Abstract
The emergence of the novel SARS-CoV2 virus, proclaimed by the World Health Organization (WHO) as a culpable agent for the pandemic situation, caught the scientific and medical communities off guard. One of the most common complications following pulmonary disease is represented by gastrointestinal (GI) disorders, especially ischemic damage. Inflammation, vasculopathy, immobility, endothelial dysfunction, and a hypercoagulable condition have all been proposed as pathophysiological factors for GI ischemia in these patients. Owing to the COVID-19 effect on a variety of GI conditions, especially ischemic changes, and the high mortality rate, physicians should always keep in mind this complication. They should take a deeper look at clinical and imaging modalities in this cohort of patients so that a proper and time-saving treatment strategy can be applied. Our study aimed to elucidate the thrombogenic mechanism in different GI disorders. Moreover, we analyzed the factors related to necrotic GI changes, by summarizing the already reported data of GI ischemia in COVID-19. To the best of our knowledge, this review is the first to incorporate all GI ischemia cases reported in the literature so far.
1. Introduction
The emergence of the novel SARS-CoV2 virus, proclaimed by the World Health Organization (WHO) as a culpable agent for the pandemic situation, caught the scientific and medical communities off guard. The world witnessed rapid viral spread that finally engulfed the entire planet, causing chaos and carnage. Despite the major respiratory complications, evidence for other organ involvement appeared as the pandemic progressed. One of the most common complications following pulmonary disease is represented by gastrointestinal (GI) disorders. Many literature studies reported GI tract involvement. A review by María-Jimena Mucino-Bermejo et al. presented data about the gastrointestinal manifestation in COVID-19 patients. They pointed out that the esophagus has more than 1% ACE2-positive epithelial cells [1]. On the other hand, gastric and liver cells have <1% ACE2-positive cell expression. Heartburn is the most common clinical sign, and standard treatment with PPIs leads to relief of the symptoms. The authors reported that ACE2 expression in ileal epithelial cells is proximately 30%. An interesting fact is that SARS-CoV-2 can spread from infected to uninfected cells in the GI tract, leading to mucosal immune cell activation [2].
However, there is scarce evidence of GI ischemia in COVID-19 patients. Furthermore, the exact pathogenic process remains obscure. Inflammation, vasculopathy, immobility, endothelial dysfunction, and a hypercoagulable condition have all been proposed as pathophysiological factors for GI ischemia in these patients [3].
Because of the high mortality percentage in GI ischemia, clinicians should implement a high clinical suspicion index to prevent its progression and promptly manage any complications, whether conservative or surgical. Therefore, we aimed to elucidate the thrombogenic mechanism in different GI disorders. Moreover, we analyzed the factors related to necrotic GI changes, by summarizing the already reported data of GI ischemia in COVID-19. To the best of our knowledge, this review is the first to incorporate all GI ischemia cases reported in the literature so far.
2. Pathogenesis in GI Ischemia
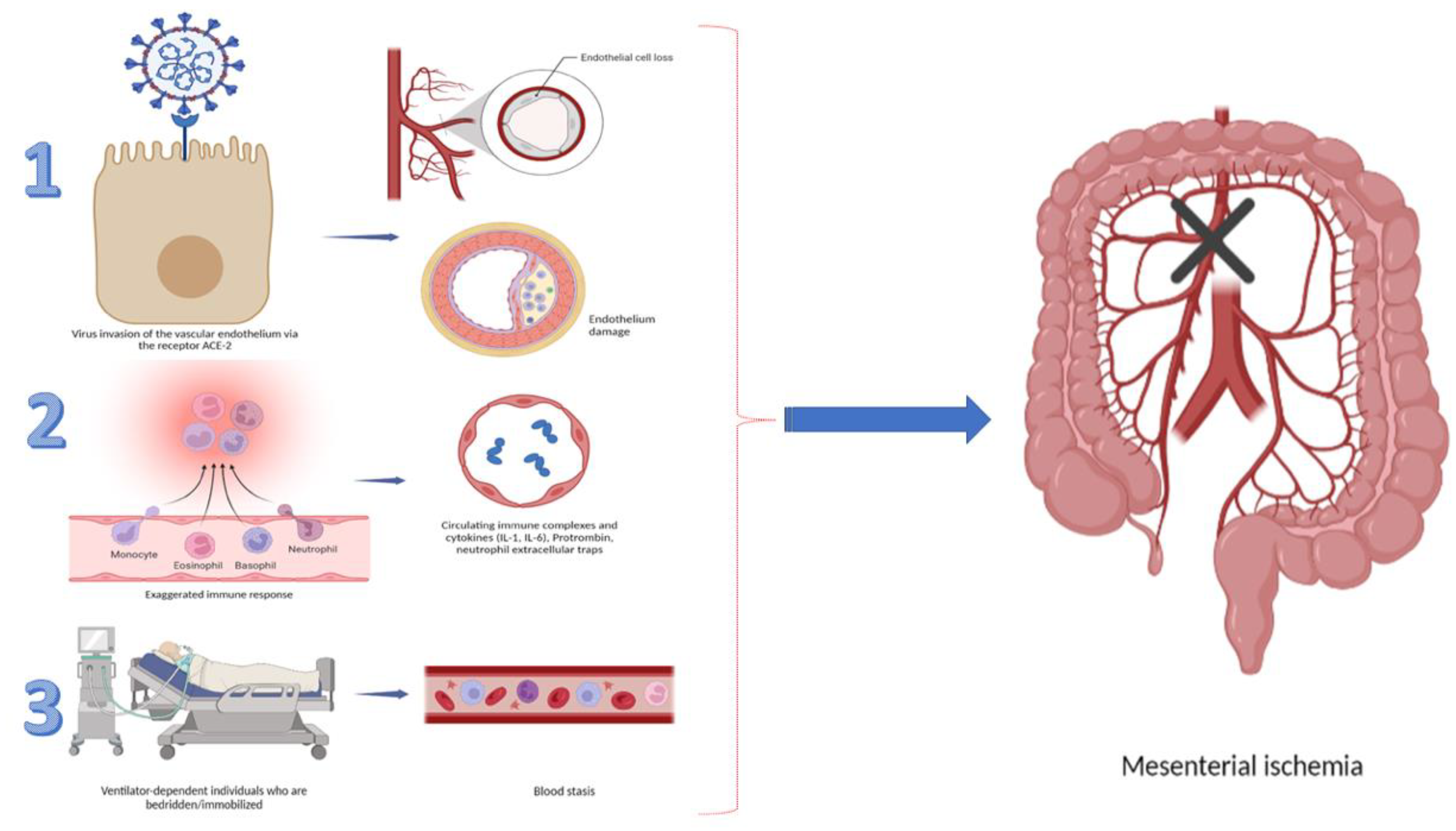
Ischemia of the gastrointestinal tract occurs when the organ’s perfusion does not meet its metabolic demands. Despite the fact that the pathophysiology of gastrointestinal complications in COVID-19 is considered to be multifactorial, the inflammation, microvascular endotheliopathy, and hypercoagulable state, leading to tissue hypoxia, have been proven to be specifically related to the COVID-19 infection. A plethora of literature data showed that coronavirus disease is associated with specific tropism to the vascular system, which leads to alteration of microcirculation and thrombotic complications [4]. This ischemic damage could involve the macro- or microvasculature and may affect any part of the gastrointestinal tract [5]. The envelope-anchored spike (S) protein of the virus has a high affinity for the human angiotensin-converting enzyme 2 (ACE2) receptor, which has been recognized as a functional cellular receptor for SARS-CoV2. ACE2 receptors are highly present in the oral mucosa, esophagus, small intestine, colon, liver, spleen, and respiratory system. An overabundance of ACE2 expression has also been established in gallbladder epithelial cells and pancreatic ductal, acinar, and islet cells. The virus causes direct endothelial damage, initiating cytokine release and the activation of various factors of coagulation. Additionally, ACE2 activation stimulates the excessive release of Von Willebrand factor, factor VIII, and plasminogen activator inhibitor-1 by endothelial cells [6,7]. This pathogenetic cascade leads to inflammation, tissue injury, fibrin deposition, vessel occlusion, and ischemia (Figure 1). A systemic review, published by Kashawartz et al., emphasized the role of macro- and microvascular involvement in the pathogenesis of intestinal ischemia in the context of SARS-CoV2 infection [3].
