Pharmaceutics 2021, 13(1), 51; https://doi.org/10.3390/pharmaceutics13010051 - 31 Dec 2020
Cited by 2 | Viewed by 2429
Abstract
The effects of tiotropium bromide soft mist inhalers (SMIs) in patients with chronic obstructive pulmonary disease (COPD) receiving mechanical ventilation remain unexplored. This study investigated the dynamic effects of a tiotropium SMI on lung mechanics and gas exchange in these patients. We analyzed
[...] Read more.
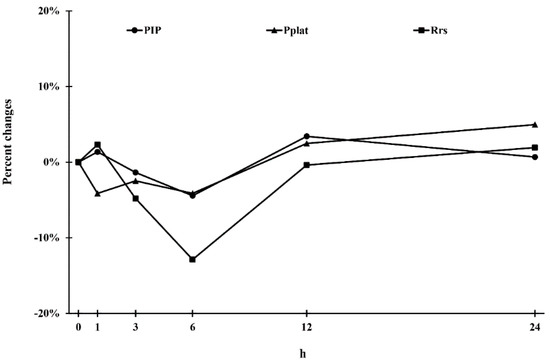
The effects of tiotropium bromide soft mist inhalers (SMIs) in patients with chronic obstructive pulmonary disease (COPD) receiving mechanical ventilation remain unexplored. This study investigated the dynamic effects of a tiotropium SMI on lung mechanics and gas exchange in these patients. We analyzed 11 mechanically ventilated and hemodynamically stable patients with COPD who experienced acute exacerbation and were ready to be weaned from the ventilator. Two puffs of tiotropium (2.5 μg/puff) were administered with a T-adaptor connected to the ventilator circuit. Lung mechanics—peak inspiratory pressure, plateau pressure, mean airway pressure, maximum respiratory resistance (Rrs), and gas exchange function—were analyzed. The two-puff tiotropium SMI treatment led to the greatest reduction in Rrs at 6 h, with the Rrs returning to baseline gradually, and significantly improved the PaO2/FiO2 ratio at 24 h. Compared with baseline values, tiotropium SMI had the strongest effect on Rrs between hours 3 and 6 but did not significantly affect hemodynamic parameters. Tiotropium SMI administration in mechanically ventilated patients with COPD achieved the greatest reduction in Rrs at 6 h and significantly improved the PaO2/FiO2 ratio at 24 h. Future studies should investigate whether the bronchodilation effect can be improved with increased dosage or frequency.
Full article
(This article belongs to the Special Issue Medical Aerosol Drug Delivery)
►
Show Figures