Glycemic Abnormalities in Pancreatic Cystic Lesions—A Single-Center Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
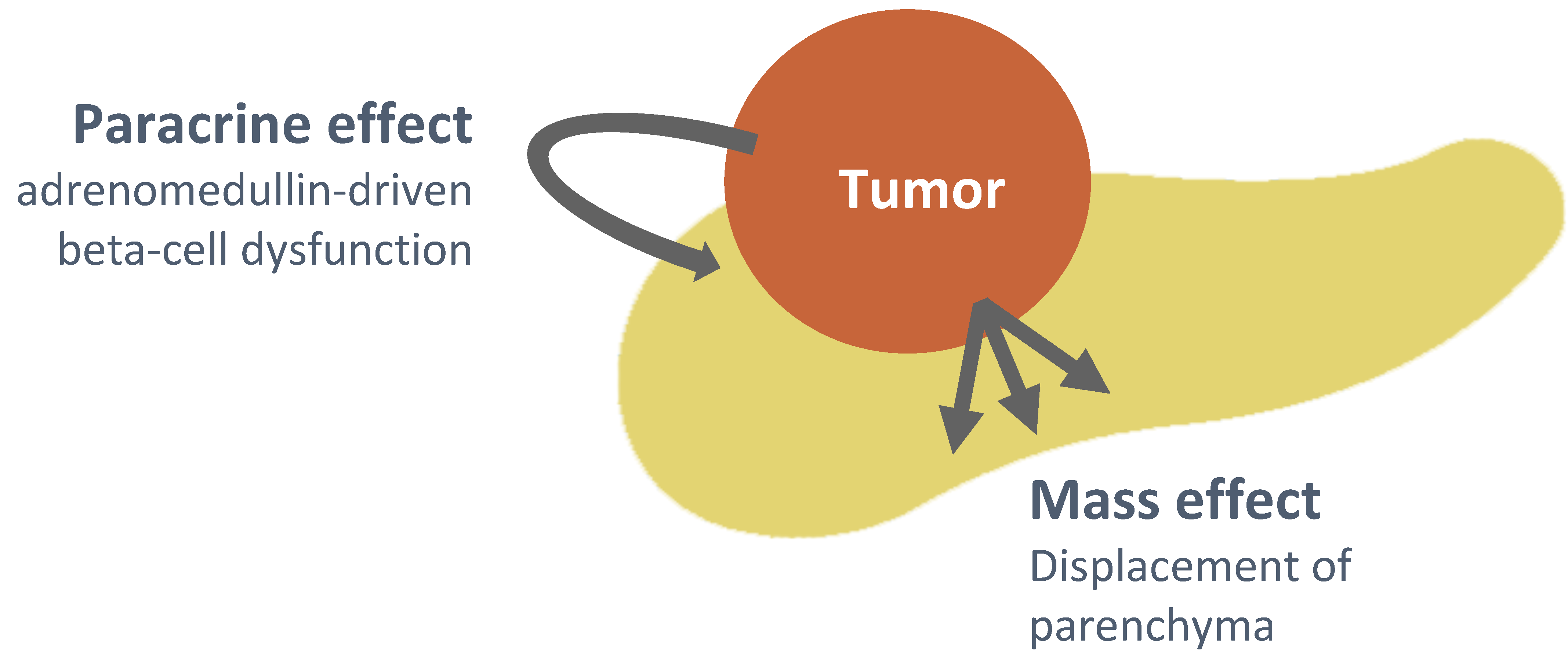
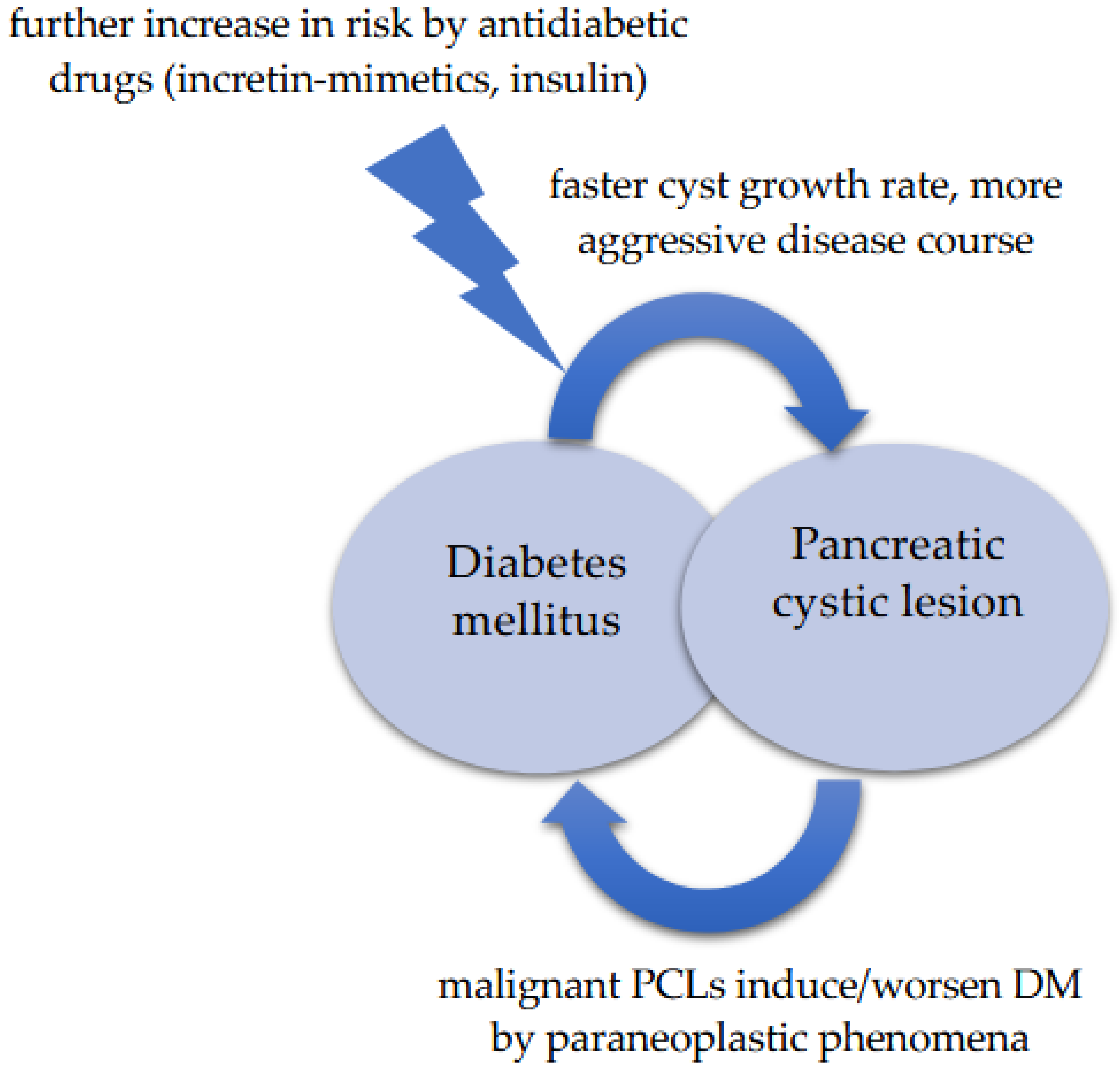
4. Discussion
| Prevalence | DM (%) | LSDM(%) | NODM (%) | |
|---|---|---|---|---|
| Pannala et al., 2008 [30] | Controls | 7.2% | 47% | 53% |
| Pancreatic cancer | 47.4% | 26% | 74% | |
| Lubetzky et al., 2009 [51] | IPMN | NS | NS | 18% |
| Leal et al., 2015 [49] | IPMN | 18% | NS | 17.9% |
| Nguyen et al., 2014 [50] | IPMN | 24.20% | NS | 1.50% |
| Morales-Oyarvide et al., 2017 [16] | IPMN | 34% | NS | NS |
| Perez-Cuadro-Robles et al., 2018 [53] | IPMN | 15% | NS | 13.3% |
| Del Chiaro et al., 2020 [54] | IPMN | 21% | NS | 2% |
| Schweber et al., 2020 [18] | MCN/IPMN | 27.60% | NS | 8.8% |
| Deng et al., 2022 [17] | IPMN, MCN, SCN | 24.1% | 9.37% | 14.73% |
| Mizuno et al., 2017 [10] | NS | 18.4% | NS | NS |
| Yoshioka et al., 2020 [50] | IPMN | 19% | NS | NS |
| Kadosh et al., 2021 [12] | PC | 34.8% | NS | NS |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciochina, M.; Balaban, D.V.; Manucu, G.; Jinga, M.; Gheorghe, C. The Impact of Pancreatic Exocrine Diseases on the β-Cell and Glucose Metabolism-A Review with Currently Available Evidence. Biomolecules 2022, 12, 618. [Google Scholar] [CrossRef]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Roy, A.; Sahoo, J.; Kamalanathan, S.; Naik, D.; Mohan, P.; Kalayarasan, R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J. Gastroenterol. 2021, 27, 4939–4962. [Google Scholar] [CrossRef]
- Duan, X.; Wang, W.; Pan, Q.; Guo, L. Type 2 Diabetes Mellitus Intersects with Pancreatic Cancer Diagnosis and Development. Front. Oncol. 2021, 11, 730038. [Google Scholar] [CrossRef]
- Mellenthin, C.; Balaban, V.D.; Dugic, A.; Cullati, S. Risk Factors for Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4684. [Google Scholar] [CrossRef]
- Gallo, M.; Adinolfi, V.; Morviducci, L.; Acquati, S.; Tuveri, E.; Ferrari, P.; Zatelli, M.C.; Faggiano, A.; Argentiero, A.; Natalicchio, A.; et al. Early prediction of pancreatic cancer from new-onset diabetes: An Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Endocrinologia (SIE)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. ESMO Open 2021, 6, 100155. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Rozek, M.; Lipinski, M.; Jozefik, E.; Znajdek, Z.; Kiziak, M.; Sznurkowska, M.; Tatur, J.; Degowska, M.; Rydzewska, G. Pancreatic cystic lesions in diabetes mellitus patients. Prz. Gastroenterol. 2021, 16, 62–66. [Google Scholar] [CrossRef]