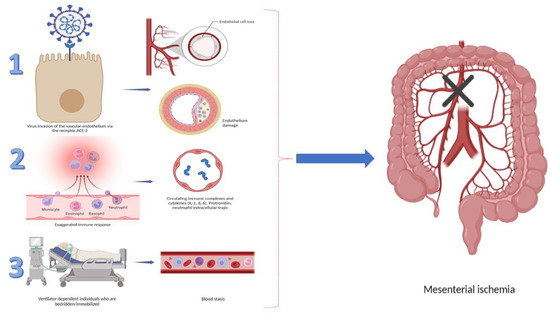
Figure 1.
Summary of factors leading to GI ischemia.
Subsequently, Silva et al. published an interesting meta-analysis highlighting the prevalence of GI symptoms associated with COVID-19 in more than 18,000 patients. The authors reported a prevalence of 30.5% (n = 1841) of any GI manifestations, pointing out diarrhea (11.5%) as the most common symptom, followed by nausea and vomiting (6.3%) and abdominal pain (2.3%) [8]. Another interesting finding is the correlation between a high frequency of COVID-19-related GI complications and the severely ill infected population. For example, Marchelo et al. published a score-matched comparison between the incidence of GI manifestation of 184 critically ill patients with and without COVID-19 infection. The results showed a statistically significant rate of manifested GI complications among COVID-19-positive patients: elevation of liver enzymes (55% vs. 27%) and ileus (48% vs. 22%) or bowel (4% vs. 0%) ischemia [9]. In addition, a study by Pascolini et al. reported that patients with severe COVID-19-associated pneumonia have a higher concentration of autoantibodies (antinuclear antibodies), which could be used as a prognostic marker in these patients. However, the authors underlined that more studies with similar designs should be conducted in order to more specifically explain the pathogenic role of antibodies in immune dysregulation in COVID-19 infection [10].
3. Esophageal and Stomach Involvement
Concerning the upper GI tract, a plethora of studies have reported a variety of endoscopic findings in COVID-19-positive patients: hemorrhagic, ischemic, and perforation complications. ACE2 receptors were shown to be strongly expressed in the epithelial cells of the esophagus, which might explain SARS-CoV2 detection in a biopsy from esophageal erosions. The direct epithelial and endothelial injury and impaired mucosal barrier systems, caused by the SARS-CoV2 virus, are considered to be the main culprit for mucosal damage, leading to necrotic ulceration and hemorrhages [11]. In a study published by Lin, six patients with GI symptoms underwent endoscopies with biopsy. SARS-CoV2 RNA was found in the tissue samples from the esophagus, stomach, duodenum, colon, and rectum [12]. Furthermore, Li et al. supported the concept of direct injury to the intestinal epithelium by publishing a case report of a 77 year old male who underwent gastroscopy due to suspicion of upper GI bleeding. The authors described herpetic erosions and ulcers in the esophagus, where viral RNA was identified in the biopsy sample [13]. Regarding COVID-19′s impact on ischemic tissue injury, Nada et al. described a patient who presented with melena 1 month after the hospital COVID-19 treatment. The endoscopy found large 1.5–2 cm wide distal esophageal ulcers without active bleeding. Histological examination revealed an ulcerated mucosa with necrosis extending to the muscularis propria. Incidentally, the patient tested positive for COVID-19 3 weeks later [14]. Another interesting case report by Deliwala et al. presented a patient under home quarantine with melena and severe anemia. The endoscopy revealed necrosis of the distal esophagus and gastroduodenal erosions. The authors suggested that the combination of hypoperfusion and thromboembolic derangements caused the necrosis [15]. In addition, Meloy et al. reported a case of an elderly patient with COVID-19 pneumonia, complicated with esophageal perforation, which led to a lethal outcome [16]. Rahamen et al. also reported a case of Boerhaave syndrome in a 53 year old patient with esophageal rupture, who was successfully treated with surgical repair and esophageal stent placement [17]. A multicentric study, published in 2021, analyzed results from 114 endoscopies, performed on 106 patients with COVID-19; 33% of them were treated in an intensive care unit, while 66.7% of endoscopies were performed urgently, due to acute GI bleeding. A total of 52 (45.6%) patients were found to have a present source of bleeding: ulcers (25.3%), erosive/ulcerative gastro-duodenopathy (16.1%), and hemorrhagic gastropathy (9.2%). According to the authors, among the patient-related variables analyzed in this study, D-dimer levels above 1850 ng/mL were related to the presence of mucosal abnormalities at endoscopy [18]. Despite the low rate of esophageal necrosis in the overall population (<0.2%) in COVID-19 cohorts of patients, the exact number could be easily underestimated, owing to the possibility of subclinical disease presentation, as well as early mucosal healing, with transient ischemic or chemical injury [19].
