Langerhans Cells—Revising Their Role in Skin Pathologies
Abstract
:1. Introduction
2. Inflammation and Inflammation-Mediated Skin Pathologies
2.1. Role of Langerhans Cells in Wound Healing
2.2. Role of Langerhans Cells in Psoriasis
2.3. The Role of Langerhans Cells in Other Inflammatory Skin Pathologies
3. Skin Cancers
4. LCs as Therapeutic Targets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collin, M.; Milne, P. Langerhans cell origin and regulation. Curr. Opin. Hematol. 2016, 23, 28–35. [Google Scholar] [CrossRef] [Green Version]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells as Immune Regulators within the Skin. Front Immunol. 2018, 8, 1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissmann, F.; Dieu-Nosjean, M.C.; Dezutter, C.; Valladeau, J.; Kayal, S.; Leborgne, M.; Brousse, N.; Saeland, S.; Davoust, J. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 2002, 196, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Ginhoux, F.; Tacke, F.; Angeli, V.; Bogunovic, M.; Loubeau, M.; Dai, X.M.; Stanley, E.R.; Randolph, G.J.; Merad, M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006, 7, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Nagao, K.; Kobayashi, T.; Moro, K.; Ohyama, M.; Adachi, T.; Kitashima, D.Y.; Ueha, S.; Horiuchi, K.; Tanizaki, H.; Kabashima, K.; et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012, 13, 744–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sere, K.; Baek, J.H.; Ober-Blobaum, J.; Muller-Newen, G.; Tacke, F.; Yokota, Y.; Zenke, M.; Hieronymus, T. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 2012, 37, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Merad, M.; Ginhoux, F.; Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008, 8, 935–947. [Google Scholar] [CrossRef]
- Raaby, L.; Rosada, C.; Langkilde, A.; Lauridsen, K.L.; Vinter, H.; Ommen, P.; Kjellerup, R.B.; Johansen, C.; Iversen, L. Langerhans cell markers CD1a and CD207 are the most rapidly responding genes in lesional psoriatic skin following adalimumab treatment. Exp. Derm. 2017, 26, 804–810. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, R.; Luo, Y.; Wang, S.; Zhao, Y.; Qiu, Z.; Zhang, Y.; Liu, X.; Yao, X.; Li, X.; et al. Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation. Immunity 2021, 54, 2305–2320.e11. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021, 371, eaba6500. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Sedgewick, A.J.; Finnegan, A.I.; Harirchian, P.; Lee, J.; Kwon, S.; Fassett, M.S.; Golovato, J.; Gray, M.; Ghadially, R.; et al. Transcriptional Programming of Normal and Inflamed Human Epidermis at Single-Cell Resolution. Cell Rep. 2018, 25, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Szretter, K.J.; Vermi, W.; Gilfillan, S.; Rossini, C.; Cella, M.; Barrow, A.D.; Diamond, M.S.; Colonna, M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012, 13, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strid, J.; Roberts, S.J.; Filler, R.B.; Lewis, J.M.; Kwong, B.Y.; Schpero, W.; Kaplan, D.H.; Hayday, A.C.; Girardi, M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008, 9, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Hsiung, B.; Pestal, K.; Procyk, E.; Raulet, D.H. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J. Exp. Med. 2012, 209, 2409–2422. [Google Scholar] [CrossRef] [Green Version]
- Strid, J.; Sobolev, O.; Zafirova, B.; Polic, B.; Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 2011, 334, 1293–1297. [Google Scholar] [CrossRef] [Green Version]
- Kimber, I.; Cumberbatch, M.; Dearman, R.J.; Bhushan, M.; Griffiths, C.E. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br. J. Derm. 2000, 142, 401–412. [Google Scholar] [CrossRef]
- Juhász, I.; Simon, M., Jr.; Herlyn, M.; Hunyadi, J. Repo.opulation of Langerhans cells during wound healing in an experimental human skin/SCID mouse model. Immunol. Lett. 1996, 52, 125–128. [Google Scholar] [CrossRef]
- Johnson, L.A.; Clasper, S.; Holt, A.P.; Lalor, P.F.; Baban, D.; Jackson, D.G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006, 203, 2763–2777. [Google Scholar] [CrossRef] [Green Version]
- Stojadinovic, O.; Yin, N.; Lehmann, J.; Pastar, I.; Kirsner, R.S.; Tomic-Canic, M. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol. Res. 2013, 57, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef]
- Antal, D.; Alimohammadi, S.; Bai, P.; Szöllősi, A.G.; Szántó, M. Antigen-Presenting Cells in Psoriasis. Life 2022, 3, 234. [Google Scholar] [CrossRef] [PubMed]
