Systematic Review of Endoscopic Management of Stricture, Fistula and Abscess in Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Search Strategy
3. Endoscopic Therapy for Strictures in IBD
3.1. Endoscopic Balloon Dilation (EBD)
3.1.1. Outcomes of EBD
3.1.2. Predictors of EBD Success and Surgery-Free Disease Course
| Number of Patients | Location, Type of Strictures | Technical Success | Clinical Success | Recurrence Rates | Complications | Repeat Dilation | Surgery on Follow Up | Median Follow Up Period (Months) | |
|---|---|---|---|---|---|---|---|---|---|
| Ferlitsch et al., 2006 [4] | 46 | Ileo-colonic Anastomotic | 95% | 89.7% | 62% | 4% | 31% | 33% | 21 |
| Nomura et al., 2006 [5] | 16 | Ileo-colonic, ileo-ileal anastomosis | 93% | 93% | 46.6% | 25% | 25% | 44% | 38.5 |
| Ajlouni et al., 2007 [6] | 37 | De novo and anastomotic Ileo-colonic | 90% | 90% | 32.2% | 3% | 21.6% | 5.4% | 20 |
| Pohl, 2007 [7] | 10 | Small bowel | 80% | 60% | - | 0% | 50% | 40% | 10 |
| Ohmiya, 2009 [8] | 16 | Small bowel | 96% | 100% | n.a | 0% | 12.5% | 18.8% | 16% |
| Despott, 2009 [9] | 11 | Small bowel | 81.8% | 72.7 | n.a | 9.1% | 22.2% | 9.1% | 20.5 |
| Steinecker et al., 2009 [10] | 25 | Lower GI tract (primary or anastomotic) | 97% | 96% | 46% | 3% | 29.2% | 16.7% | 81 |
| Hirai, 2010 [11] | 25 | Small bowel | 72% | 72% | 17% | 8% | 22.2% | 28% | 11.4% |
| Mueller et al., 2010 [12] | 55 | Duodenum, terminal ileum, colon, ileo-colonic anastomosis | 95% | 76% | 9.2% | 1.8% | 47% | 24% | 44 |
| Thienpont et al., 2010 [13] | 138 | Ileal, Ileocolonic | 97% | - | 55.8% | 5.1% per patient analysis | 46% | 24% | 69.6 |
| Gustavsson et al., 2012 [14] | 178 | Anastomotic, upper GI, small bowel, ileo-colonic | 89% | 77% | 66.4% | 5.3% | 66% | 36% | 144 |
| De Angelis et al., 2013 [15] | 26 | Anastomotic, upper GI, small bowel, ileo-colonic | 100% | 81.5% | 54.2% | 0% | 54% | 8% | 40.7 |
| Endo et al., 2013 [16] | 30 | De novo and anastomotic | 93.6% | 93.6% | 60.5% | 10.6% | 60.5% | 37% | 26 |
| Atreja et al., 2014 [17] | 128 | De novo and anastomotic Ileo-colonic | 83% | - | 73.4% | 3.1% | 58.6% | 32.8% | 21.6 |
| Bhalme et al., 2014 [18] | 79 | Anastomotic, upper GI, small bowel, ileo-colonic | 95% | 43% | 66% | 4% | 66% | 23% | 26.8% |
| Chen et al., 2014 [19] | 60 | Anastomosis, ileo-colonic | 94% | - | 16.7% | 0% | 31.7% | 33.3% | 50 |
| Navaneethan et al., 2014 [20] | 8 | Small bowel | 75% | - | - | n.a | 66.6% | n.a | n.a |
| Gill et al., 2014 [21] | 10 | Small bowel | 100% | 80% | - | 20% | 40% | 30% | 16 |
| Hirai, 2014 [22] | 65 | Small bowel | 80% | 80% | - | 4.6% | 50% | 26.2% | 40.3 |
| Lian et al., 2015 [23] | 185 | Ileo-colonic Anastomotic | 91% | - | - | 1.1% | - | 35.7% | 46.8 |
| Ding et al., 2015 [24] | 54 | Anastomotic | 98% | 98% | 68.5% | 1.8% | 68.5% | 18.5% | 72 |
| Guo et al., 2016 [25] | 24 | Upper GI | 92.5% | 95.8% | 79.2% | 8.4% | 79.2% | 24% | 23 |
| Sunada et al., 2016 [26] | 85 | Small bowel | - | - | - | 5.9% | 75.3% | 24.7% | 41.9 |
| Bettenworth et al., 2017 [27] | 1463 | Ileal (98.6%) and anastomotic (62%) | 89.1% | 80.8% | 47.5% | 2.8% | 73.5% | 42.9% | 24 |
| Lian et al., 2017 [28] | 176 | Ileo-colonic Anastomotic | 90.3% | - | - | 8.8%% | - | 51.7% | 21.6 |
| Reutmann et al., 2017 [29] | 135 | De novo and anastomotic Ileo-colonic | 74% | - | - | 0.7% | - | 28.1% | 41.7 |
| Singh et al., 2017 [30] | 35 | Stomach, Duodenum | 93% | 87% | 75% | 4% | 93% | 34% | 15.1 |
| Nishida et al., 2017 [31] | 37 | Small bowel | - | - | - | 8.1% | - | 48.6% | 27.1 |
| Lee et al., 2018 [32] | 30 | Stomach (n = 1), small bowel (n = 5), colon (n= 36) both ulcerative colitis and Crohn’s disease | 86.7% | 93.3% | 26.7% | 6.7% | 26.7% | 3.3% | 134.8 |
| Shivashankar et al., 2018 [33] | 273 | Entire GI tract, Pouch, anastomosis | 91.3% | 91.3% | 41.8% | 2.1% | 41.8% | 30% | 31.2 |
| Winder et al., 2019 [34] | 64 | Primary, Anastomotic, Ileo-colonic. | 89.9% | 84.7% | - | 5% | - | 32.8% | 39.6 |
| Chang et al., 2020 [35] | 26 | Ileo-colonic, upper GI | 96.2% | 83.3% | 17.1% | 2.4% | - | 26.9% | 75 |
| Andujar et al., 2020 [36] | 187 | Anastomotic, pouch, ileo-colonic | 79.5% | 55.3% | - | 1.3% | 49.7% | 20.9% | 40 |
| Sivasailam et al., 2021 [37] | 99 | Ileo-colonic, anastomotic | 75% | - | 52% | 3.3% | 52% | 33% | 62 |
| Wewer et al., 2022 [38] | 90 | Small bowel, de novo and anastomotic | - | - | 45.5% | - | 14% | 27% | 60 |
| Watanabe et al., 2022 [39] | 75 | Small bowel, large bowel, anastomosis | - | 78.5% | 68% | 1.1% | - | 40.5% | 82 |
| Pal et al., 2022 [40] | 44 | Upper GI, Small bowel, Large bowel, pouch, anastomosis | 81.8% | 95.4% | 27.3% | 9.1% | 22.7% | 2.3% | 5 |
| Lee et al., 2022 [41] | 114 | Upper GI, Small bowel, Large bowel | 96.4% | 54.3% | - | 0.8% | 16.7% | 18.4% | >6 |
| Ladron et al., 2022 [42] | 32 | Anastomotic | 63.5% | 62.5% | - | 3.2% | 47% | 37.5% | 72 |
| Hibiya et al., 2022 [43] | 98 | Small bowel | 98.3% | - | - | 2% | 75% | 24.5% | 12 |
4. Endoscopic Stricturotomy
4.1. Method of ES
4.2. Indications of ES Comparison with Other Techniques
| Study, Year of Publication | Etiology | Technical Success | Clinical Success | Recurrence Rates | Complications | Repeat Interventions | Surgery on Follow Up | Median Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|