4. Colon Ischemia
A study by Wichmann et al. published data about the results from autopsies in COVID-19 patients, which revealed that, in three of the first 12 COVID-19 autopsies, patients had severe intestinal ischemic lesions [20]. The studies demonstrated that acute mesenteric ischemia (AMI) is one of the possible complications in patients with severe SARS-CoV2 infection. In general, AMI is classified as arterial thrombosis, arterial embolism, venous thrombosis, and nonocclusive mesenteric ischemia [21]. AMI has a high mortality rate (about 80%) in patients who do not undergo revascularization [22]. It was established that the frequency of AMI in COVID-19 patients admitted to intensive care units is about 0.7%, with even higher rates for those with acute respiratory syndrome (ARDS) (4.3%) [23]. In COVID-19 patients, the most common cause of AMI is acute occlusion of the superior mesenteric artery (SMA). Clinical presentation varies from poorly localized abdominal pain to fulminant bowel ischemia [24,25]. The manifestation and severity of abdominal pain depend on the occlusion degree, type of artery, collateral blood flow compensation, and intensity of presentation [25]. For patients with SARS-CoV2 infection and AMI, elevated levels of CRP, lactate, and D-dimer have low negative predictive value as possible prognostic factors because they could be detected in severe COVID-19 infection [22]. However, the normal levels of D-dimer in COVID-19 patients could be a useful diagnostic test for the exclusion of acute thromboembolic occlusion of the SMA [26]. Several authors reported intriguing endoscopic changes in COVID-19 patients with lower GI bleeding. The most frequent finding among colonoscopies performed was ischemic-like colitis (33.3%). Half showed acute mucosal injuries, and more than one-third of lower GI endoscopies had features of ischemic colitis [18]. Another interesting review by Kerawala et al. included 41 COVID-19 patients with bowel necrosis. The authors elucidated that the exact mechanism of ischemic injury is based on disruption of the classic Virchow’s triad. Furthermore, they underlined the importance of the direct viral bowel damage via binding with ACE2 receptors on enterocytes, as well as hemodynamic instability with hypotension, which could be the reason for nonocclusive mesenteric ischemia in these patients [27]. Any patient who has COVID-19 and is experiencing gastrointestinal symptoms should be monitored for acute mesenteric ischemia. Therefore, the most important moment in developing AMI is a prompt and timely diagnosis establishment, based on clinical symptoms and imaging findings. On the other hand, the diagnostic algorithm is a contrast-enhanced CT scan which is considered a very useful tool in COVID-19 patients with suspected AMI. This imaging technique can detect intestinal ischemia, which is characterized by the following signs: bowel wall thickening, edema, and dilatation of the colon. When the diameter of the bowel is more than 3 cm, AMI is very likely to be present [28]. Another specific CT sign for AMI is pneumatosis intestinalis, which is detected in 6–28% of patients with AMI. Pneumatosis is a very specific marker for intestinal ischemia, but unfortunately with very low sensitivity [29]. A study by Parry et al. showed that, in severe COVID-19 patients with AMI, there was a 15% higher venous gas incidence in comparison with patients without infection [30]. Treatment strategies for managing patients suffering from both AMI and COVID-19 represent huge challenges. There are no published therapeutic guidelines for this issue so far. Thus, the therapeutic options for these patients are generally the same, despite SARS-CoV2 infection being a comorbidity. It is of great importance to treat patients with AMI with revascularization before intestinal gangrene occurs. If the patients have a clinical sign of peritonitis, tissue death is very likely to unfold. In that case, immediate intestinal surgery or damage control surgery should be performed [29]. The recent data published in many studies showed that better outcomes in these patients are observed when surgery is performed as a first-line treatment option. Surgery allows clinicians to directly visualize intestinal viability, as well as remove necrotic bowel tissue [31]. Another enduring hypothesis is the potential role of gut microbiota modulation as a promising and safe therapeutic approach to prevent major COVID-19-related complications. A study by Odun-Ayo et al. reported on the potential role of gut microbiota in SARS-CoV2-infected patients and the implication of probiotics in their treatment. The authors highlighted that administration of probiotics in COVID-19 illness may lead to normal balance in the gut microbiota and prevent subsequent bacterial infection in severe SARS-CoV2 infection [32]. Recently, many studies were conducted on patients with dysbiosis and COVID-19 infections. The authors aimed to determine the correlation between gut dysbiosis in COVID-19 infection and the severity of the illness, as well as its implication as a diagnostic and prognostic marker [33].