- Surcel, M.; Huică, R.I.; Munteanu, A.N.; Isvoranu, G.; Pîrvu, I.R.; Ciotaru, D.; Constantin, C.; Bratu, O.; Căruntu, C.; Neagu, M.; et al. Phenotypic changes of lymphocyte populations in psoriasiform dermatitis animal model. Exp. Med. 2019, 17, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshiki, R.; Kabashima, K.; Honda, T.; Nakamizo, S.; Sawada, Y.; Sugita, K.; Yoshioka, H.; Ohmori, S.; Malissen, B.; Tokura, Y.; et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing gammadelta T cells. J. Investig. Derm. 2014, 134, 1912–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohn, C.; Ober-Blöbaum, J.L.; Haak, S.; Pantelyushin, S.; Cheong, C.; Zahner, S.P.; Onderwater, S.; Kant, M.; Weighardt, H.; Holzmann, B.; et al. Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10723–10728. [Google Scholar] [CrossRef] [Green Version]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Singh, T.; Zhang, H.; Borek, I.; Wolf, P.; Hedrick, M.N.; Singh, S.P.; Kelsall, B.L.; Clausen, B.E.; Farber, J.M. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat. Commun. 2016, 7, 13581. [Google Scholar] [CrossRef] [Green Version]
- Gordon, K.B.; Colombel, J.F.; Hardin, D.S. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2016, 375, 2102. [Google Scholar] [CrossRef]
- Kim, J.H.; Hu, Y.; Yongqing, T.; Kim, J.; Hughes, V.A.; Le Nours, J.; Marquez, E.A.; Purcell, A.W.; Wan, Q.; Sugita, M.; et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 2016, 17, 1159–1166. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Lu, Y.; Sun, X.Y.; Ze, K.; Zhou, Y.Q.; Li, W.; Li, X.; Li, H.J.; Li, B. Celastrol gel ameliorates imiquimod-induced psoriasis-like dermatitis in mice by targeting Langerhans cells. Biomed. Pharmacother. 2022, 147, 112644. [Google Scholar] [CrossRef]
- Kase, M.; Fujita, Y.; Ota, A.; Shimizu, S.; Itoi-Ochi, S.; Sano, S. Loss of epidermal Langerhans cells in psoriasiform lesions of de novo induced or worsened pre-existing psoriasis following uses of immune checkpoint inhibitors. J. Dermatol. 2022, 49, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.A.; Ho, J.; De La Cruz Diaz, J.S.; Nishimura, S.; Kaplan, D.H. TGFβ activating integrins β6 and β8 are dysregulated in inflammatory skin disease and cutaneous melanoma. J. Derm. Sci. 2022, 106, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Garces, S.; Yin, C.C.; Miranda, R.N.; Patel, K.P.; Li, S.; Xu, J.; Thakral, B.; Poppiti, R.J.; Medina, A.M.; Sriganeshan, V.; et al. Clinical, histopathologic, and immunoarchitectural features of dermatopathic lymphadenopathy: An update. Mod. Pathol. 2020, 33, 1104–1121. [Google Scholar] [CrossRef] [PubMed]
- Boda, D.; Neagu, M.; Constantin, C.; Voinescu, R.N.; Caruntu, C.; Zurac, S.; Spandidos, D.A.; Drakoulis, N.; Tsoukalas, D.; Tsatsakis, A.M. HPV strain distribution in patients with genital warts in a female population sample. Oncol. Lett. 2016, 12, 1779–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperling, T.; Ołdak, M.; Walch-Rückheim, B.; Wickenhauser, C.; Doorbar, J.; Pfister, H.; Malejczyk, M.; Majewski, S.; Keates, A.C.; Smola, S. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012, 8, e1002833. [Google Scholar] [CrossRef]
- de Koning, H.D.; Bergboer, J.G.; van den Bogaard, E.H.; van Vlijmen-Willems, I.M.; Rodijk-Olthuis, D.; Simon, A.; Zeeuwen, P.L.; Schalkwijk, J. Strong induction of AIM2 expression in human epidermis in acute and chronic inflammatory skin conditions. Exp. Dermatol. 2012, 21, 961–964. [Google Scholar] [CrossRef]
- Marschall, P.; Wei, R.; Segaud, J.; Yao, W.; Hener, P.; German, B.F.; Meyer, P.; Hugel, C.; Ada Da Silva, G.; Braun, R.; et al. Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1778–1794. [Google Scholar] [CrossRef]
- Dubrac, S.; Schmuth, M.; Ebner, S. Atopic dermatitis: The role of Langerhans cells in disease pathogenesis. Immunol. Cell Biol. 2010, 88, 400–409. [Google Scholar] [CrossRef]
- Iwamoto, K.; Nümm, T.J.; Koch, S.; Herrmann, N.; Leib, N.; Bieber, T. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy 2018, 73, 2205–2213. [Google Scholar] [CrossRef]
- Kabashima, K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J. Derm. Sci. 2013, 70, 3–11. [Google Scholar] [CrossRef]
- Cancer Facts and Statistics—American Cancer Society. Available online: www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-andfigures-2021.pdf (accessed on 1 January 2020).