| Lan et al., 2017 [48] | 85 Ileal pouch (n = 50), Crohn’s disease (n = 35) (14 combined EBD) | 100% | 54.7% (29/53 with immediate clinical follow up) | 60.6% | 3.7% (bleeding 3.3%, perforation 0.4%) | 60.6% | 15.3% | 11 |
| Lan et al., 2018 [49] | Anastomotic strictures | 100% | 72.7% (vs. EBD 45.4%) | 61.9% | 14.3% (bleeding which required transfusion) | 61.9% | 9.5% | 9 |
| Zhang et al., 2020 [50] | 49 IBD related | 100% | IBD (67.6%) | 34.7% | 4.7% (bleeding) | 49% additional ES, 20.4% additional EBD | 12.2% | 11 |
| Navaneethan U et al., 2020 [51] | 2 Crohn’s disease | 100% | 100% | - | 0% | - | - | - |
| Mohy-ud-din et al., 2020 [52] | 11 (IBD, including pouch) | 92% | 92% | - | 9% (self limiting bleeding) | 8% repeat ES | 9% | 5 |
| Moroi et al., 2020 [53] | CD-4 Anastomotic and 1 primary stricture | 100% | 100% | - | 20% (delayed bleeding) | - | - | - |
| Lan et al., 2020 [54] | Crohn’s de novo distal ileal strictures (n = 13), (versus ileo-cecal resection, n = 32) | 100% | ES (50.0%) (90% with ileo-cecal resection) | 38.5% | 6.9% | 15.4% (surgery) | 15.4% | 21 |
| Lan et al., 2021 [55] | 40 Pouch strictures (vs. EBD-160) | 100% | 42.3% (vs. 13.2% EBD) | 44.4% (vs. 41.3% EBD) | 4.7% (bleeding) (vs. 0.8% EBD) | 22.5% | 22.5% (vs. 20.6%) | 7 |
5. Endoscopic Stenting
5.1. Indications and Types
| Name of Stent | Diameter (mm) | Length (cm) | Stent Type | Specifics |
|---|---|---|---|---|
| Niti S enteral colonic covered stent | 18–22 | 6–15 | Fully covered enteral stent | High migration rates |
| HANARO stent | 20 (26 at ends) | 2.4, 5.4, 7.4 (6, 9, 11) | Partially covered self-expanding metal stent | Lower migration rates |
| Axios stent | 10–20 (21–29 for flanges) | 1 (saddle length) | Lumen apposing metal stent | Short delivery catheter (not for proximal stenosis) |
| SX-ELLA-Biodegradable stents | 18, 20, 23, 25 (23, 25, 28, 31) | 6, 8, 10 | Biodegradable stent | Not through the scope (TTS), made of polydioxanone, degraded in 10–12 weeks |
5.2. Technical Tips for Endoscopic Stenting in IBD
5.3. Results of Endoscopic Stenting
| Author/Year | No. of Patients | Length | Stent Type | Technical Success | Clinical Success | Recurrence | Adverse Events/Migration | Repeat Intervention | Duration of Stenting (Weeks) | Surgery | Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole et al., 1998 [60] | 1 Colon, CD | - | Tracheo-bronchial Wallstents | 100% | 100% | - | - | 100% | 3 | Used as bridge to surgery | 0.75 |
| Matsuhashi et al., 2000 [61] | 2 Colon, IC Anastomosis, Post EBD | - | FCSEMS (specially modified) | 100% | 100% | 0% | 100% (migration) | 0% | 4 and 22 | 0% | 54 |
| Suzuki et al., 2004 [62] | 2 Colon | - | USCEMS | YES | yes | Yes | Fistula in 1 | Surgery and repeat stenting | 3 and 104 | 1/2 | 3 and 26 |
| Bickston et al., 2005 [63] | 1 ileo-cecal Post EBD | - | 2 UCSEMS | yes | yes | - | - | - | 8 | Used s bridge to surgery | 2 |
| Wada et al., 2005 [64] | 1 Colon | - | UCSEMS | Yes | yes | Restenosis | Perforation, fistula | Yes- surgery | 139 | Yes | 8 |
| Dafnis et al., 2007 [65] | 1 colon | 5 cm | 4UCSEMS | yes | yes | Yes | - | 4 times | 14 | No | 1 |
| Martines et al., 2008 [66] | 1 IC anastomosis Post EBD | 6 cm | FCSEMS | Yes | yes | - | - | - | 1 | Used as bridge to surgery | 0.25 |
| Small et al., 2008 [67] | 1 rectum | - | 2 PCSEMS | yes | yes | - | - | - | 1 | Used as bridge to surgery | - |
| Keranen et al., 2010 [68] | 2 Anastomosis | - | FCSEMS UCSEMS | yes | yes | - | Perforation-1 | Surgery 1 | 6 and 221 | 1/2 | - |
| Rejchrt et al., 2011 [69] | 11 CD Post EBD 07 | 1.5–5 | Polydioxanone biodegradable stent | 90% | 63% | 36.3% | 27% early stent migration | - | 16 | - | 16 |
| Attar et al., 2012 [70] | 11 CD Post EBD-9 | 1–4 cm | FCSEMS | 90% | 36% | 63.6% (1 year), total 90% | 10% proximal migration, 70% migration | 18.2% | <4 | 18.2% | 26 |
| Branche et al., 2012 [71] | 7 CD Ileo-colonic (IC) anastomosis Post EBD | <5 cm | PCSEMS | 100% | 71.4% | 28.5 | 42.8% pain | 14% (EBD) | 1 | 0% | 10 |
| Levin et al., 2012 [72] | 5 IC anastomosis Post EBD-2 | <5 cms | UCSEMS | 100% | 80% | 20% | 0% | 20% | 3 (1 patient at 9 years) | 20% | 28 |
| Loras et al., 2012 [73] | 17 CD Post EBD 14 | 2–6 cm | PCSEMS/FCSEM | 94.1% | 64.7% | 31% | 5.9% spontaneous migration 52% migration | - | Mean-4 | 43.7% | 12 |
| Karstensen et al., 2016 [74] | 6 CD Post EBD | 2–10 | Polydioxanone monofilament, biodegradable stent | 83% | 20% | 80% | 17% stent migration | - | - | - | 4–42 |
| Axelrad et al., 2018 [75] | 1 Rectal-colon anastomosis Post EBD | 1 cm | LAMS | Yes | Yes | 0% | No | - | 8 | - | 3 |
| Oztas et al., 2018 [76] | 1 IC anastomosis | 3 cm | UCSEMS | Yes | Yes | Yes | 0% | Yes, (PC-SEMS within FC SEMS) | 24 (UC SEMS), 52 (PC SEMS) | - | 12 months |
| Ouali et al., 2019 [77] | 1 Pouch inlet stricture Post EBD/ES | 10 cm | FC-SEMS | yes | yes | Yes | spontaneous migration | EBD, ES | 1 | 0% | - |
| Fung et al., 2020 [78] | 1 Descending colon | - | UCSEMS | yes | yes | No | spontaneous migration | No | < 1 | No | 10 |
| Das et al., 2020 [79] | 21 CD | <6 cm | PCSEMS | 95.8% | 54.2% | 12.5% | 21.7% (2 pain, 3 migration) | 9.5% restenting | 1 | - | 3–50 |
| Lamazza et al., 2021 [80] | 4 rectum Post EBD | - | FCSEMS | 100% | 100% | 75% | 25% migration | 75% (2 EBD, 1 surgery) | 2–12 | 25% | 12 |
