Gag extension: Conformational changes require simultaneous interaction with membrane and nucleic acid. J. Mol. Biol. 2011, 406, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sturgeon, T.; Chen, C.; Watkins, S.C.; Weisz, O.A.; Montelaro, R.C. Distinct intracellular trafficking of Equine Infectious Anemia Virus and human immunodeficiency virus type 1 Gag during viral assembly and budding revealed by bimolecular fluorescence complementation assays. J. Virol. 2007, 81, 11226–11235. [Google Scholar] [CrossRef] [PubMed]
- Mariani, R.; Rutter, G.; Harris, M.E.; Hope, T.J.; Kräusslich, H.G.; Landau, N.R. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 2000, 74, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Naji, S.; Ambrus, G.; Cimermancic, P.; Reyes, J.R.; Johnson, J.R.; Filbrandt, R.; Huber, M.D.; Vesely, P.; Krogan, N.J.; Yates, J.R.; et al. Host cell interactome of HIV-1 Rev includes RNA helicases involved in multiple facets of virus production. Mol. Cell. Proteom. 2012, 11, M111.015313. [Google Scholar] [CrossRef] [PubMed]
- Muriaux, D.; Mirro, J.; Harvin, D.; Rein, A. RNA is a structural element in retrovirus particles. Proc. Natl. Acad. Sci. USA 2001, 98, 5246–5251. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Noonan, K.; Aldovini, A. Nucleocapsid-RNA interactions are essential to structural stability but not to assembly of retroviruses. J. Virol. 2004, 78, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Houzet, L.; Morichaud, Z.; Didierlaurent, L.; Muriaux, D.; Darlix, J.L.; Mougel, M. Nucleocapsid mutations turn HIV-1 into a DNA-containing virus. Nucleic Acids Res. 2008, 36, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Bosche, W.J.; Shatzer, T.L.; Johnson, D.G.; Gorelick, R.J. Mutations in human immunodeficiency virus type 1 Nucleocapsid protein zinc fingers cause premature reverse transcription. J. Virol. 2008, 82, 9318–9328. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.S.; Liang, C.; Wainberg, M.A. Is HIV-1 RNA dimerization a prerequisite for packaging? Yes, no, probably? Retrovirology 2004, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, M.; Clerté, C.; Chamontin, C.; Basyuk, E.; Lainé, S.; Hottin, J.; Bertrand, E.; Margeat, E.; Mougel, M. Imaging HIV-1 RNA dimerization in cells by multicolor super-resolution and fluctuation microscopies. Nucleic Acids Res. 2016, gkw511. [Google Scholar] [CrossRef] [PubMed]
- Borodavka, A.; Tuma, R.; Stockley, P.G. Evidence that viral RNAs have evolved for efficient, two-stage packaging. Proc. Natl. Acad. Sci. 2012, 109, 15769–15774. [Google Scholar] [CrossRef] [PubMed]
- Stockley, P.G.; Twarock, R.; Bakker, S.E.; Barker, A.M.; Borodavka, A.; Dykeman, E.; Ford, R.J.; Pearson, A.R.; Phillips, S.E.V.; Ranson, N.A.; et al. Packaging signals in single-stranded RNA viruses: Nature’s alternative to a purely electrostatic assembly mechanism. J. Biol. Phys. 2013, 39, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Stockley, P.G.; White, S.J.; Dykeman, E.; Manfield, I.; Rolfsson, O.; Patel, N.; Bingham, R.; Barker, A.; Wroblewski, E.; Chandler-Bostock, R.; et al. Bacteriophage MS2 genomic RNA encodes an assembly instruction manual for its capsid. Bacteriophage 2016, 6, e1157666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, S.J.; Dokland, T.; Ojala, P.M.; Bamford, D.H.; Fuller, S.D. Intermediates in the assembly pathway of the double-stranded RNA virus φ6. EMBO J. 1997, 16, 4477–4487. [Google Scholar] [CrossRef] [PubMed]
- Huiskonen, J.T.; de Haas, F.; Bubeck, D.; Bamford, D.H.; Fuller, S.D.; Butcher, S.J. Structure of the bacteriophage φ6 Nucleocapsid suggests a mechanism for sequential RNA packaging. Structure 2006, 14, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Mindich, L.; Qiao, X.; Qiao, J. Packaging of multiple copies of reduced-size genomic segments by bacteriophage φ6. Virology 1995, 212, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Campsteijn, C.; Vietri, M.; Stenmark, H. Novel ESCRT functions in cell biology: Spiraling out of control? Curr. Opin. Cell Biol. 2016, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Ip, N.C.Y.; Prestwood, L.J.; Abbink, T.E.M.; Lever, A.M.L. Evidence that the endosomal sorting complex required for transport-II (ESCRT-II) is required for efficient human immunodeficiency virus-1 (HIV-1) production. Retrovirology 2015, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Ghoujal, B.; Milev, M.P.; Ajamian, L.; Abel, K.; Mouland, A.J. ESCRT-II’s involvement in HIV-1 genomic RNA trafficking and assembly. Biol. Cell 2012, 104, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Sette, P.; Dussupt, V.; Bouamr, F. Identification of the HIV-1 NC binding interface in Alix Bro1 reveals a role for RNA. J. Virol. 2012, 86, 11608–11615. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nikolaitchik, O.; Singh, J.; Wright, A.; Bencsics, C.E.; Coffin, J.M.; Ni, N.; Lockett, S.; Pathak, V.K.; Hu, W.-S. High efficiency of HIV-1 genomic RNA packaging and heterozygote formation revealed by single virion analysis. Proc. Natl. Acad. Sci. USA 2009, 106, 13535–13540. [Google Scholar] [CrossRef] [PubMed]