- Ilie, M.A.; Caruntu, C.; Tampa, M.; Georgescu, S.-R.; Lixandru, D.; Constantin, C.; Neagu, M.; Zurac, S.A.; Boda, D. In vivo Confocal Laser Scanning Microscopy Imaging of Skin Inflammation: Clinical Applications and Research Directions. Exp. Ther. Med. 2019, 17, 1004–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilie, M.A.; Caruntu, C.; Rosca, A.E.; Kaleshi, H.; Caruntu, A.; Moraru, L.; Docea, A.O.; Zurac, S.A.; Boda, D.; Neagu, M.; et al. Reflectance confocal microscopy and dermoscopy for in vivo, noninvasive skin imaging of superficial basal cell carcinoma. Oncol. Lett. 2016, 11, 3019–3024. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, P.; Carrasco, C. Langerhans cell histiocytosis. In Bone Tumors; Paulos, J., Poitout, D.G., Eds.; Springer: London, UK, 2021. [Google Scholar] [CrossRef]
- Abla, O.; Weitzman, S. Treatment of Langerhans cell histiocytosis: Role of BRAF/MAPK inhibition. Hematol. Am. Soc. Hematol. Educ. Program. 2015, 2015, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, M.; Néron, A.; Duvert-Lehembre, S.; Amine Larabi, I.; Barkaoui, M.; Emile, J.F.; Seigneurin, A.; Boralevi, F.; Donadieu, J. Cutaneous adverse events in children treated with vemurafenib for refractory BRAF(V600E) mutated Langerhans cell histiocytosis. Pediatr. Blood Cancer 2021, 68, e29140. [Google Scholar] [CrossRef]
- Oneal, P.A.; Kwitkowski, V.; Luo, L.; Shen, Y.L.; Subramaniam, S.; Shord, S.; Goldberg, K.B.; McKee, A.E.; Kaminskas, E.; Farrell, A.; et al. FDA Approval Summary: Vemurafenib for the Treatment of Patients with Erdheim-Chester Disease with the BRAFV600 Mutation. Oncologist 2018, 23, 1520–1524. [Google Scholar] [CrossRef] [Green Version]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Goto, K.; Yoshikawa, S.; Kiyohara, Y.; Kukita, Y.; Miura, K.; Oishi, T. Co-existence of BRAF V600E-mutated malignant melanoma and BRAF V600E-mutated Langerhans cell histiocytosis: A case report. J. Cutan. Pathol. 2022, 49, 393–398. [Google Scholar] [CrossRef]
- Alhaj Moustafa, M.; Jiang, L.; Kuhlman, J.J.; Jones, J.; Lou, Y.; Sokumbi, O.; Tun, H.W. BRAF p.V600E associated poly-neoplastic syndrome. Rare Tumors. 2021, 13, 20363613211012929. [Google Scholar] [CrossRef]