| Attar et al., 2021 [81] | 46 CD Post EBD-36 | Mean 3.9 cm (all <5 cm) | PCSEMS (Hanaro stent) | 100% | 58.7% | 6.5% | 15.2% (4 pain, 3 proximal migration) | 34.8% | 1 | 17.3% | 26 |
| Heden strom et al., 2021 [82] | 7 CD | - | PC SEMS | 100% | 86% | - | 71.4% (4 pain, 1 bleeding) | 14.2% | 1 | 14.2% | 69 |
| Andújar et al., 2022 [83] | 39 CD | Mean 4 cm (all <9 cm) | FCSEMS | 92.3% | 51% | - | 7.7% (2 proximal migration, 1 perforation) | 49% | <1 | - | 12 |
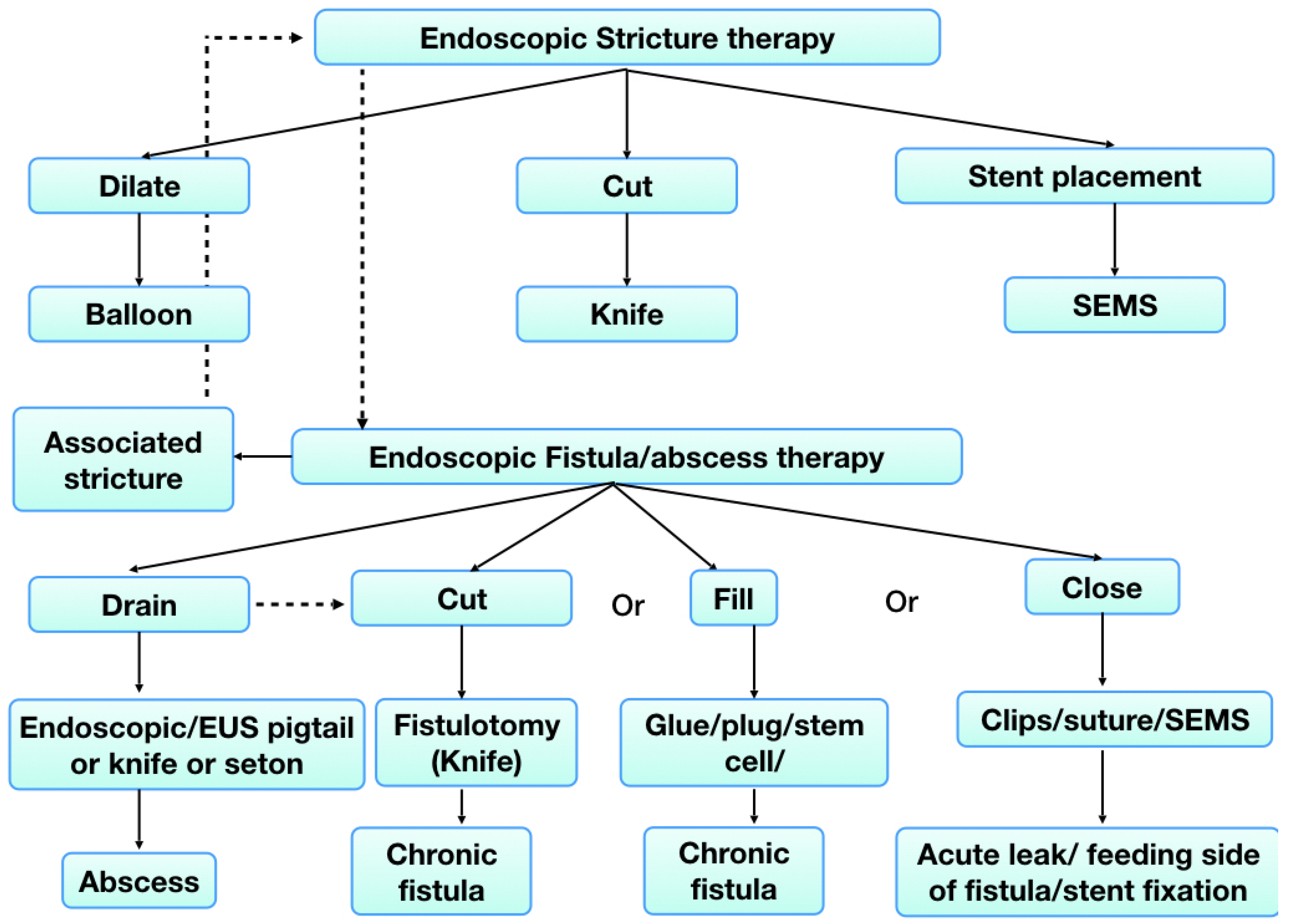
6. Endoscopic Management of Fistula and Abscesses
6.1. Endoscopic Drainage
6.2. Endoscopic Fistulotomy
6.3. Injection of Filling Materials
6.3.1. Glue
6.3.2. Fistula Plug
6.3.3. Stem Cells
6.3.4. Sclerosing Agents
6.4. Endoscopic Closure
6.4.1. Endoscopic Clipping
6.4.2. Endoscopic Suturing
6.4.3. Endoscopic Stenting
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.P. Long-term evolution of disease behavior of Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef]
- Navaneethan, U.; Lourdusamy, D. Endoscopic Stricturotomy and Strictureplasty. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Shen, B. Endoscopic Therapy for Fistulas and Abscesses in Crohn’s Disease. Endosc. Clin. N. Am. 2022, 32, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Ferlitsch, A.; Reinisch, W.; Püspök, A.; Dejaco, C.; Schillinger, M.; Schöfl, R.; Pötzi, R.; Gangl, A.; Vogelsang, H. Safety and efficacy of endoscopic balloon dilation for treatment of Crohn’s disease strictures. Endoscopy 2006, 38, 483–487. [Google Scholar] [CrossRef]
- Nomura, E.; Takagi, S.; Kikuchi, T.; Negoro, K.; Takahashi, S.; Kinouchi, Y.; Hiwatashi, N.; Shimosegawa, T. Efficacy and safety of endoscopic balloon dilation for Crohn’s strictures. Dis. Colon Rectum 2006, 49, S59–S67. [Google Scholar] [CrossRef]
- Ajlouni, Y.; Iser, J.H.; Gibson, P.R. Endoscopic balloon dilatation of intestinal strictures in Crohn’s disease: Safe alternative to surgery. J. Gastroenterol. Hepatol. 2007, 22, 486–490. [Google Scholar] [CrossRef]
- Pohl, J.; May, A.; Nachbar, L.; Ell, C. Diagnostic and therapeutic yield of push-and-pull enteroscopy for symptomatic small bowel Crohn’s disease strictures. Eur. J. Gastroenterol. Hepatol. 2007, 19, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, N.; Arakawa, D.; Nakamura, M.; Honda, W.; Shirai, O.; Taguchi, A.; Itoh, A.; Hirooka, Y.; Niwa, Y.; Maeda, O.; et al. Small-bowel obstruction: Diagnostic comparison between double-balloon endoscopy and fluoroscopic enteroclysis, and the outcome of enteroscopic treatment. Gastrointest. Endosc. 2009, 69, 84–93. [Google Scholar] [CrossRef]
- Despott, E.J.; Gupta, A.; Burling, D.; Tripoli, E.; Konieczko, K.; Hart, A.; Fraser, C. Effective dilation of small-bowel strictures by double-balloon enteroscopy in patients with symptomatic Crohn’s disease (with video). Gastrointest. Endosc. 2009, 70, 1030–1036. [Google Scholar] [CrossRef]
- Stienecker, K.; Gleichmann, D.; Neumayer, U.; Glaser, H.J.; Tonus, C. Long-term results of endoscopic balloon dilatation of lower gastrointestinal tract strictures in Crohn’s disease: A prospective study. World J. Gastroenterol. 2009, 15, 2623–2627. [Google Scholar] [CrossRef]
- Hirai, F.; Beppu, T.; Sou, S.; Seki, T.; Yao, K.; Matsui, T. Endoscopic balloon dilatation using double-balloon endoscopy is a useful and safe treatment for small intestinal strictures in Crohn’s disease. Dig. Endosc. 2010, 22, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Rieder, B.; Bechtner, G.; Pfeiffer, A. The response of Crohn’s strictures to endoscopic balloon dilation. Aliment. Pharmacol. Ther. 2010, 31, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Thienpont, C.; D’Hoore, A.; Vermeire, S.; Demedts, I.; Bisschops, R.; Coremans, G.; Rutgeerts, P.; Van Assche, G. Long-term outcome of endoscopic dilatation in patients with Crohn’s disease is not affected by disease activity or medical therapy. Gut 2010, 59, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Magnuson, A.; Blomberg, B.; Andersson, M.; Halfvarson, J.; Tysk, C. Endoscopic dilation is an efficacious and safe treatment of intestinal strictures in Crohn’s disease. Aliment. Pharmacol. Ther. 2012, 36, 151–158. [Google Scholar] [CrossRef]
- de’Angelis, N.; Carra, M.C.; Borrelli, O.; Bizzarri, B.; Vincenzi, F.; Fornaroli, F.; De Caro, G.; de’Angelis, G.L. Short- and long-term efficacy of endoscopic balloon dilation in Crohn’s disease strictures. World J. Gastroenterol. 2013, 19, 2660–2667. [Google Scholar] [CrossRef]
- Endo, K.; Takahashi, S.; Shiga, H.; Kakuta, Y.; Kinouchi, Y.; Shimosegawa, T. Short and long-term outcomes of endoscopic balloon dilatation for Crohn’s disease strictures. World J. Gastroenterol. 2013, 19, 86–91. [Google Scholar] [CrossRef]
- Atreja, A.; Aggarwal, A.; Dwivedi, S.; Rieder, F.; Lopez, R.; Lashner, B.A.; Brzezinski, A.; Vargo, J.J.; Shen, B. Safety and efficacy of endoscopic dilation for primary and anastomotic Crohn’s disease strictures. J. Crohns Colitis 2014, 8, 392–400. [Google Scholar] [CrossRef]
- Bhalme, M.; Sarkar, S.; Lal, S.; Bodger, K.; Baker, R.; Willert, R.P. Endoscopic balloon dilatation of Crohn’s disease strictures: Results from a large United kingdom series. Inflamm. Bowel Dis. 2014, 20, 265–270. [Google Scholar] [CrossRef]
- Chen, M.; Shen, B. Comparable short- and long-term outcomes of colonoscopic balloon dilation of Crohn’s Disease and benign non-Crohn’s Disease strictures. Inflamm. Bowel Dis. 2014, 20, 1739–1746. [Google Scholar] [CrossRef]