5. Pancreas
As an extrapulmonary manifestation of COVID-19, acute pancreatitis might be an unusual complication of viral infection. It was estimated that, in patients with COVID-19 pneumonia, pancreatic injury was evaluated in 17% [34]. It is well established that the pancreas expresses high levels of ACE2 receptor and transmembrane protease serine 2 (TMPRSS2), which facilitates viral entry into the exocrine glands and islets of the pancreas [35]. The putative pathogenetic mechanism of acute pancreatitis is thought to be linked with the direct viral cytopathic effect or an immune response. Microvascular injury and thrombosis are common complications of COVID-19 infection, which might cause gastrointestinal hypoperfusion, leading to pancreatic injury [36].
Several factors, such as the direct effect of SARS-CoV2, cytokine storm, dehydration, and multiple organ failure, could lead to developing acute pancreatitis (AP) in COVID-19 patients [37]. Furthermore, many studies have demonstrated that the activation of the coagulation cascade, caused by SARS-CoV2 infection, is an additional pathogenetic mechanism leading to injury of the pancreatic gland [38]. The inflammation caused by COVID-19 results in endothelial dysfunction and platelet activation, which are the major risk factors leading to venous thromboembolism [39]. The aforementioned statement is supported by a study from Warzecha et al., which demonstrated that the severity of COVID-19 AP strongly correlates with the hemostasis imbalance in these patients. The authors showed in experimental models that inhibition of the coagulation process decreased the severity of the clinical manifestation of AP [40].
It is of great importance to notice that clinical manifestation of AP in COVID-19 patients could be very heterogeneous. Some patients may present with abdominal pain and vomiting at the beginning of SARS-CoV2 infection, while others could develop AP days after the beginning of infection. It was established that the severity of the disease is strongly associated with the viral load and the multiple organ dysfunction of patients with COVID-19 infection. Nevertheless, there is no conclusive evidence of an increased incidence of AP during the COVID-19 pandemic [41]. It has to be underlined that the exact frequency of patients with COVID-19 AP is not correctly estimated, because, quite often, the pancreatic enzymes of these patients are not examined at admission. A study by Bulthuis, which included 433 patients with COVID-19, reported a ~2% rate of AP, meeting the Atlanta criteria for AP [42]. In another study by Bruno et al. which included 70 COVID-19 patients, six presented with pancreatic abnormalities including elevated serum amylase and lipase. Despite the elevated enzymes, none of the patients met the Atlanta criteria [43]. A retrospective cohort analysis of 11,883 COVID-19-positive hospitalized patients from 12 hospitals in the United States established 32 patients with AP, with a point frequency of 0.2% [44]. Other retrospective studies from Spain by Miro et al. involving more than 63,000 patients with COVID-19 reported a frequency of acute pancreatitis of 0.07 [45]. Isolated ischemic damage to the pancreas is not a common finding in COVID-19 infection. Involvement of the pancreas is most likely to be presented in severe cases of SARS-CoV2 patients. Further studies are needed to obtain guidelines for the surveillance of patients after COVID-19 AP, as well as the incidence of virus-induced diabetes [46].
6. Liver
In COVID-19 patients, liver injury is the result of many different factors such as inflammation, ischemia, thrombosis, direct cytopathic effect, and drug-induced adverse events [47]. It was established that the expression of ACE2 in the liver is mainly in the endothelial cells lining the endothelium of small blood vessels. Their expression in cholangiocytes is about 59.7%. It is a common finding for patients with COVID-19 to have abnormal liver function because of the overabundance of ACE2 receptor expression [48]. According to the available literature, COVID-19-related hepatic injury is considered when aminotransferase (ALT or AST) levels are 3× the ULN, and when cholestatic markers such as ALP, GGT, or TBIL have levels greater than 2× the ULN [49]. The pattern of hepatocellular injury is more prevalent [50,51], while elevated GGT and TBIL are less prevalent as an abnormal liver test. In mild and moderate COVID-19 cases, transient elevations in AST and ALT may be expected findings during hospitalization. It has been postulated that systemic hypoxia in COVID-19 could play a role in AST elevation, as previously documented with other viral types of pneumonia, where aminotransferase levels rise with decreasing peripheral oxygen saturation [51]. Treatment strategies with various antibiotics, antivirals, and antifungals, as well as systemic corticosteroids, could have a hepatotoxic effect. These factors could be one of the possible components in the pathophysiology process of liver damage in COVID-19 patients [52,53].
Another case scenario is hepatic steatosis as the most common abnormal liver finding, according to a study by Legana et al. The authors published a study involving 40 deceased patients from COVID-19 complications. They reported that these patients had mild acute hepatitis and portal inflammation from histopathological reports [54]. Despite these histological changes, Elsoukkary et al. found that widespread microscopic thromboses in many organs including the liver can be a typical finding in many COVID-19 patients [55].
Substantial data have been published in the literature showing a higher risk of liver damage in patients with prolonged hospitalization and severe SARS-CoV2 infection [56]. Up to date, there are scarce data on isolated liver ischemia due to COVID-19. Thus, further studies are needed to evaluate the short- and long-term effects of the COVID-19 infection on the liver.
7. Gallbladder
Another interesting and tardive complication reported in COVID-19 patients might be ischemic gangrenous cholecystitis, which is characterized by a disrupted host inflammatory response with activation of the coagulation cascade and subsequent thrombosis of medium-sized arteries. Bruni et al. published such a case report of a 59 year old male patient with severe SARS-CoV2 infection, who developed peritonitis due to a perforated gangrenous gallbladder. Histopathology and immunohistochemistry data revealed vasculitis of the gallbladder with endothelial overexpression of medium-size vessels (anti-CD31) and an overabundance of macrophages (anti-CD68) and T-helper lymphocytes (anti-CD4), which injured the gallbladder vessels [57].