- Chilosi, M.; Facchetti, F.; Caliò, A.; Zamò, A.; Brunelli, M.; Martignoni, G.; Rossi, A.; Montagna, L.; Piccoli, P.; Dubini, A.; et al. Oncogene-induced senescence distinguishes indolent from aggressive forms of pulmonary and non-pulmonary Langerhans cell histiocytosis. Leuk. Lymphoma. 2014, 55, 2620–2626. [Google Scholar] [CrossRef]
- Nelson, D.S.; van Halteren, A.; Quispel, W.T.; van den Bos, C.; Bovee, J.V.; Patel, B.; Badalian-Very, G.; van Hummelen, P.; Ducar, M.; Lin, L.; et al. MAP2K1 and MAP3K1 mutations in langerhans cell histiocytosis. Genes Chromosom. Cancer 2015, 54, 361–368. [Google Scholar] [CrossRef]
- Chakraborty, R.; Hampton, O.A.; Shen, X.; Simko, S.J.; Shih, A.; Abhyankar, H.; Lim, K.P.; Covington, K.R.; Trevino, L.; Dewal, N.; et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood 2014, 124, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Furtado, L.V.; Betz, B.L.; Kiel, M.J.; Weigelin, H.C.; Lim, M.S.; Elenitoba-Johnson, K.S. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood 2014, 124, 1655–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, T.; Li, C.; Wang, H. Trametinib in the treatment of multiple malignancies harboring MEK1 mutations. Cancer Treat. Rev. 2019, 81, 101907. [Google Scholar] [CrossRef] [PubMed]
- Maraee, A.; Farag, A.G.A.; Gadallah, M.M.; Abdou, A.G. Tumour-infiltrating Langerhans cells in non-melanoma skin cancer, a clinical and immunohistochemical study. Ecancer Med. Sci. 2020, 14, 1045. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Yoshida, Y.; Yamada, N.; Adachi, K.; Nanba, E.; Yamamoto, O. Nagashima-type palmoplantar keratosis with melanoma: Absence of epidermal Langerhans cells in hyperkeratotic skin. Eur. J. Dermatol. 2017, 27, 210–212. [Google Scholar] [CrossRef]
- Ilie, M.A.; Caruntu, C.; Lupu, M.; Tampa, M.; Georgescu, S.-R.; Bastian, A.; Constantin, C.; Neagu, M.; Zurac, S.A.; Boda, D. Current and future applications of confocal laser scanning microscopy imaging in skin oncology. Oncol. Lett. 2019, 17, 4102–4111. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, P.; Pulitzer, M.P.; Scope, A.; Kovalyshyn, I.; Halpern, A.C.; Marghoob, A.A. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: A challenge for melanoma diagnosis. J. Am. Acad. Dermatol. 2012, 66, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Chuchvara, N.; Berger, L.; Reilly, C.; Maghari, A.; Rao, B.K. Langerhans Cells as Morphologic Mimickers of Atypical Melanocytes on Reflectance Confocal Microscopy: A Case Report and Review of the Literature. Dermatol. Pract. Concept. 2021, 11, e2021078. [Google Scholar] [CrossRef]