- Navaneethan, U.; Vargo, J.J.; Menon, K.V.; Sanaka, M.R.; Tsai, C.J. Impact of balloon-assisted enteroscopy on the diagnosis and management of suspected and established small-bowel Crohn’s disease. Endosc. Int. Open 2014, 2, E201–E206. [Google Scholar] [CrossRef] [Green Version]
- Gill, R.S.; Kaffes, A.J. Small bowel stricture characterization and outcomes of dilatation by double-balloon enteroscopy: A single-centre experience. Ther. Adv. Gastroenterol. 2014, 7, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Beppu, T.; Takatsu, N.; Yano, Y.; Ninomiya, K.; Ono, Y.; Hisabe, T.; Matsui, T. Long-term outcome of endoscopic balloon dilation for small bowel strictures in patients with Crohn’s disease. Dig. Endosc. 2014, 26, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Stocchi, L.; Shen, B.; Liu, X.; Ma, J.; Zhang, B.; Remzi, F. Prediction of need for surgery after endoscopic balloon dilation of ileocolic anastomotic stricture in patients with Crohn’s disease. Dis. Colon Rectum 2015, 58, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.S.; Yip, W.M.; Choi, C.H.; Saunders, B.; Thomas-Gibson, S.; Arebi, N.; Humphries, A.; Hart, A. Endoscopic Dilatation of Crohn’s Anastomotic Strictures is Effective in the Long Term, and Escalation of Medical Therapy Improves Outcomes in the Biologic Era. J. Crohns Colitis 2016, 10, 1172–1178. [Google Scholar] [CrossRef]
- Guo, F.; Huang, Y.; Zhu, W.; Wang, Z.; Cao, L.; Chen, A.; Guo, Z.; Li, Y.; Gong, J.; Li, J. Efficacy and Safety of Endoscopic Balloon Dilation for Upper Gastrointestinal Strictures of Crohn’s Disease. Dig. Dis. Sci. 2016, 61, 2977–2985. [Google Scholar] [CrossRef]
- Sunada, K.; Shinozaki, S.; Nagayama, M.; Yano, T.; Takezawa, T.; Ino, Y.; Sakamoto, H.; Miura, Y.; Hayashi, Y.; Sato, H.; et al. Long-term Outcomes in Patients with Small Intestinal Strictures Secondary to Crohn’s Disease After Double-balloon Endoscopy-assisted Balloon Dilation. Inflamm. Bowel Dis. 2016, 22, 380–386. [Google Scholar] [CrossRef]
- Bettenworth, D.; Gustavsson, A.; Atreja, A.; Lopez, R.; Tysk, C.; van Assche, G.; Rieder, F. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 133–142. [Google Scholar] [CrossRef]
- Lian, L.; Stocchi, L.; Remzi, F.H.; Shen, B. Comparison of Endoscopic Dilation vs Surgery for Anastomotic Stricture in Patients with Crohn’s Disease Following Ileocolonic Resection. Clin. Gastroenterol. Hepatol. 2017, 15, 1226–1231. [Google Scholar] [CrossRef]
- Reutemann, B.A.; Turkeltaub, J.A.; Al-Hawary, M.; Waljee, A.K.; Higgins, P.D.R.; Stidham, R.W. Endoscopic Balloon Dilation Size and Avoidance of Surgery in Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 1803–1809. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, N.; Kurada, S.; Lopez, R.; Kessler, H.; Philpott, J.; Shen, B.; Lashner, B.; Rieder, F. Efficacy, Safety, and Long-term Outcome of Serial Endoscopic Balloon Dilation for Upper Gastrointestinal Crohn’s Disease-associated Strictures-A Cohort Study. J. Crohns Colitis 2017, 11, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Hosomi, S.; Yamagami, H.; Yukawa, T.; Nagami, Y.; Tanaka, F.; Kamata, N.; Tanigawa, T.; Shiba, M.; Watanabe, T.; et al. Analysis of the Risk Factors of Surgery after Endoscopic Balloon Dilation for Small Intestinal Strictures in Crohn’s Disease Using Double-balloon Endoscopy. Intern. Med. 2017, 56, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, S.J.; Jeon, S.R.; Ye, B.D.; Park, J.J.; Cheon, J.H.; Kim, T.I.; Kim, W.H. Long-Term Outcomes of Endoscopic Balloon Dilation for Benign Strictures in Patients with Inflammatory Bowel Disease. Gut Liver 2018, 12, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, R.; Edakkanambeth Varayil, J.; Scott Harmsen, W.; Faubion, W.A.; Wong Kee Song, L.M.; Bruining, D.H.; Schroeder, K.W.; Kisiel, J.; Loftus, E.V., Jr.; Nayantara, C.P.; et al. Outcomes of Endoscopic Therapy for Luminal Strictures in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 1575–1581. [Google Scholar] [CrossRef]
- Winder, O.; Fliss-Isakov, N.; Winder, G.; Scapa, E.; Yanai, H.; Barnes, S.; Dekel, R.; Dotan, I.; Maharshak, N. Clinical outcomes of endoscopic balloon dilatation of intestinal strictures in patients with Crohn’s disease. Medicine 2019, 98, e16864. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Tu, C.H.; Chou, J.W.; Huang, T.Y.; Hsu, W.H.; Wang, Y.P.; Chen, C.C.; Chung, C.S.; Lin, C.P.; Lin, W.C.; et al. Endoscopic management of strictures in patients with Crohn’s disease—A multi-center experience in Taiwan. J. Formos Med. Assoc. 2020, 119, 1500–1505. [Google Scholar] [CrossRef]
- Andújar, X.; Loras, C.; González, B.; Socarras, M.; Sanchiz, V.; Boscà, M.; Domenech, E.; Calafat, M.; Rodríguez, E.; Sicilia, B.; et al. Efficacy and safety of endoscopic balloon dilation in inflammatory bowel disease: Results of the large multicenter study of the ENEIDA registry. Surg. Endosc. 2020, 34, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Sivasailam, B.; Manski, S.; Wentz, A.; Cross, R.K. Presence of Obstructive Symptoms and Absence of Perianal Crohn Disease Is Predictive of Surgery After Endoscopic Balloon Dilation. Inflamm. Bowel Dis. 2021, 27, 1230–1236. [Google Scholar] [CrossRef]