- Mizuno, S.; Isayama, H.; Nakai, Y.; Yoshikawa, T.; Ishigaki, K.; Matsubara, S.; Yamamoto, N.; Ijichi, H.; Tateishi, K.; Tada, M.; et al. Prevalence of Pancreatic Cystic Lesions Is Associated with Diabetes Mellitus and Obesity: An Analysis of 5296 Individuals Who Underwent a Preventive Medical Examination. Pancreas 2017, 46, 801–805. [Google Scholar] [CrossRef]
- Pergolini, I.; Schorn, S.; Jäger, C.; Göß, R.; Novotny, A.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Diabetes mellitus in intraductal papillary mucinous neoplasms: A systematic review and meta-analysis. Surgery 2021, 169, 411–418. [Google Scholar] [CrossRef]
- Kadosh, D.; Ascherman, B.; Khurana, S.; Robbins, D. S5 Prevalence and Growth Rates of Pancreatic Cysts in Patients with Diabetes Mellitus. Am. J. Gastroenterol. 2021, 116, S3. [Google Scholar] [CrossRef]
- Sofi, A.A.; Ahmad, S.; Peerzada, M.; Hackett, L. Diabetes mellitus and the risk of progression or malignancy of pancreatic cystic neoplasms in patients undergoing surveillance: A systematic review and meta-analysis. Pancreatology 2022, 22, 1195–1201. [Google Scholar] [CrossRef]
- Marchegiani, G.; Crippa, S.; Perri, G.; Rancoita, P.M.V.; Caravati, A.; Belfiori, G.; Dall’olio, T.; Aleotti, F.; Partelli, S.; Bassi, C.; et al. Surgery for Intraductal Papillary Mucinous Neoplasms of the Pancreas: Preoperative Factors Tipping the Scale of Decision-Making. Ann. Surg. Oncol. 2022, 29, 3206–3214. [Google Scholar] [CrossRef]
- Pergolini, I.; Jäger, C.; Safak, O.; Göß, R.; Novotny, A.; Ceyhan, G.O.; Friess, H.; Demir, I.E. Diabetes and Weight Loss Are Associated with Malignancies in Patients with Intraductal Papillary Mucinous Neoplasms. Clin. Gastroenterol. Hepatol. 2021, 19, 171–179. [Google Scholar] [CrossRef]
- Morales-Oyarvide, V.; Mino-Kenudson, M.; Ferrone, C.R.; Sahani, D.V.; Pergolini, I.; Negreros-Osuna, A.A.; Warshaw, A.L.; Lillemoe, K.D.; Castillo, C.F.-D. Diabetes mellitus in intraductal papillary mucinous neoplasm of the pancreas is associated with high-grade dysplasia and invasive carcinoma. Pancreatology 2017, 17, 920–926. [Google Scholar] [CrossRef]