- Mignogna, C.; Scali, E.; Camastra, C.; Presta, I.; Zeppa, P.; Barni, T.; Donato, G.; Bottoni, U.; Di Vito, A. Innate immunity in cutaneous melanoma. Clin. Exp. Dermatol. 2017, 42, 243–250. [Google Scholar] [CrossRef]
- Kumar, V. Going, Toll-like receptors in skin inflammation and inflammatory diseases. EXCLI J. 2021, 20, 52–79. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Zurac, S. Immune parameters in the prognosis and therapy monitoring of cutaneous melanoma patients: Experience, role, and limitations. Biomed. Res. Int. 2013, 2013, 107940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Ohno, Y.; Toyoshima, Y.; Ohtake, J.; Homma, S.; Kawamura, H.; Takahashi, N.; Taketomi, A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017, 108, 1947–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlini, G.; Di Gennaro, P.; Pimpinelli, N.; Sestini, S.; Borgognoni, L. Tolerogenic IDO1(+)CD83(-) Langerhans Cells in Sentinel Lymph Nodes of Patients with Melanoma. Int. J. Mol. Sci. 2022, 23, 3441. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, P.; Romoli, M.R.; Gerlini, G.; D’Amico, M.; Brandani, P.; Pimpinelli, N.; Borgognoni, L. IDO and CD83 expression in human epidermal Langerhans cells. J. Dermatol. Sci. 2014, 73, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.R.; Di Gennaro, P.; Gerlini, G.; Sestini, S.; Brandani, P.; Ferrone, S.; Borgognoni, L. High Antigen Processing Machinery component expression in Langerhans cells from melanoma patients’ sentinel lymph nodes. Cell Immunol. 2017, 320, 29–37. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Investig. 2007, 117, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- von Bubnoff, D.; Bausinger, H.; Matz, H.; Koch, S.; Häcker, G.; Takikawa, O.; Bieber, T.; Hanau, D.; de la Salle, H. Human epidermal Langerhans cells express the immuno regulatory enzyme indoleamine 2,3-dioxygenase. J. Investig. Dermatol. 2004, 123, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, J.; Beura, L.K.; Bobr, A.; Astry, B.; Chicoine, B.; Kashem, S.W.; Welty, N.E.; Igyártó, B.Z.; Wijeyesinghe, S.; Thompson, E.A.; et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat. Immunol. 2016, 17, 414–421. [Google Scholar] [CrossRef]