- Wewer, M.D.; Karstensen, J.G.; Burisch, J. Endoscopic small bowel balloon dilations in patients with Crohn’s disease: A Danish nationwide cohort study, 1997–2015. Eur. J. Gastroenterol. Hepatol. 2022, 34, 831–837. [Google Scholar] [CrossRef]
- Watanabe, K.; Kamikozuru, K.; Sato, T.; Kawai, M.; Yokoyama, Y. Efficacy of Endoscopic Monitoring after Endoscopic Balloon Dilation in Patients with Crohn’s Disease. Dig. Endosc. 2022, 34, 87. [Google Scholar]
- Pal, P.; Ramchandani, M.; Banerjee, R.; Inavolu, P.; Nabi, Z.; Rughwani, H.; Singh, A.P.H.; Patel, R.; Vijayalaxmi, P.; Singh, J.R.; et al. Role of Interventional Inflammatory Bowel Disease (IBD) in the Management of Complex IBD: Initial Prospective Experience from a Tertiary Center in India. J. Dig. Endosc. 2022, 13, 207–217. [Google Scholar] [CrossRef]
- Lee, H.S.; Chiorean, M.V.; Boden, E.; Lord, J.; Irani, S.; Kozarek, R.; Larsen, M.; Ross, A. Usefulness of Fluoroscopy for Endoscopic Balloon Dilation of Crohn’s Disease-Related Strictures. Dig. Dis. Sci. 2022, 67, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Ladrón Abia, P.; Alonso, N.; Mínguez Sabater, A.; Gimeno Torres, M.; Bastida, G.; Aguas, M.; Beltrán, B.; Sáez-González, E.; Pons, V.; Nos, P.; et al. The characteristics of the stricture, but not the ongoing treatment, could influence the outcome of endoscopic dilation in recurrent Crohn’s disease. Gastroenterol. Hepatol. 2022, 45, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hibiya, S.; Ohtsuka, K.; Takenaka, K.; Kawamoto, A.; Matsuyama, Y.; Udagawa, Y.; Motobayashi, M.; Shimizu, H.; Fujii, T.; Saito, E.; et al. Mucosal healing of small intestinal stricture is associated with improved prognosis post-dilation in Crohn’s disease. BMC Gastroenterol. 2022, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bettenworth, D.; Bokemeyer, A.; Kou, L.; Lopez, R.; Bena, J.F.; El Ouali, S.; Mao, R.; Kurada, S.; Bhatt, A.; Beyna, T.; et al. Systematic review with meta-analysis: Efficacy of balloon-assisted enteroscopy for dilation of small bowel Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2020, 52, 1104–1116. [Google Scholar] [CrossRef]
- Bossuyt, P.; Debeuckelaere, C.; Ferrante, M.; de Buck van Overstraeten, A.; Vanbeckevoort, D.; Billiet, T.; Wolthuis, A.; Cleynen, I.; Van Assche, G.; D’Hoore, A.; et al. Risk Stratification for Surgery in Stricturing Ileal Crohn’s Disease: The BACARDI Risk Model. J. Crohns Colitis 2018, 12, 32–38. [Google Scholar] [CrossRef]
- El Ouali, S.; Baker, M.E.; Lyu, R.; Fletcher, J.G.; Bruining, D.H.; Holubar, S.D.; Click, B.; Qazi, T.; Cohen, B.L.; Rieder, F. Validation of stricture length, duration and obstructive symptoms as predictors for intervention in ileal stricturing Crohn’s disease. United Eur. Gastroenterol. J. 2022, 10, 958–972. [Google Scholar] [CrossRef]
- Lee, K.E.; Lim, F.; Faye, A.S.; Shen, B.; Hur, C. Endoscopic Balloon Dilation Is Cost-Effective for Crohn’s Disease Strictures. Dig. Dis. Sci. 2022, 67, 5462–5471. [Google Scholar] [CrossRef]
- Lan, N.; Shen, B. Endoscopic Stricturotomy with Needle Knife in the Treatment of Strictures from Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 502–513. [Google Scholar] [CrossRef]
- Lan, N.; Shen, B. Endoscopic Stricturotomy Versus Balloon Dilation in the Treatment of Anastomotic Strictures in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 897–907. [Google Scholar] [CrossRef]
- Zhang, L.J.; Lan, N.; Wu, X.R.; Shen, B. Endoscopic stricturotomy in the treatment of anastomotic strictures in inflammatory bowel disease (IBD) and non-IBD patients. Gastroenterol. Rep. 2020, 8, 143–150. [Google Scholar] [CrossRef]
- Navaneethan, U. Endoscopic Stricturotomy for Refractory Anal Strictures in Crohn’s Disease. Inflamm. Bowel Dis. 2020, 26, e99–e100. [Google Scholar] [CrossRef] [PubMed]
- Mohy-ud-din, N.; Kochhar, G.S. Endoscopic Stricturotomy Is an Efficacious Option for Management of Strictures in Patients With Inflammatory Bowel Disease. Crohn’s Colitis 360 2020, 2, otaa069. [Google Scholar] [CrossRef]
- Moroi, R.; Shiga, H.; Kuroha, M.; Kanazawa, Y.; Nochioka, K.; Kakuta, Y.; Kinouchi, Y.; Masamune, A. Endoscopic radial incision and cutting for Crohn’s Disease-associated intestinal stricture: A pilot study. Endosc. Int. Open 2020, 8, e81–e86. [Google Scholar] [CrossRef]
- Lan, N.; Hull, T.L.; Shen, B. Endoscopic stricturotomy and ileo-colonic resection in patients with primary Crohn’s disease-related distal ileum strictures. Gastroenterol. Rep. 2020, 8, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Wu, J.J.; Wu, X.R.; Hull, T.L.; Shen, B. Endoscopic treatment of pouch inlet and afferent limb strictures: Stricturotomy vs. balloon dilation. Surg. Endosc. 2021, 35, 1722–1733. [Google Scholar] [CrossRef]
- Lukas, M.; Kolar, M.; Ryska, O.; Juhas, S.; Juhasova, J.; Kalvach, J.; Pazin, J.; Kocisova, T.; Foltan, O.; Kristianova, H.; et al. Novel porcine model of Crohn’s disease anastomotic stricture suitable for evaluation and training of advanced endoscopic techniques. Gastrointest. Endosc. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Ali, S.E.; Bhakta, A.; Bautista, R.M.; Sherif, A.; Frandah, W. Endoscopic stricturotomy with pulsed argon plasma and balloon dilation for refractory benign colorectal strictures: A case series. Transl. Gastroenterol. Hepatol. 2022, 7, 32. [Google Scholar] [CrossRef]