- Deng, J.; Guo, Y.; Gu, J.; Du, J.; Kong, L.; Tao, B.; Li, J.; Fu, D. The Role of Diabetes Mellitus in the Malignant Pancreatic Cyst Neoplasm Diagnosis and Prognosis. Cancer Manag. Res. 2022, 14, 2091–2104. [Google Scholar] [CrossRef]
- Schweber, A.B.; Brooks, C.; Agarunov, E.; Sethi, A.; Poneros, J.M.; Schrope, B.A.; Kluger, M.D.; Chabot, J.A.; Gonda, T.A. New onset diabetes predicts progression of low risk pancreatic mucinous cysts. Pancreatology 2020, 20, 1755–1763. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Maitra, A.; Sharma, A.; Brand, R.E.; Van Den Eeden, S.K.; Fisher, W.E.; Hart, P.A.; Hughes, S.J.; Mather, K.J.; Pandol, S.J.; Park, W.G.; et al. A Prospective Study to Establish a New-Onset Diabetes Cohort: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018, 47, 1244–1248. [Google Scholar] [CrossRef]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients with New-Onset Diabetes. Gastroenterology. Gastroenterology 2018, 155, 730–739.e3. [Google Scholar] [CrossRef]
- Khan, S.; Safarudin, R.F.; Kupec, J.T. Validation of the ENDPAC model: Identifying new-onset diabetics at risk of pancreatic cancer. Pancreatology 2021, 21, 550–555. [Google Scholar] [CrossRef]
- Oldfield, L.; Evans, A.; Rao, R.G.; Jenkinson, C.; Purewal, T.; Psarelli, E.E.; Menon, U.; Timms, J.F.; Pereira, S.P.; Ghaneh, P.; et al. Blood levels of adiponectin and IL-1Ra distinguish type 3c from type 2 diabetes: Implications for earlier pancreatic cancer detection in new-onset diabetes. EBiomedicine 2022, 75, 103802. [Google Scholar] [CrossRef]
- Perera, C.J.; Falasca, M.; Chari, S.T.; Greenfield, J.R.; Xu, Z.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Role of Pancreatic Stellate Cell-Derived Exosomes in Pancreatic Cancer-Related Diabetes: A Novel Hypothesis. Cancers 2021, 13, 5224. [Google Scholar] [CrossRef]
- Javeed, N.; Sagar, G.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Bhattacharya, S.; Truty, M.; Petersen, G.M.; Kaufman, R.J.; Chari, S.T.; et al. Pancreatic Cancer-Derived Exosomes Cause Paraneoplastic β-cell Dysfunction. Clin. Cancer Res. 2015, 21, 1722–1733. [Google Scholar] [CrossRef]