- Weng, W.H.; Liao, Y.H.; Tsai, M.R.; Wei, M.L.; Huang, H.Y.; Sun, C.K. Differentiating intratumoral melanocytes from Langerhans cells in nonmelanocytic pigmented skin tumors in vivo by label-free third-harmonic generation microscopy. J. Biomed. Opt. 2016, 21, 76009. [Google Scholar] [CrossRef] [Green Version]
- Pellacani, G.; De Pace, B.; Reggiani, C.; Cesinaro, A.M.; Argenziano, G.; Zalaudek, I.; Soyer, H.P.; Longo, C. Distinct melanoma types based on reflectance confocal microscopy. Exp. Dermatol. 2014, 23, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Noordegraaf, M.; Martina, C.A.; Clausen, B.E. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J. Immunol. 2007, 179, 6830–6835. [Google Scholar] [CrossRef] [Green Version]
- Kwiek, B.; Leśniewska, A.; Kowalewski, C.; Woźniak, K. Langerhans cells are predominant high affinity immunoglobulin E receptor bearing cells in the epidermis of bullous pemphigoid skin. J. Derm. Sci. 2017, 85, 60–63. [Google Scholar] [CrossRef] [PubMed]
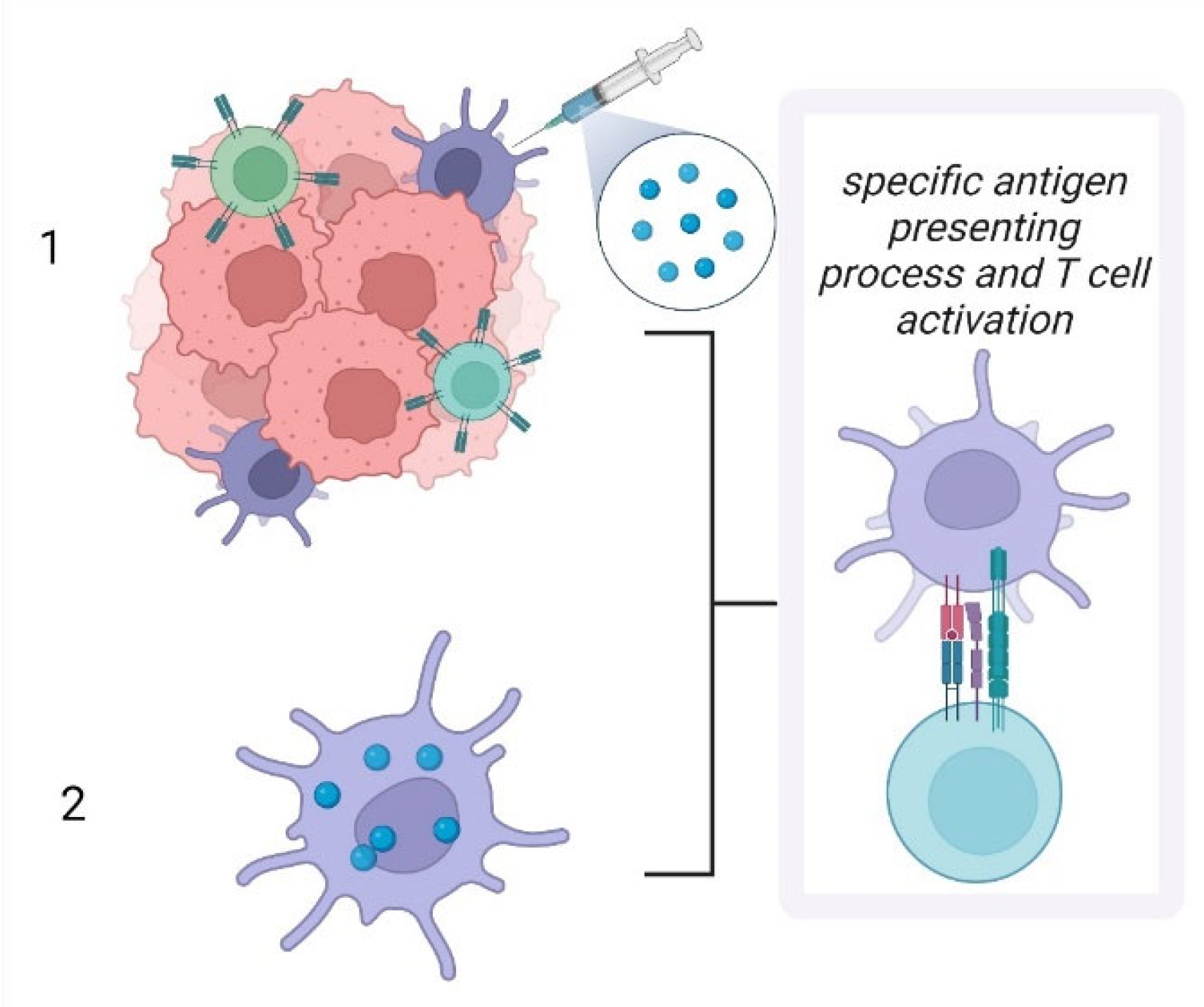
- Kozaka, S.; Tahara, Y.; Wakabayashi, R.; Nakata, T.; Ueda, T.; Kamiya, N.; Goto, M. Transcutaneous Cancer Vaccine Using a Reverse Micellar Antigen Carrier. Mol. Pharm. 2020, 17, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.M.; Corthésy, B.; Merkle, H.P. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv. Drug Deliv. Rev. 2013, 65, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Tajpara, P.; Schuster, C.; Schön, E.; Kienzl, P.; Vierhapper, M.; Mildner, M.; Elbe-Bürger, A. Epicutaneous administration of the pattern recognition receptor agonist polyinosinic-polycytidylic acid activates the MDA5/MAVS pathway in Langerhans cells. FASEB J. 2018, 32, 4132–4144. [Google Scholar] [CrossRef] [Green Version]
- Duinkerken, S.; Horrevorts, S.K.; Kalay, H.; Ambrosini, M.; Rutte, L.; de Gruijl, T.D.; Garcia-Vallejo, J.J.; van Kooyk, Y. Glyco-Dendrimers as Intradermal Anti-Tumor Vaccine Targeting Multiple Skin DC Subsets. Theranostics 2019, 9, 5797–5809. [Google Scholar] [CrossRef]