- Karstensen, J.G.; Christensen, K.R.; Brynskov, J.; Rønholt, C.; Vilmann, P.; Hendel, J. Biodegradable stents for the treatment of bowel strictures in Crohn’s disease: Technical results and challenges. Endosc. Int. Open 2016, 4, e296–e300. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Oliveira, A.; Santos, L.; Pires, E.; Deus, J. Biodegradable stent for the treatment of a colonic stricture in Crohn’s disease. World J. Gastrointest. Endosc. 2013, 5, 265–269. [Google Scholar] [CrossRef]
- Wholey, M.H.; Levine, E.A.; Ferral, H.; Castaneda-Zuniga, W. Initial clinical experience with colonic stent placement. Am. J. Surg. 1998, 175, 194–197. [Google Scholar] [CrossRef]
- Matsuhashi, N.; Nakajima, A.; Suzuki, A.; Yazaki, Y.; Takazoe, M. Long-term outcome of non-surgical strictureplasty using metallic stents for intestinal strictures in Crohn’s disease. Gastrointest. Endosc. 2000, 51, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Saunders, B.P.; Thomas-Gibson, S.; Akle, C.; Marshall, M.; Halligan, S. Colorectal stenting for malignant and benign disease: Outcomes in colorectal stenting. Dis. Colon Rectum 2004, 47, 1201–1207. [Google Scholar] [CrossRef]
- Bickston, S.J.; Foley, E.; Lawrence, C.; Rockoff, T.; Shaffer, H.A., Jr.; Yeaton, P. Terminal ileal stricture in Crohn’s disease: Treatment using a metallic enteral endoprosthesis. Dis. Colon Rectum 2005, 48, 1081–1085. [Google Scholar] [CrossRef]
- Wada, H.; Mochizuki, Y.; Takazoe, M.; Matsuhashi, N.; Kitou, F.; Fukushima, T. A case of perforation and fistula formation resulting from metallic stent for sigmoid colon stricture in Crohn’s disease. Tech. Coloproctol. 2005, 9, 53–56. [Google Scholar] [CrossRef]
- Dafnis, G. Repeated coaxial colonic stenting in the palliative management of benign colonic obstruction. Eur. J. Gastroenterol. Hepatol. 2007, 19, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Martines, G.; Ugenti, I.; Giovanni, M.; Memeo, R.; Iambrenghi, O.C. Anastomotic stricture in Crohn’s disease: Bridge to surgery using a metallic endoprosthesis. Inflamm. Bowel Dis. 2008, 14, 291–292. [Google Scholar] [CrossRef]
- Small, A.J.; Young-Fadok, T.M.; Baron, T.H. Expandable metal stent placement for benign colorectal obstruction: Outcomes for 23 cases. Surg. Endosc. 2008, 22, 454–462. [Google Scholar] [CrossRef]
- Keränen, I.; Lepistö, A.; Udd, M.; Halttunen, J.; Kylänpää, L. Outcome of patients after endoluminal stent placement for benign colorectal obstruction. Scand. J. Gastroenterol. 2010, 45, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Rejchrt, S.; Kopacova, M.; Brozik, J.; Bures, J. Biodegradable stents for the treatment of benign stenoses of the small and large intestines. Endoscopy 2011, 43, 911–917. [Google Scholar] [CrossRef]
- Attar, A.; Maunoury, V.; Vahedi, K.; Vernier-Massouille, G.; Vida, S.; Bulois, P.; Colombel, J.F.; Bouhnik, Y.; GETAID. Safety and efficacy of extractible self-expandable metal stents in the treatment of Crohn’s disease intestinal strictures: A prospective pilot study. Inflamm. Bowel Dis. 2012, 18, 1849–1854. [Google Scholar] [CrossRef]
- Branche, J.; Attar, A.; Vernier-Massouille, G.; Bulois, P.; Colombel, J.F.; Bouhnik, Y.; Maunoury, V. Extractible self-expandable metal stent in the treatment of Crohn’s disease anastomotic strictures. Endoscopy 2012, 44 (Suppl. S2), E325–E326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, R.A.; Wasvary, H.; Kadro, O. Endoprosthetic management of refractory ileocolonic anastomotic strictures after resection for Crohn’s disease: Report of nine-year follow-up and review of the literature. Inflamm. Bowel Dis. 2012, 18, 506–512. [Google Scholar] [CrossRef]
- Loras, C.; Pérez-Roldan, F.; Gornals, J.B.; Barrio, J.; Igea, F.; González-Huix, F.; González-Carro, P.; Pérez-Miranda, M.; Espinós, J.C.; Fernández-Bañares, F.; et al. Endoscopic treatment with self-expanding metal stents for Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2012, 36, 833–839. [Google Scholar] [CrossRef]
- Karstensen, J.G.; Vilmann, P.; Hendel, J. Successful endoscopic treatment of a 12-cm small-bowel Crohn stricture with a custom-made biodegradable stent. Endoscopy 2014, 46 (Suppl. S1), E227–E228. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Lichtiger, S.; Sethi, A. Treatment of Crohn’s Disease Anastomotic Stricture With a Lumen-apposing Metal Stent. Clin. Gastroenterol. Hepatol. 2018, 16, A25–A26. [Google Scholar] [CrossRef] [PubMed]
- Öztaş, E.; Akpınar, M.Y.; Özderin Özin, Y.; Dişibeyaz, S. Self-expandable metallic stent as a rescue therapy in stenotic Crohn’s Disease. Turk. J. Gastroenterol. 2019, 30, 381–382. [Google Scholar] [CrossRef] [PubMed]
- El Ouali, S.; Kessler, H.; Shen, B. Self-Expandable Metal Stent in the Treatment of Refractory Long Pouch Inlet Stricture. Inflamm. Bowel Dis. 2019, 25, e13–e14. [Google Scholar] [CrossRef]
- Fung, B.M.; Chen, F.C.; Tabibian, J.H. Clear cap-assisted luminal stenting may improve technical success in gastroduodenal and colonic obstruction. Endosc. Int. Open 2020, 8, E1429–E1434. [Google Scholar] [CrossRef]
- Das, R.; Singh, R.; Din, S.; Lund, J.; Krishnamoorthy, R.; Hearing, S.; Norton, B.; Williams, J.; Fraser, C.; Goddard, A.; et al. Therapeutic resolution of focal, predominantly anastomotic Crohn’s disease strictures using removable stents: Outcomes from a single-center case series in the United Kingdom. Gastrointest. Endosc. 2020, 92, 344–352. [Google Scholar] [CrossRef]