- Korc, M. Pancreatic cancer-associated diabetes is an “exosomopathy”. Clin. Cancer Res. 2015, 21, 1508–1510. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, F.; Jiang, M.; Han, M.; Huang, B. Roles of osteoprotegerin in endocrine and metabolic disorders through receptor activator of nuclear factor kappa-B ligand/receptor activator of nuclear factor kappa-B signaling. Front. Cell Dev. Biol. 2022, 10, 1005681. [Google Scholar] [CrossRef]
- Shi, W.; Qiu, W.; Wang, W.; Zhou, X.; Zhong, X.; Tian, G.; Deng, A. Osteoprotegerin is up-regulated in pancreatic cancers and correlates with cancer-associated new-onset diabetes. Biosci. Trends 2014, 8, 322–326. [Google Scholar] [CrossRef]
- Permert, J.; Ihse, I.; Jorfeldt, L.; von Schenck, H.; Arnquist, H.J.; Larsson, J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br. J. Surg. 1993, 80, 1047–1050. [Google Scholar] [CrossRef]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Visrodia, K.; Farrell, J.J.; Gonda, T.A. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J. Gastroenterol. 2019, 25, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef]
- Buerlein, R.C.D.; Shami, V.M. Management of pancreatic cysts and guidelines: What the gastroenterologist needs to know. Ther. Adv. Gastrointest. Endosc. 2021, 14, 26317745211045770. [Google Scholar] [CrossRef]
- Lanke, G.; Lee, J.H. Similarities and differences in guidelines for the management of pancreatic cysts. World J. Gastroenterol. 2020, 26, 1128–1141. [Google Scholar] [CrossRef]
- Cheesman, A.R.; Zhu, H.; Liao, X.; Szporn, A.H.; Kumta, N.A.; Nagula, S.; DiMaio, C.J. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest. Endosc. 2020, 91, 1095–1104. [Google Scholar] [CrossRef]
- Balaban, V.D.; Cazacu, I.M.; Pinte, L.; Jinga, M.; Bhutani, M.S.; Saftoiu, A. EUS-through-the-needle microbiopsy forceps in pancreatic cystic lesions: A systematic review. Endosc. Ultrasound 2021, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wietrzykowska-Grishanovich, D.; Pawlik, E.; Neubauer, K. Biochemical Intracystic Biomarkers in the Differential Diagnosis of Pancreatic Cystic Lesions. Medicina 2022, 58, 994. [Google Scholar] [CrossRef]
- Pușcașu, C.I.; Rimbaş, M.; Mateescu, R.B.; Larghi, A.; Cauni, V. Advances in the Diagnosis of Pancreatic Cystic Lesions. Diagnostics 2022, 12, 1779. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Marfella, R.; Rizzo, M.R.; Sasso, F.C. Pancreatic cancer and diabetes: A two-way relationship in the perspective of diabetologist. Int. J. Surg. 2015, 21 (Suppl. S1), S72–S77. [Google Scholar] [CrossRef]