Subsequently, Bhayana et al. published a retrospective cross-sectional study, which analyzed the abdominal imaging findings of 412 patients with SARS-CoV2 infection. The authors found that half of the patients with CT scans (54%) had gallbladder bile stasis, and those who underwent laparotomy often had ischemia, possibly due to small-vessel thrombosis [58].
Furthermore, Lovece et al. reported a case of a 42 year old male patient with COVID-19 infection and micro-perforation of acalculous cholecystitis. The pathology findings demonstrated transmural necrosis of the gallbladder wall [59].
Despite the published literature data, the exact pathophysiological mechanism is still disputable. One of the hypotheses discusses extended fasting as the possible cause of bile stasis and consequent wall ischemia [60]. Another probable theory is that systemic inflammation or SARS-CoV2- induced immunosuppression may contribute to the late manifestation of cholecystitis either directly or indirectly through an opportunistic infection [61]. It is unclear whether COVID-19 subclinical coagulopathy can lead to gallbladder wall ischemia and small-vessel thrombosis. More convincing and unambiguous data on the impact of SARS-CoV2 on the clinical outcome are needed to reveal the main culprit behind ischemic gallbladder changes in these patients.
8. Results
Our research showed that there were 34 published studies including 48 patients with ischemic GI injuries (Table 1). However, the vast majority were ischemic bowel changes (n = 40), followed by an equal percentage of necrotic esophageal injuries and ischemic gangrenous cholecystitis (8.3%). As for the gender distribution, there was a male preponderance (n = 36). The mean age of the affected patients was 55.4 years. Most of the patients had cardiovascular problems (22.9% HTN, 2% AF), followed by obesity and diabetes mellitus as concomitant diseases. With respect to symptoms, the most common was abdominal pain (73.5%), followed by vomiting (29.4%) and nausea (26.5%). In <15% of the patients, fever and diarrhea were established. In patients with upper GI ischemia, melena was the most frequent symptom. In the group with bowel ischemia, 33 patients were treated surgically, while only 17.5% received conservative treatment with anticoagulants only. In the gallbladder group, only one patient was treated conservatively, while the others underwent cholecystectomy. Furthermore, in patients with necrotic esophageal injuries, the diagnosis was established endoscopically, but the treatment algorithm included high doses of PPI, with antibiotic and antimycotic medications. The mortality rate was highest in patients with bowel necrosis (35%), followed by esophageal and gallbladder injuries.

Table 1.
Gastrointestinal ischemic injuries in COVID-19 patients.
9. Conclusions
Owing to the COVID-19 effect on a variety of GI conditions, especially ischemic changes, and the high mortality rate, physicians should always keep in mind this complication. They should take a deeper look at clinical and imaging modalities in this cohort of patients so that a proper and time-saving treatment strategy can be applied. However, more convincing and unambiguous data on COVID-19 GI ischemia and its impact on the clinical outcome should be obtained to clarify all the ambiguities.
Author Contributions
M.P.-S. and M.P. were involved in conceptualization. M.P.-S., I.B., M.S., M.Z. and M.P. were involved in writing the draft. M.S. and M.P.-S. constructed the table. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mucino-Bermejo, M.-J. COVID-19 and the Gastrointestinal Tract. Gastroenterol. Insights 2021, 12, 394–404. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshavarz, P.; Rafiee, F.; Kavandi, H.; Goudarzi, S.; Heidari, F.; Gholamrezanezhad, A. Ischemic gastrointestinal complications of COVID-19: A systematic review on imaging presentation. Clin. Imaging 2021, 73, 86–95. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Hoteit, L.; Deeb, A.-P.; Andraska, E.A.; Kaltenmeier, C.; Yazdani, H.O.; Tohme, S.; Neal, M.D.; Mota, R.I. The Pathobiological Basis for Thrombotic Complications in COVID-19: A Review of the Literature. Curr. Pathobiol. Rep. 2021, 8, 107–117. [Google Scholar] [CrossRef]
- Alam, W. Hypercoagulability in COVID-19: A review of the potential mechanisms underlying clotting disorders. SAGE Open Med. 2021, 9, 20503121211002996. [Google Scholar] [CrossRef]
- Song, M.; Li, Z.-L.; Zhou, Y.-J.; Tian, G.; Ye, T.; Zeng, Z.-R.; Deng, J.; Wan, H.; Li, Q.; Liu, J.-B. Gastrointestinal involvement of COVID-19 and potential faecal transmission of SARS-CoV-2. J. Zhejiang Univ. Sci. B 2020, 21, 749–751. [Google Scholar] [CrossRef]
- Da Silva, F.A.F.; De Brito, B.B.; Santos, M.L.C.; Marques, H.S.; Júnior, R.T.D.S.; De Carvalho, L.S.; Vieira, E.S.; Oliveira, M.V.; De Melo, F.F. COVID-19 gastrointestinal manifestations: A systematic review. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200714. [Google Scholar] [CrossRef]