- Lamazza, A.; Fiori, E.; Carati, M.V.; Pronio, A.M.; Antoniozzi, A.; Sterpetti, A.V. Self-Expandable Metal Stents for Refractory Complete Rectal Obstruction in Patients With Crohn Disease. Inflamm. Bowel Dis. 2021, 27, e136–e137. [Google Scholar] [CrossRef]
- Attar, A.; Branche, J.; Coron, E.; Privat, J.; Caillo, L.; Chevaux, J.B.; Vuitton, L.; Amiot, A.; Belkhodja, H.; Dray, X.; et al. An Anti-migration Self-expandable and Removable Metal Stent for Crohn’s Disease Strictures: A Nationwide Study from GETAID and SFED. J. Crohns Colitis 2021, 15, 521–528. [Google Scholar] [CrossRef]
- Hedenström, P.; Stotzer, P.-O. Endoscopic treatment of Crohn-related strictures with a self-expandable stent compared with balloon dilation: A prospective, randomised, controlled study. BMJ Open Gastroenterol. 2021, 8, e000612. [Google Scholar] [CrossRef]
- Loras, C.; Andújar, X.; Gornals, J.B.; Sanchiz, V.; Brullet, E.; Sicilia, B.; Martín-Arranz, M.D.; Naranjo, A.; Barrio, J.; Dueñas, C.; et al. Self-expandable metal stents versus endoscopic balloon dilation for the treatment of strictures in Crohn’s disease (ProtDilat study): An open-label, multicentre, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Chandan, S.; Dhindsa, B.S.; Khan, S.R.; Deliwala, S.; Kassab, L.L.; Mohan, B.P.; Chandan, O.C.; Loras, C.; Shen, B.; Kochhar, G.S. Endoscopic Stenting in Crohn’s Disease-related Strictures: A Systematic Review and Meta-analysis of Outcomes. Inflamm. Bowel Dis. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Gopan, A.; Sundaram, S.; Kale, A. Efficacy and Safety of Endoscopic Stenting for Crohn’s Disease Related Strictures: A Systematic Review and Meta-analysis. Korean J. Gastroenterol. 2022, 80, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, G.; Shen, B. Endoscopic fistulotomy in inflammatory bowel disease (with video). Gastrointest. Endosc. 2018, 88, 87–94. [Google Scholar] [CrossRef]
- Chidi, V.; Shen, B. Endoscopic needle knife fistulotomy technique for ileal pouch-to-pouch fistula. Endoscopy 2015, 47 (Suppl. S1), E261. [Google Scholar] [CrossRef]
- Lee, H.; Shen, B. Endoscopic Fistulotomy Heals a Y-Shaped Entero-Entero-Cutaneous Fistula. ACG Case Rep. J. 2017, 4, e60. [Google Scholar] [CrossRef]
- Sze, G.; Khan, F.; Shen, B. Combined Endoscopic Fistulotomy and Clipping for the Treatment of Fistula from the Tip of the J-Pouch to Anastomosis. ACG Case Rep. J. 2019, 6, 1–3. [Google Scholar] [CrossRef]
- Ellis, C.N.; Clark, S. Fibrin glue as an adjunct to flap repair of anal fistulas: A randomized, controlled study. Dis. Colon Rectum 2006, 49, 1736–1740. [Google Scholar] [CrossRef]
- Grimaud, J.C.; Munoz-Bongrand, N.; Siproudhis, L.; Abramowitz, L.; Sénéjoux, A.; Vitton, V.; Gambiez, L.; Flourié, B.; Hébuterne, X.; Louis, E.; et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology 2010, 138, 2275–2281, 2281.e2271. [Google Scholar] [CrossRef] [PubMed]
- Vidon, M.; Munoz-Bongrand, N.; Lambert, J.; Maggiori, L.; Zeitoun, J.D.; Corte, H.; Panis, Y.; Seksik, P.; Treton, X.; Abramowitz, L.; et al. Long-term efficacy of fibrin glue injection for perianal fistulas in patients with Crohn’s disease. Color. Dis. 2021, 23, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, L.; Brochard, C.; Pigot, F.; Roumeguere, P.; Pillant, H.; Vinson Bonnet, B.; Faucheron, J.L.; Senéjoux, A.; Bonnaud, G.; Meurette, G.; et al. Surgical closure, mainly with glue injection and anti-tumour necrosis factor α, in fistulizing perianal Crohn’s disease: A multicentre randomized controlled trial. Color. Dis. 2022, 24, 210–219. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Champagne, B.J.; Ferguson, M.A.; Orangio, G.R.; Schertzer, M.E.; Armstrong, D.N. Efficacy of anal fistula plug in closure of Crohn’s anorectal fistulas. Dis. Colon Rectum 2006, 49, 1569–1573. [Google Scholar] [CrossRef]
- Senéjoux, A.; Siproudhis, L.; Abramowitz, L.; Munoz-Bongrand, N.; Desseaux, K.; Bouguen, G.; Bourreille, A.; Dewit, O.; Stefanescu, C.; Vernier, G.; et al. Fistula Plug in Fistulising Ano-Perineal Crohn’s Disease: A Randomised Controlled Trial. J. Crohns Colitis 2016, 10, 141–148. [Google Scholar] [CrossRef]
- Aho Fält, U.; Zawadzki, A.; Starck, M.; Bohe, M.; Johnson, L.B. Long-term outcome of the Surgisis(®) (Biodesign(®)) anal fistula plug for complex cryptoglandular and Crohn’s fistulas. Color. Dis. 2021, 23, 178–185. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2018, 154, 1334–1342. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Gilaberte, I.; Binek, M.; D’hoore, A.J.; Lindner, D.; Selvaggi, F.; Spinelli, A.; Panés, J. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn’s Disease: ADMIRE-CD phase 3 randomized controlled trial. Dis. Colon Rectum 2022, 65, 713–720. [Google Scholar] [CrossRef]
- Panés, J.; Bouma, G.; Ferrante, M.; Kucharzik, T.; Nachury, M.; de la Portilla de Juan, F.; Reinisch, W.; Selvaggi, F.; Tschmelitsch, J.; Brett, N.R.; et al. INSPECT: A Retrospective Study to Evaluate Long-term Effectiveness and Safety of Darvadstrocel in Patients With Perianal Fistulizing Crohn’s Disease Treated in the ADMIRE-CD Trial. Inflamm. Bowel Dis. 2022, 28, 1737–1745. [Google Scholar] [CrossRef]