- Karacostas, V.; Nagashima, K.; Gonde, M.A.; Moss, B. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc. Natl. Acad. Sci. USA 1989, 86, 8964–8967. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.H.; Manchester, M.; Swanstrom, R. The activity of the protease of human immunodeficiency virus type 1 is initiated at the membrane of infected cells before the release of viral proteins and is required for release to occur with maximum efficiency. J. Virol. 1994, 68, 6782–6786. [Google Scholar] [PubMed]
- Lee, S.-K.; Potempa, M.; Swanstrom, R. The choreography of HIV-1 proteolytic processing and virion assembly. J. Biol. Chem. 2012, 287, 40867–40874. [Google Scholar] [CrossRef] [PubMed]
- Mattei, S.; Schur, F.K.M.; Briggs, J.A.G. Retrovirus maturation—An extraordinary structural transformation. Curr. Opin. Virol. 2016, 18, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bendjennat, M.; Saffarian, S. The race against Protease activation defines the role of ESCRTs in HIV budding. PLOS Pathog. 2016, 12, e1005657. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, K.; Rutter, G.; Kottler, H.; Tessmer, U.; Hohenberg, H.; Kräusslich, H.G. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J. Virol. 1998, 72, 2846–2854. [Google Scholar] [PubMed]
- Li, F.; Goila-Gaur, R.; Salzwedel, K.; Kilgore, N.R.; Reddick, M.; Matallana, C.; Castillo, A.; Zoumplis, D.; Martin, D.E.; Orenstein, J.M.; et al. PA-457: A potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc. Natl. Acad. Sci. USA 2003, 100, 13555–13560. [Google Scholar] [CrossRef] [PubMed]
- Pettit, S.C.; Moody, M.D.; Wehbie, R.S.; Kaplan, A.H.; Nantermet, P.V.; Klein, C.A.; Swanstrom, R. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 1994, 68, 8017–8027. [Google Scholar] [PubMed]
- Liang, C.; Rong, L.; Laughrea, M.; Kleiman, L.; Wainberg, M.A. Compensatory point mutations in the human immunodeficiency virus type 1 Gag region that are distal from deletion mutations in the dimerization initiation site can restore viral replication. J. Virol. 1998, 72, 6629–6636. [Google Scholar] [PubMed]
- Liang, C.; Rong, L.; Quan, Y.; Laughrea, M.; Kleiman, L.; Wainberg, M.A. Mutations within four distinct Gag proteins are required to restore replication of human immunodeficiency virus type 1 after deletion mutagenesis within the dimerization initiation site. J. Virol. 1999, 73, 7014–7020. [Google Scholar] [PubMed]
- Liang, C.; Rong, L.; Russell, R.S.; Wainberg, M.A. Deletion mutagenesis downstream of the 5’ long terminal repeat of human immunodeficiency virus type 1 is compensated for by point mutations in both the U5 region and Gag gene. J. Virol. 2000, 74, 6251–6261. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Russell, R.S.; Hu, J.; Guan, Y.; Kleiman, L.; Liang, C.; Wainberg, M.A. Hydrophobic amino acids in the human immunodeficiency virus type 1 p2 and Nucleocapsid proteins can contribute to the rescue of deleted viral RNA packaging signals. J. Virol. 2001, 75, 7230–7243. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.S.; Roldan, A.; Detorio, M.; Hu, J.; Wainberg, M.A.; Liang, C. Effects of a single amino acid substitution within the p2 region of human immunodeficiency virus type 1 on packaging of spliced viral RNA. J. Virol. 2003, 77, 12986–12995. [Google Scholar] [CrossRef] [PubMed]
- Potempa, M.; Nalivaika, E.; Ragland, D.; Lee, S.-K.; Schiffer, C.A.; Swanstrom, R. A direct interaction with RNA dramatically enhances the catalytic activity of the HIV-1 Protease in vitro. J. Mol. Biol. 2015, 427, 2360–2378. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellmund, C.; Lever, A.M.L. Coordination of Genomic RNA Packaging with Viral Assembly in HIV-1. Viruses 2016, 8, 192. https://doi.org/10.3390/v8070192
Hellmund C, Lever AML. Coordination of Genomic RNA Packaging with Viral Assembly in HIV-1. Viruses. 2016; 8(7):192. https://doi.org/10.3390/v8070192
Chicago/Turabian StyleHellmund, Chris, and Andrew M. L. Lever. 2016. "Coordination of Genomic RNA Packaging with Viral Assembly in HIV-1" Viruses 8, no. 7: 192. https://doi.org/10.3390/v8070192
APA StyleHellmund, C., & Lever, A. M. L. (2016). Coordination of Genomic RNA Packaging with Viral Assembly in HIV-1. Viruses, 8(7), 192. https://doi.org/10.3390/v8070192