- El Moheb, M.; Naar, L.; Christensen, M.A.; Kapoen, C.; Maurer, L.R.; Farhat, M.; Kaafarani, H.M.A. Gastrointestinal Complications in Critically Ill Patients With and Without COVID-19. JAMA 2020, 324, 1899–1901. [Google Scholar] [CrossRef]
- Pascolini, S.; Vannini, A.; Deleonardi, G.; Ciordinik, M.; Sensoli, A.; Carletti, I.; Veronesi, L.; Ricci, C.; Pronesti, A.; Mazzanti, L.; et al. COVID-19 and Immunological Dysregulation: Can Autoantibodies be Useful? Clin. Transl. Sci. 2020, 14, 502–508. [Google Scholar] [CrossRef]
- Ribeiro-Junior, M.A.F.; Augusto, S.D.S.; Elias, Y.G.B.; Costa, C.T.K.; Néder, P.R. Gastrointestinal complications of coronavirus disease (COVID-19). Arq. Bras. Cir. Dig. 2022, 34, e1620. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, S.; Lu, J.; Lai, R.; Zhang, Z.; Lin, X.; Zheng, X.; Shan, H. Upper Gastrointestinal Bleeding Caused by SARS-CoV-2 Infection. Am. J. Gastroenterol. 2020, 115, 1541–1542. [Google Scholar] [CrossRef]
- Mustafa, N.F.; Jafri, N.S.; Holtorf, H.L.; Shah, S.K. Acute oesophageal necrosis in a patient with recent SARS-CoV-2. BMJ Case Rep. 2021, 14, e244164. [Google Scholar] [CrossRef]
- Deliwala, S.S.; Gurvits, G.E. Acute Esophageal Necrosis in a Patient with COVID-19. Am. J. Gastroenterol. 2021, 116, 1977. [Google Scholar] [CrossRef] [PubMed]
- Meloy, P.; Bhambri, A. Esophageal Rupture Associated With COVID-19: A Novel Case Report. Cureus 2020, 12, e12256. [Google Scholar] [CrossRef]
- Rahim, F.; Subramanian, S.K.; Larson, S. Case Report of Acute Esophageal Necrosis (Gurvits Syndrome) in Vaccinated, COVID-19-Infected Patient. Cureus 2022, 14, e22241. [Google Scholar] [CrossRef]
- Vanella, G.; Capurso, G.; Burti, C.; Fanti, L.; Ricciardiello, L.; Lino, A.S.; Boskoski, I.; Bronswijk, M.; Tyberg, A.; Nair, G.K.K.; et al. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: An international multicentre study. BMJ Open Gastroenterol. 2021, 8, e000578. [Google Scholar] [CrossRef]
- Gurvits, G.E. Black esophagus: Acute esophageal necrosis syndrome. World J. Gastroenterol. 2010, 16, 3219–3225. [Google Scholar] [CrossRef]
- Wichmann, D. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020, 173, 1030. [Google Scholar] [CrossRef]
- Clair, D.G.; Beach, J.M. Mesenteric Ischemia. N. Engl. J. Med. 2016, 374, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kärkkäinen, J.M.; Acosta, S. Acute mesenteric ischemia (part I)—Incidence, etiologies, and how to improve early diagnosis. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.-W.; Shi, P.-F.; Qian, S.-X. Acute Mesenteric Ischemia in Patients with COVID-19: Review of the literature. J. Natl. Med Assoc. 2021, 114, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.B.; Mostafavi, K.; Craven, T.E.; Ayerdi, J.; Edwards, M.S.; Hansen, K.J. Clinical course of mesenteric artery stenosis in elderly americans. Arch. Intern. Med. 2006, 166, 2095–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kärkkäinen, J.M.; Lehtimäki, T.T.; Manninen, H.; Paajanen, H. Acute Mesenteric Ischemia Is a More Common Cause than Expected of Acute Abdomen in the Elderly. J. Gastrointest. Surg. 2015, 19, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Kerawala, A.A.; Das, B.; Solangi, A. Mesenteric ischemia in COVID-19 patients: A review of current literature. World J. Clin. Cases 2021, 9, 4700–4708. [Google Scholar] [CrossRef]
- Acosta, S.; Nilsson, T.K.; Björck, M. D-dimer testing in patients with suspected acute thromboembolic occlusion of the superior mesenteric artery. Br. J. Surg. 2004, 91, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, W.; Khurana, B.; Ji, H.; Ros, P.R. CT of acute bowel ischemia. Radiology 2003, 226, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.H.; Wani, A.H.; Yaseen, M. Acute Mesenteric Ischemia in Severe Coronavirus-19 (COVID-19): Possible Mechanisms and Diagnostic Pathway. Acad. Radiol. 2020, 27, 1190. [Google Scholar] [CrossRef]
- Norsa, L.; Valle, C.; Morotti, D.; Bonaffini, P.A.; Indriolo, A.; Sonzogni, A. Intestinal ischemia in the COVID-19 era. Dig. Liver Dis. 2020, 52, 1090–1091. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Reddy, L. Gastrointestinal Microbiota Dysbiosis Associated with SARS-CoV-2 Infection in Colorectal Cancer: The Implication of Probiotics. Gastroenterol. Insights 2022, 13, 35–59. [Google Scholar] [CrossRef]
- Scarpellini, E.; Scarcella, L.; Romanelli, G.; Basilico, M.; Lattanzi, E.; Rasetti, C.; Abenavoli, L.; Santori, P. Nutritional Status and the Critically Ill Patient: Gut Microbiota and Immuno-Nutrition in I.C.U. at the Time of SARS-COV2 Pandemic. Gastroenterol. Insights 2021, 12, 259–269. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Fan, J.; Zhang, Y.; Wang, H.; Zhao, Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology 2020, 159, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128–2130.e2. [Google Scholar] [CrossRef]
- Esmon, C.T. Crosstalk between inflammation and thrombosis. Maturitas 2008, 61, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Pretreatment with low doses of acenocoumarol inhibits the development of acute ischemia/reperfusion-induced pancreatitis. J. Physiol. Pharmacol. 2015, 66, 731–740. [Google Scholar] [PubMed]