- Andreini, M.; Doknic, D.; Sutkeviciute, I.; Reina, J.J.; Duan, J.; Chabrol, E.; Thepaut, M.; Moroni, E.; Doro, F.; Belvisi, L.; et al. Second generation of fucose-based DC-SIGN ligands: Affinity improvement and specificity versus Langerin. Org. Biomol. Chem. 2011, 9, 5778–5786. [Google Scholar] [CrossRef] [Green Version]
- Stolk, D.A.; de Haas, A.; Vree, J.; Duinkerken, S.; Lübbers, J.; van de Ven, R.; Ambrosini, M.; Kalay, H.; Bruijns, S.; van der Vliet, H.J.; et al. Lipo-Based Vaccines as an Approach to Target Dendritic Cells for Induction of T- and iNKT Cell Responses. Front. Immunol. 2020, 11, 990. [Google Scholar] [CrossRef]
- Li, R.E.; Hogervorst, T.P.; Achilli, S.; Bruijns, S.C.M.; Spiekstra, S.; Vivès, C.; Thépaut, M.; Filippov, D.V.; van der Marel, G.A.; van Vliet, S.J.; et al. Targeting of the C-Type Lectin Receptor Langerin Using Bifunctional Mannosylated Antigens. Front. Cell Dev. Biol. 2020, 8, 556. [Google Scholar] [CrossRef]
- Meneveau, M.O.; Petroni, G.R.; Salerno, E.P.; Lynch, K.T.; Smolkin, M.; Woodson, E.; Chianese-Bullock, K.A.; Olson, W.C.; Deacon, D.; Patterson, J.W.; et al. Immunogenicity in humans of a transdermal multipeptide melanoma vaccine administered with or without a TLR7 agonist. J. Immunother. Cancer. 2021, 9, e002214. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.J.; Carvajal, R.D.; Postow, M.A.; Sharma, S.; Pronschinske, K.B.; Shyer, J.A.; Singh-Kandah, S.; Dickson, M.A.; D’Angelo, S.P.; Wolchok, J.D.; et al. Langerhans-type dendritic cells electroporated with TRP-2 mRNA stimulate cellular immunity against melanoma: Results of a phase I vaccine trial. Oncoimmunology 2017, 7, e1372081. [Google Scholar] [CrossRef] [PubMed]
- Gerlini, G.; Sestini, S.; Di Gennaro, P.; Urso, C.; Pimpinelli, N.; Borgognoni, L. Dendritic cells recruitment in melanoma metastasis treated by electrochemotherapy. Clin. Exp. Metastasis 2013, 30, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff, D.; Sluijter, B.J.; Hangalapura, B.N.; de Gruijl, T.D. The dermis as a portal for dendritic cell-targeted immunotherapy of cutaneous melanoma. Curr. Top. Microbiol. Immunol. 2012, 351, 181–220. [Google Scholar] [CrossRef]

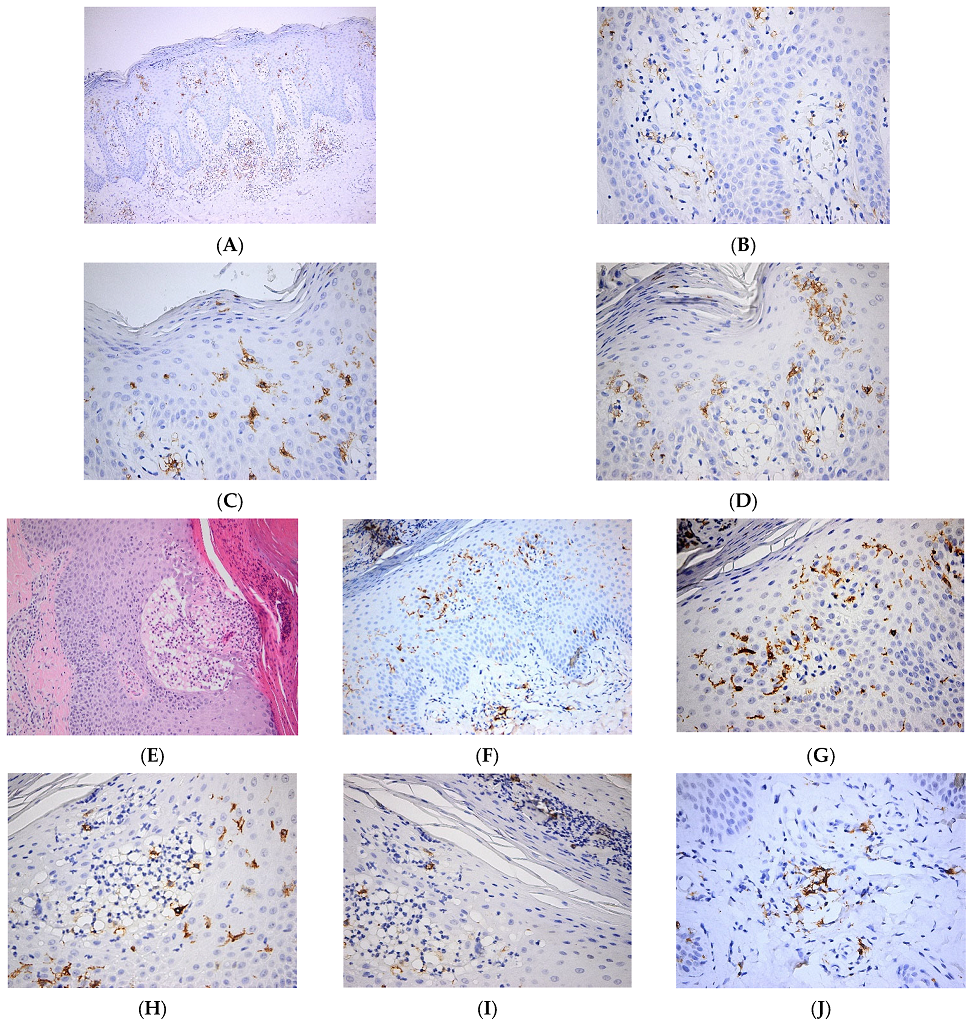

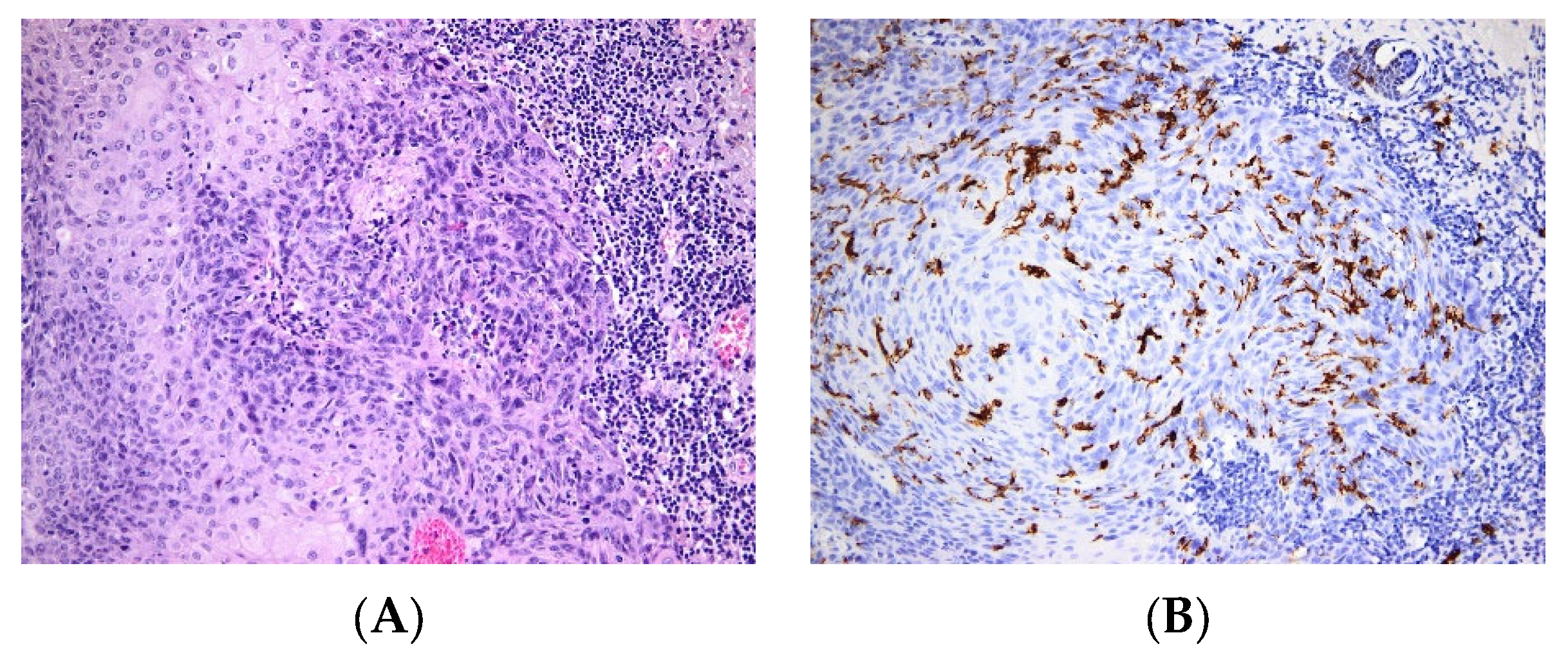
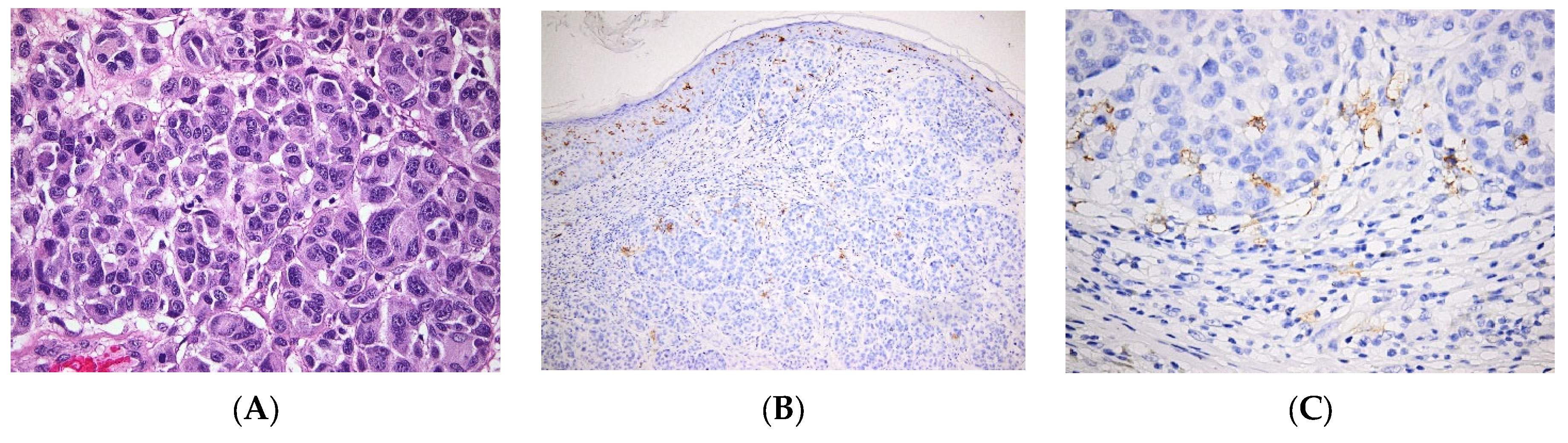