- Keung, C.; Nguyen, T.C.; Lim, R.; Gerstenmaier, A.; Sievert, W.; Moore, G.T. Local Peri-Fistula Injection of A Novel Placenta-Derived Cell Is a safe and Promising Treatment for Refractory Complex Fistulizing Perianal Crohn’s Disease: A Phase I Open Label Study. Gastroenterology 2022, 162, S4–S5. [Google Scholar] [CrossRef]
- Lightner, A.L.; Reese, J.; Ream, J.; Nachand, D.; Jia, X.; Dadgar, N.; Steele, S.R.; Hull, T. A Phase IB/IIA Study of Ex Vivo Expanded Allogeneic Bone Marrow Derived Mesenchymal Stem Cells for the Treatment of Perianal Fistulizing Crohn’s Disease. Dis. Colon Rectum 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Johnson, S.; Hoch, J.S.; Halabi, W.J.; Ko, J.; Nolta, J.; Dave, M. Mesenchymal Stem/Stromal Cell Therapy Is More Cost-Effective Than Fecal Diversion for Treatment of Perianal Crohn’s Disease Fistulas. Front. Immunol. 2022, 13, 859954. [Google Scholar] [CrossRef] [PubMed]
- Dozois, E.; Lightner, A.L.; Fletcher, J.G.; Lee, Y.; Friton, J.; Faubion, W.; Dietz, A. Mesenchymal Stem/Stromal Cells: Durable Response Seen in Patients with Refractory Fistulizing Perianal Crohn’s Disease Using Autologous Mesenchymal Stem Cells on a Dissolvable Matrix: Results from the Phase I Stem Cell on Matrix Plug (Stomp) Trial. Cytotherapy 2022, 24, S50. [Google Scholar] [CrossRef]
- Dozois, E.J.; Lightner, A.L.; Dietz, A.B.; Fletcher, J.G.; Lee, Y.S.; Friton, J.J.; Faubion, W.A. Durable Response in Patients With Refractory Fistulizing Perianal Crohn’s Disease Using Autologous Mesenchymal Stem Cells on a Dissolvable Matrix: Results from the Phase I Stem Cell on Matrix Plug Trial. Dis. Colon Rectum 2023, 66, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Nyabanga, C.T.; Obusez, E.C.; Purysko, A.; Shen, B. Healing of a chronic anal stump sinus after administration of combined high-concentration dextrose and doxycycline solution. Int. J. Colorectal. Dis. 2016, 31, 775–776. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, R.; Laukötter, M.; Senninger, N.; Rijcken, E. The OTSC(®) proctology clip system for the closure of refractory anal fistulas. Tech. Coloproctol. 2015, 19, 241–246. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.F.; Zhu, W.M. Endoscopic closure instead of surgery to close an ileal pouch fistula with the over-the-scope clip system. World J. Gastrointest. Endosc. 2017, 9, 95–98. [Google Scholar] [CrossRef]
- Abdalla, M.; Kothari, T. Successful closure of Crohn’s associated anovaginal fistula involving the dentate line using over-the-scope-clip (OTSC). Inflamm. Bowel Dis. 2016, 22, S10. [Google Scholar] [CrossRef]
- Kochhar, G.S.; Shen, B. Use of over-the-scope-clip system to treat ileocolonic transverse staple line leak in patients with Crohn’s disease. Inflamm. Bowel Dis. 2018, 24, 666–667. [Google Scholar] [CrossRef]
- Tong, Y.; Trilling, B.; Sage, P.Y.; Girard, E.; Faucheron, J.L. Short-term outcomes of the over-the-scope clip proctology system for rectovaginal fistula repair: A prospective study. Tech. Coloproctol. 2019, 23, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Savioli, F.; Komolafe, O. Endoscopic Closure of of Late-Presenting Defect in Ileal Pouch Anal Anastomosis Using Over-The-Scope-Clip (OTSC). Br. J. Surg. 2020, 107, 82. [Google Scholar] [CrossRef]
- Yzet, C.; Brazier, F.; Sabbagh, C.; Le Mouel, J.P.; Hakim, S.; Nguyen-Khac, E.; Fumery, M. Endoscopic treatment of enterocutaneous fistulas in Crohn’s disease. Dis. Colon Rectum 2022, 65, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Wallenhorst, T.; Jacques, J.; Bouguen, G.; Pagenault, M.; D’Halluin, P.N.; Siproudhis, L.; Pioche, M. Successful Closure of a Rectal Fistula of Crohn’s Disease Using Endoscopic Submucosal Dissection Combined with an Over-the-Scope Clip. Am. J. Gastroenterol. 2019, 114, 1416. [Google Scholar] [CrossRef]
- Cereatti, F.; Fiocca, F.; Dumont, J.L.; Ceci, V.; Vergeau, B.M.; Tuszynski, T.; Meduri, B.; Donatelli, G. Fully covered self-expandable metal stent in the treatment of postsurgical colorectal diseases: Outcome in 29 patients. Ther. Adv. Gastroenterol. 2016, 9, 180–188. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, P.; Kanaganti, S.; Banerjee, R.; Ramchandani, M.; Nabi, Z.; Reddy, D.N.; Tandan, M. Systematic Review of Endoscopic Management of Stricture, Fistula and Abscess in Inflammatory Bowel Disease. Gastroenterol. Insights 2023, 14, 45-63. https://doi.org/10.3390/gastroent14010006
Pal P, Kanaganti S, Banerjee R, Ramchandani M, Nabi Z, Reddy DN, Tandan M. Systematic Review of Endoscopic Management of Stricture, Fistula and Abscess in Inflammatory Bowel Disease. Gastroenterology Insights. 2023; 14(1):45-63. https://doi.org/10.3390/gastroent14010006
Chicago/Turabian StylePal, Partha, Swathi Kanaganti, Rupa Banerjee, Mohan Ramchandani, Zaheer Nabi, Duvvuru Nageshwar Reddy, and Manu Tandan. 2023. "Systematic Review of Endoscopic Management of Stricture, Fistula and Abscess in Inflammatory Bowel Disease" Gastroenterology Insights 14, no. 1: 45-63. https://doi.org/10.3390/gastroent14010006
APA StylePal, P., Kanaganti, S., Banerjee, R., Ramchandani, M., Nabi, Z., Reddy, D. N., & Tandan, M. (2023). Systematic Review of Endoscopic Management of Stricture, Fistula and Abscess in Inflammatory Bowel Disease. Gastroenterology Insights, 14(1), 45-63. https://doi.org/10.3390/gastroent14010006