- De-Madaria, E.; Capurso, G. COVID-19 and acute pancreatitis: Examining the causality. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Bulthuis, M.C.; Boxhoorn, L.; Beudel, M.; Elbers, P.W.G.; Kop, M.P.M.; van Wanrooij, R.L.J.; Besselink, M.G.; Voermans, R.P. Acute pancreatitis in COVID-19 patients: True risk? Scand. J. Gastroenterol. 2021, 56, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Fabrizio, C.; Santoro, C.R.; Buccoliero, G.B. Pancreatic injury in the course of coronavirus disease 2019: A not-so-rare occurrence. J. Med Virol. 2020, 93, 74–75. [Google Scholar] [CrossRef]
- Inamdar, S.; Benias, P.C.; Liu, Y.; Sejpal, D.V.; Satapathy, S.K.; Trindade, A.J.; The Northwell COVID-19 Research Consortium. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients With Coronavirus Disease 2019 Presenting as Acute Pancreatitis. Gastroenterology 2020, 159, 2226–2228.e2. [Google Scholar] [CrossRef] [PubMed]
- Miró, O.; Llorens, P.; Jiménez, S.; Piñera, P.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; del Castillo, J.G. Frequency of five unusual presentations in patients with COVID-19: Results of the UMC-19-S1. Epidemiol. Infect. 2020, 148, e189. [Google Scholar] [CrossRef] [PubMed]
- De Sá, T.C.; Soares, C.; Rocha, M. Acute pancreatitis and COVID-19: A literature review. World J. Gastrointest. Surg. 2021, 13, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, D.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Li, Z.; Zhou, G.; Gou, J.; Qu, J.; et al. COVID-19: Abnormal liver function tests. J. Hepatol. 2020, 73, 566–574. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Papic, N.; Pangercic, A.; Vargovic, M.; Barsic, B.; Vince, A.; Kuzman, I. Liver involvement during influenza infection: Perspective on the 2009 influenza pandemic. Influ. Other Respir. Viruses 2011, 6, e2–e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Du, Q.; Yan, S.; Guo, X.-G.; He, Y.; Zhu, G.; Zhao, K.; Ouyang, S. Liver injury in COVID-19: Clinical features and treatment management. Virol. J. 2021, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.T.N.; Thu, N.T.N.; Barrail-Tran, A.; Duc, N.H.; Lan, N.N.; Laureillard, D.; Lien, T.T.X.; Borand, L.; Quillet, C.; Connolly, C.; et al. Randomised pharmacokinetic trial of rifabutin with lopinavir/ritonavir-antiretroviral therapy in patients with HIV-associated tuberculosis in Vietnam. PLoS ONE 2014, 9, e84866. [Google Scholar] [CrossRef] [PubMed]
- Lagana, S.M.; Kudose, S.; Iuga, A.C.; Lee, M.J.; Fazlollahi, L.; Remotti, H.E.; Del Portillo, A.; De Michele, S.; De Gonzalez, A.K.; Saqi, A.; et al. Hepatic pathology in patients dying of COVID-19: A series of 40 cases including clinical, histologic, and virologic data. Mod. Pathol. 2020, 33, 2147–2155. [Google Scholar] [CrossRef]
- Elsoukkary, S.S.; Mostyka, M.; Dillard, A.; Berman, D.R.; Ma, L.X.; Chadburn, A.; Yantiss, R.K.; Jessurun, J.; Seshan, S.V.; Borczuk, A.C.; et al. Autopsy Findings in 32 Patients with COVID-19: A Single-Institution Experience. Pathobiology 2020, 88, 56–68. [Google Scholar] [CrossRef]
- Ahmed, J.; Rizwan, T.; Malik, F.; Akhter, R.; Malik, M.; Ahmad, J.; Khan, A.W.; Chaudhary, M.A.; Usman, M.S. COVID-19 and Liver Injury: A Systematic Review and Meta-Analysis. Cureus 2020, 12, e9424. [Google Scholar] [CrossRef]
- Bruni, A.; Garofalo, E.; Zuccalà, V.; Currò, G.; Torti, C.; Navarra, G.; De Sarro, G.; Navalesi, P.; Longhini, F.; Ammendola, M. Histopathological findings in a COVID-19 patient affected by ischemic gangrenous cholecystitis. World J. Emerg. Surg. 2020, 15, 43. [Google Scholar] [CrossRef]
- Bhayana, R.; Som, A.; Li, M.D.; Carey, D.E.; Anderson, M.A.; Blake, M.A.; Catalano, O.; Gee, M.S.; Hahn, P.F.; Harisinghani, M.; et al. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology 2020, 297, E207–E215. [Google Scholar] [CrossRef]
- Lovece, A.; Asti, E.; Bruni, B.; Bonavina, L. Subtotal laparoscopic cholecystectomy for gangrenous gallbladder during recovery from COVID-19 pneumonia. Int. J. Surg. Case Rep. 2020, 72, 335–338. [Google Scholar] [CrossRef]
- Huffman, J.L.; Schenker, S. Acute acalculous cholecystitis: A review. Clin. Gastroenterol. Hepatol. 2010, 8, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, D.L.; Andrus, C.H.; German, D.; Deshpande, Y.G. The role of prostanoids in the production of acute acalculous cholecystitis by platelet-activating factor. Ann. Surg. 1990, 212, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Beccara, L.A.; Pacioni, C.; Ponton, S.A. Arterial Mesenteric Thrombosis as a Complication of SARS-CoV-2 Infection. Eur. J. Case Rep. Intern. Med. 2020, 7, 001690. [Google Scholar] [CrossRef] [PubMed]
- Ignat, M.; Philouze, G.; Aussenac-Belle, L.; Faucher, V.; Collange, O.; Mutter, D.; Pessaux, P. Small bowel ischemia and SARS-CoV-2 infection: An underdiagnosed distinct clinical entity. Surgery 2020, 168, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Gandet, F.F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensiv. Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Rondi, P.; Botturi, E.; Renzulli, M.; Borghesi, A.; Guelfi, D.; Ravanelli, M. Gastrointestinal: Bowel ischemia in a suspected coronavirus disease (COVID-19) patient. J. Gastroenterol. Hepatol. 2020, 36, 41. [Google Scholar] [CrossRef] [PubMed]