- Bonelli, L.; Aste, H.; Bovo, P.; Cavallini, G.; Felder, M.; Gusmaroli, R.; Morandini, E.; Ravelli, P.; Briglia, R.; Lombardo, L. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: A case-control study in northern Italy. Pancreas 2003, 27, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, F.M.; Saleh, M.A.; Kim, W.; Alkhayyat, M.; Habash, A.; Almomani, A.; Simons-Linares, C.R.; Garber, A.; Chahal, P. S0070 Diabetes Mellitus Is Associated with Higher Rates of Pancreatic Cysts: A U.S. Population-Based Study. Am. J. Gastroenterol. 2020, 115, S34. [Google Scholar] [CrossRef]
- Bar-Mashiah, A.; Aronson, A.; Naparst, M.; DiMaio, C.J.; Lucas, A.L. Elevated hemoglobin A1c is associated with the presence of pancreatic cysts in a high-risk pancreatic surveillance program. BMC Gastroenterol. 2020, 20, 161. [Google Scholar] [CrossRef]
- Leal, J.N.; Kingham, T.P.; D’Angelica, M.I.; DeMatteo, R.P.; Jarnagin, W.R.; Kalin, M.F.; Allen, P.J. Intraductal Papillary Mucinous Neoplasms and the Risk of Diabetes Mellitus in Patients Undergoing Resection Versus Observation. J. Gastrointest. Surg. 2015, 19, 1974–1981. [Google Scholar] [CrossRef]
- Yoshioka, T.; Shigekawa, M.; Ikezawa, K.; Tamura, T.; Sato, K.; Urabe, M.; Sueyoshi, H.; Yamai, T.; Suda, T.; Sakamori, R.; et al. Risk Factors for Pancreatic Cancer and the Necessity of Long-Term Surveillance in Patients with Pancreatic Cystic Lesions. Pancreas 2020, 49, 552–560. [Google Scholar] [CrossRef]
- Lubezky, N.; Ben-Haim, M.; Nakache, R.; Lahat, G.; Blachar, A.; Brazowski, E.; Santo, E.; Klausner, J.M. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World J Surg. World J. Surg. 2010, 34, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; Toste, P.A.; Farrell, J.J.; Clerkin, B.M.; Williams, J.; Muthusamy, V.R.; Watson, R.R.; Tomlinson, J.S.; Hines, O.J.; Reber, H.A.; et al. Current recommendations for surveillance and surgery of intraductal papillary mucinous neoplasms may overlook some patients with cancer. J. Gastrointest. Surg. 2014, 19, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cuadrado-Robles, E.; Uribarri-González, L.; Borbath, I.; Vila, J.J.; López-López, S.; Deprez, P.H. Risk of advanced lesions in patients with branch-duct IPMN and relative indications for surgery according to European evidence-based guidelines. Dig. Liver Dis. 2019, 51, 882–886. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Beckman, R.; Ateeb, Z.; Orsini, N.; Rezaee, N.; Manos, L.; Valente, R.; Yuan, C.; Ding, D.; Margonis, G.A.; et al. Main Duct Dilatation Is the Best Predictor of High-grade Dysplasia or Invasion in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2020, 272, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; Klauss, M.; Bergmann, F.; Hackert, T.; Hartwig, W.; Strobel, O.; Bundy, B.D.; Büchler, M.W.; Werner, J. Small (Sendai negative) branch-duct IPMNs: Not harmless. Ann. Surg. 2012, 256, 313–320. [Google Scholar] [CrossRef]
- Wong, J.; Weber, J.; Centeno, B.A.; Vignesh, S.; Harris, C.L.; Klapman, J.B.; Hodul, P. High-grade dysplasia and adenocarcinoma are frequent in side-branch intraductal papillary mucinous neoplasm measuring less than 3 cm on endoscopic ultrasound. J. Gastrointest. Surg. 2013, 17, 78–84. [Google Scholar] [CrossRef]
- Duconseil, P.; Adham, M.; Sauvanet, A.; Autret, A.; Périnel, J.; Chiche, L.; Mabrut, J.-Y.; Tuech, J.-J.; Mariette, C.; Turrini, O. Fukuoka-Negative Branch-Duct IPMNs: When to Worry? A Study from the French Surgical Association (AFC). Ann. Surg. Oncol. 2018, 25, 1017–1025. [Google Scholar] [CrossRef]
- Crippa, S.; Fogliati, A.; Valente, R.; Sadr-Azodi, O.; Arnelo, U.; Capurso, G.; Halimi, A.; Partelli, S.; Ateeb, Z.; Arcidiacono, P.G.; et al. A tug-of-war in intraductal papillary mucinous neoplasms management: Comparison between 2017 International and 2018 European guidelines. Dig. Liver Dis. 2021, 53, 998–1003. [Google Scholar] [CrossRef]
- Sadr-Azodi, O.; Sanders, D.S.; Murray, J.A.; Ludvigsson, J.F. Patients with celiac disease have an increased risk for pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 1136–1142.e3. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Dai, M.; Xing, C.; Shi, N.; Wang, S.; Wu, G.; Liao, Q.; Zhang, T.; Chen, G.; Wu, W.; Guo, J.; et al. Risk factors for new-onset diabetes mellitus after distal pancreatectomy. BMJ Open Diabetes Res. Care 2020, 8, e001778. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Cefalo, C.M.A.; Cinti, F.; Quero, G.; Pontecorvi, A.; Alfieri, S.; Holst, J.J.; Giaccari, A. Endocrine and Metabolic Insights from Pancreatic Surgery. Trends Endocrinol. Metab. 2020, 31, 760–772. [Google Scholar] [CrossRef]