| Pathology | LC Involvement | |
|---|---|---|
| Bacterial and viral infections | Expression of pathogen-recognition receptors, secretion of cytokines, migration to skin draining lymph nodes to generate the adaptive immune response [2] | |
| Wounds | Increased LC migration to skin draining lymph nodes mediated by enhanced expression of MICA B by keratinocytes [14,15]; TNF-mediated LC repopulation of the epidermis contributes to tissue remodeling [17,18] | |
| Other skin pathologies | Psoriasis | Pro-inflammatory role of LCs via increased secretion of IL-23, over-expression of CD1a and increased interaction with γδT cells [27,30] |
| Contact dermatitis | Uptake of allergens and immune response via antigen presentation to T cells [74] | |
| Atopic dermatitis | Activated LCs migrate to skin draining lymph nodes to prime Th2 T cells, increased expression of the high-affinity immunoglobulin (Ig)E receptor [37,38,39,40,75] | |
| Skin cancers | BCC | LC are low in BCC [57] |
| SCC | Various levels of LC infiltration in SCC, alternatively low [56] or high densities [54]. | |
| Melanoma | Tolerogeneic vs. immunogenic role of LCs, infiltration of the tumor with LCs is a marker of good outcome [68] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, M.; Constantin, C.; Jugulete, G.; Cauni, V.; Dubrac, S.; Szöllősi, A.G.; Zurac, S. Langerhans Cells—Revising Their Role in Skin Pathologies. J. Pers. Med. 2022, 12, 2072. https://doi.org/10.3390/jpm12122072
Neagu M, Constantin C, Jugulete G, Cauni V, Dubrac S, Szöllősi AG, Zurac S. Langerhans Cells—Revising Their Role in Skin Pathologies. Journal of Personalized Medicine. 2022; 12(12):2072. https://doi.org/10.3390/jpm12122072
Chicago/Turabian StyleNeagu, Monica, Carolina Constantin, Gheorghita Jugulete, Victor Cauni, Sandrine Dubrac, Attila Gábor Szöllősi, and Sabina Zurac. 2022. "Langerhans Cells—Revising Their Role in Skin Pathologies" Journal of Personalized Medicine 12, no. 12: 2072. https://doi.org/10.3390/jpm12122072
APA StyleNeagu, M., Constantin, C., Jugulete, G., Cauni, V., Dubrac, S., Szöllősi, A. G., & Zurac, S. (2022). Langerhans Cells—Revising Their Role in Skin Pathologies. Journal of Personalized Medicine, 12(12), 2072. https://doi.org/10.3390/jpm12122072