- Azouz, E.; Yang, S.; Monnier-Cholley, L.; Arrivé, L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensiv. Care Med. 2020, 46, 1464–1465. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, P.; Jacob, S.; Davenport, R.A. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br. J. Haematol. 2020, 189, 1053–1054. [Google Scholar] [CrossRef]
- Fraissé, M.; Logre, E.; Pajot, O.; Mentec, H.; Plantefève, G.; Contou, D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: A French monocenter retrospective study. Crit Care 2020, 24, 275. [Google Scholar] [CrossRef]
- Bianco, F.; Ranieri, A.J.; Paterniti, G.; Pata, F.; Gallo, G. Acute intestinal ischemia in a patient with COVID-19. Tech. Coloproctol. 2020, 24, 1217–1218. [Google Scholar] [CrossRef]
- Do Carmo Filho, A.; da Silva Cunha, B. Case Report—Inferior Mesenteric Vein Thrombosis and COVID-19. Preprints 2020, 2020060282. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Rakheja, D.; Gopal, P. SARS-CoV-2-related Hypercoagulable State Leading to Ischemic Enteritis Secondary to Superior Mesenteric Artery Thrombosis. Clin. Gastroenterol. Hepatol. 2020, 19, e111. [Google Scholar] [CrossRef] [PubMed]
- English, W.; Banerjee, S. Coagulopathy and mesenteric ischaemia in severe SARS-CoV-2 infection. ANZ J. Surg. 2020, 90, 1826. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Quiwa, J.C.; Pillai, A.; Onwu, C.; Tharayil, Z.J.; Gupta, R. Superior Mesenteric Artery Thrombosis and Acute Intestinal Ischemia as a Consequence of COVID-19 Infection. Am. J. Case Rep. 2020, 21, e925753. [Google Scholar] [CrossRef] [PubMed]
- De Barry, O.; Mekki, A.; Diffre, C.; Seror, M.; El Hajjam, M.; Carlier, R.-Y. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol. Case Rep. 2020, 15, 1054–1057. [Google Scholar] [CrossRef]
- Kraft, M.; Pellino, G.; Jofra, M.; Sorribas, M.; Solís-Peña, A.; Biondo, S.; Espín-Basany, E. Incidence, features, outcome and impact on health system of de-novo abdominal surgical diseases in patients admitted with COVID-19. Surgeon 2021, 19, e53–e58. [Google Scholar] [CrossRef]
- Besutti, G.; Bonacini, R.; Iotti, V.; Marini, G.; Riva, N.; Dolci, G.; Maiorana, M.; Spaggiari, L.; Monelli, F.; Ligabue, G.; et al. Abdominal Visceral Infarction in 3 Patients with COVID-19. Emerg. Infect. Dis. 2020, 26, 1926–1928. [Google Scholar] [CrossRef]
- Sehhat, S.; Talebzadeh, H.; Hakamifard, A.; Melali, H.; Shabib, S.; Rahmati, A.; Larki-Harchegani, A. Acute Mesenteric Ischemia in a Patient with COVID-19: A Case Report. Arch. Iran. Med. 2020, 23, 639–643. [Google Scholar] [CrossRef] [PubMed]
- De Roquetaillade, C.; Chousterman, B.; Tomasoni, D.; Zeitouni, M.; Houdart, E.; Guedon, A.; Reiner, P.; Bordier, R.; Gayat, E.; Montalescot, G.; et al. Unusual arterial thrombotic events in COVID-19 patients. Int. J. Cardiol. 2020, 323, 281–284. [Google Scholar] [CrossRef]
- Singh, B.; Mechineni, A.; Kaur, P.; Ajdir, N.; Maroules, M.; Shamoon, F.; Bikkina, F.S.A.M. Acute Intestinal Ischemia in a Patient with COVID-19 Infection. Korean J. Gastroenterol. 2020, 76, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Lari, E.; Lari, A.; AlQinai, S.; Abdulrasoul, M.; AlSafran, S.; Ameer, A.; Al-Sabah, S. Severe ischemic complications in COVID-19—A case series. Int. J. Surg. Case Rep. 2020, 75, 131–135. [Google Scholar] [CrossRef]
- Thuluva, S.K.; Zhu, H.; Tan, M.M.L.; Gupta, S.; Yeong, K.Y.; Wah, S.T.C.; Lin, L.; Yap, E.S. A 29-Year-Old Male Construction Worker from India Who Presented with Left- Sided Abdominal Pain Due to Isolated Superior Mesenteric Vein Thrombosis Associated with SARS-CoV-2 Infection. Am. J. Case Rep. 2020, 21, e926785. [Google Scholar] [CrossRef] [PubMed]
- Levolger, S.; Bokkers, R.P.; Wille, J.; Kropman, R.H.; de Vries, J.-P.P. Arterial thrombotic complications in COVID-19 patients. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nakamura, R.M.; Gonzalez-Calatayud, M.; Martinez, A.R.M. Acute mesenteric thrombosis in two patients with COVID-19. Two cases report and literature review. Int. J. Surg. Case Rep. 2020, 76, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Khesrani, L.S.; Chana, K.; Sadar, F.Z.; Dahdouh, A.; Ladjadj, Y.; Bouguermouh, D. Intestinal ischemia secondary to COVID-19. J. Pediatr. Surg. Case Rep. 2020, 61, 101604. [Google Scholar] [CrossRef]
- Ucpinar, B.A.; Sahin, C. Superior Mesenteric Artery Thrombosis in a Patient with COVID-19: A Unique Presentation. J. Coll. Physicians Surg. Pak. 2020, 30, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Karna, S.T.; Panda, R.; Maurya, A.P.; Kumari, S. Superior Mesenteric Artery Thrombosis in COVID-19 Pneumonia: An Underestimated Diagnosis-First Case Report in Asia. Indian J. Surg. 2020, 82, 1235–1237. [Google Scholar] [CrossRef]
- Hurtado, F.B.; Arrabal, E.G.; Delgado, A.B.; Rodríguez, A.J.R. SARS-CoV-2 infection presenting as acute acalculous cholecystitis. Rev. Esp. Quimioter. 2022, 35, 87–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).