- Kaiser, J.; Scheifele, C.; Hinz, U.; Leonhardt, C.S.; Hank, T.; Koenig, A.K.; Tjaden, C.; Hackert, T.; Bergmann, F.; Büchler, M.W.; et al. IPMN-associated pancreatic cancer: Survival, prognostic staging and impact of adjuvant chemotherapy. Eur. J. Surg. Oncol. 2022, 48, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.X.; Chok, A.Y.; Zheng, H.L.; Tan, C.S.; Goh, B.K.P. Systematic review and meta-analysis comparing the surgical outcomes of invasive intraductal papillary mucinous neoplasms and conventional pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 2782–2800. [Google Scholar] [CrossRef] [PubMed]
- Mas, L.; Lupinacci, R.M.; Cros, J.; Bachet, J.B.; Coulet, F.; Svrcek, M. Intraductal Papillary Mucinous Carcinoma versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights. Int. J. Mol. Sci. 2021, 22, 6756. [Google Scholar] [CrossRef]
- Waters, J.A.; Schnelldorfer, T.; Aguilar-Saavedra, J.R.; Chen, J.H.; Yiannoutsos, C.T.; Lillemoe, K.D.; Farnell, M.B.; Sarr, M.G.; Schmidt, C.M. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: A multi-institutional comparison according to American Joint Committee on Cancer Stage. J. Am. Coll. Surg. 2011, 213, 275–283. [Google Scholar] [CrossRef]
- Woo, S.M.; Ryu, J.K.; Lee, S.H.; Yoo, J.W.; Park, J.K.; Kim, Y.T.; Yoon, Y.B. Survival and prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas: Comparison with pancreatic ductal adenocarcinoma. Pancreas 2008, 36, 50–55. [Google Scholar] [CrossRef]
- Kang, M.J.; Lee, K.B.; Jang, J.Y.; Kwon, W.; Park, J.W.; Chang, Y.R.; Kim, S.W. Disease spectrum of intraductal papillary mucinous neoplasm with an associated invasive carcinoma invasive IPMN versus pancreatic ductal adenocarcinoma-associated IPMN. Pancreas 2013, 42, 1267–1274. [Google Scholar] [CrossRef]
- Chen, W.; Butler, R.K.; Lustigova, E.; Chari, S.T.; Wu, B.U. Validation of the Enriching New-Onset Diabetes for Pancreatic Cancer Model in a Diverse and Integrated Healthcare Setting. Dig. Dis. Sci. 2021, 66, 78–87. [Google Scholar] [CrossRef]
- Boursi, B.; Patalon, T.; Webb, M.; Margalit, O.; Beller, T.; Yang, Y.X.; Chodick, G. Validation of the Enriching New-Onset Diabetes for Pancreatic Cancer Model: A Retrospective Cohort Study Using Real-World Data. Pancreas 2022, 51, 196–199. [Google Scholar] [CrossRef]
- Sharma, A.; Smyrk, T.C.; Levy, M.J.; Topazian, M.A.; Chari, S.T. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology 2018, 155, 490–500.e2. [Google Scholar] [CrossRef] [PubMed]
- Lemanska, A.; Price, C.A.; Jeffreys, N.; Byford, R.; Dambha-Miller, H.; Fan, X.; Hinton, W.; Otter, S.; Rice, R.; Stunt, A.; et al. BMI and HbA1c are metabolic markers for pancreatic cancer: Matched case-control study using a UK primary care database. PLoS ONE 2022, 17, e0275369. [Google Scholar] [CrossRef] [PubMed]

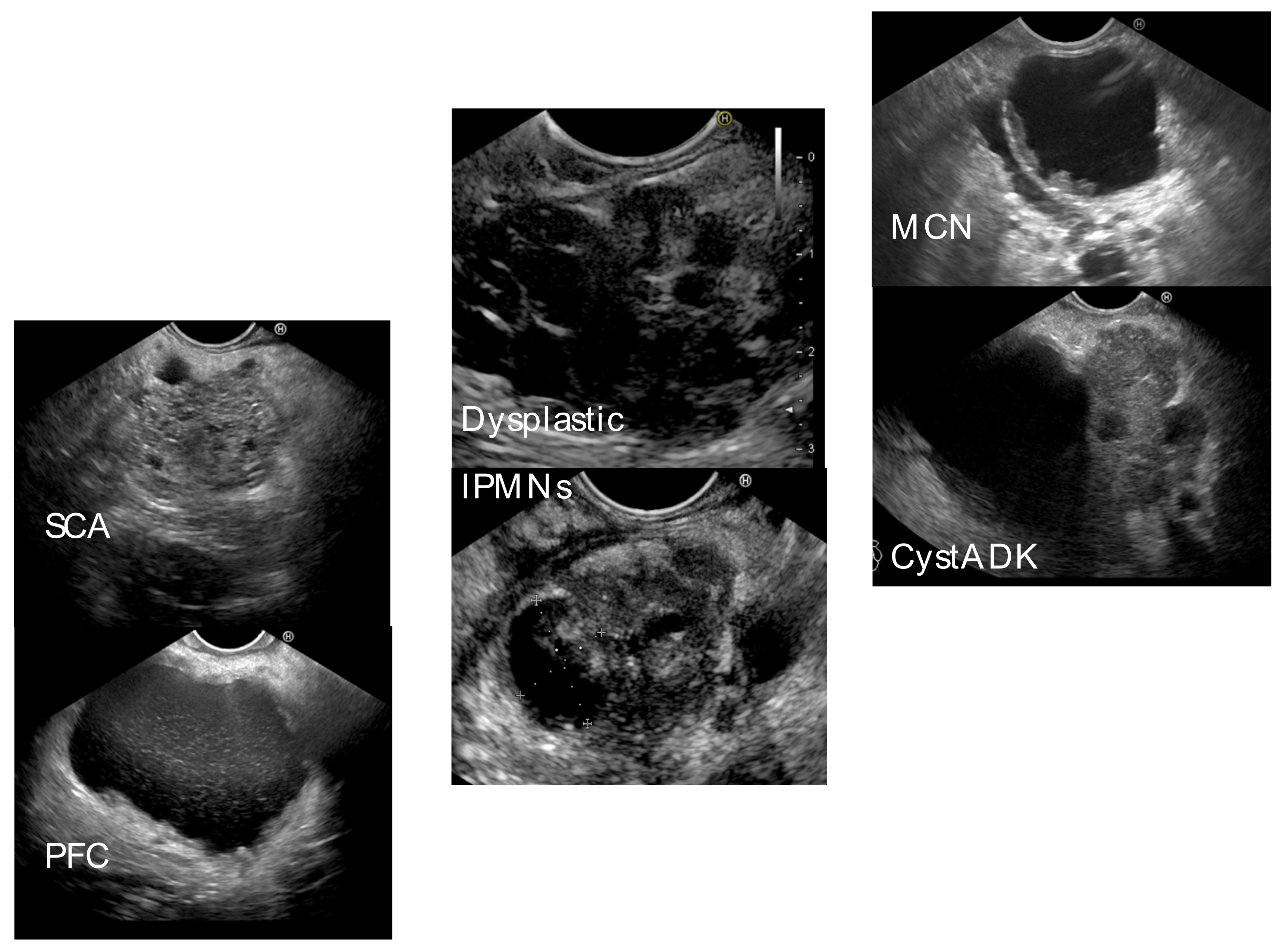
| n = 81 (%) | |
| Demographics | |
| Male sex | 44 (54.3) |
| Age (median, range) | 66, 21–88 years |
| Type of cystic lesion | |
| Pseudocyst | 25 (30.9) |
| WON | 2 (2.5) |
| IPMN (total) | 35 (43.2) |
| MD-IPMN | 9 |
| SB-IPMN | 25 |
| Mixed-IPMN | 1 |
| MCN | 9 (11.1) |
| Cystadenocarcinoma | 4 (4.9) |
| SCA | 5 (6.2) |
| NET | 1 (1.2) |
| Glycemic Abnormality Stratification | ||||
|---|---|---|---|---|
| Non-DM (%) | IFG (%) | Long-Lasting DM (%) | NOD (%) | |
| Pseudocyst + WON | 11 (40.7) | 11 (40.7) | 3 (11.1) | 2 (7.4) |
| SCA | 5 (100) | |||
| IPMN | 16 (45.7) | 11(31.4) | 7 (20) | 1 (2.8) |
| MD-IPMN | 3 (33.3) | 3 (33.3) | 2 (22.2) | 1 (11.1) |
| SB-IPMN | 12 (48) | 8 (32) | 5 (20) | |
| Mixed-IPMN | 1 (100) | |||
| MCN | 2 (22.2) | 3 (33.3) | 3 (33.3) | 1 (11.1) |
| CystADK | 1 (25) | 2 (50) | 1 (25) | |
| NET | 1 (100) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaban, D.V.; Coman, L.; Balaban, M.; Zoican, A.; Pușcașu, D.A.; Ayatollahi, S.; Mihălțeanu, E.; Costache, R.S.; Ioniță-Radu, F.; Jinga, M. Glycemic Abnormalities in Pancreatic Cystic Lesions—A Single-Center Retrospective Analysis. Gastroenterol. Insights 2023, 14, 191-203. https://doi.org/10.3390/gastroent14020015
Balaban DV, Coman L, Balaban M, Zoican A, Pușcașu DA, Ayatollahi S, Mihălțeanu E, Costache RS, Ioniță-Radu F, Jinga M. Glycemic Abnormalities in Pancreatic Cystic Lesions—A Single-Center Retrospective Analysis. Gastroenterology Insights. 2023; 14(2):191-203. https://doi.org/10.3390/gastroent14020015
Chicago/Turabian StyleBalaban, Daniel Vasile, Laura Coman, Marina Balaban, Andreea Zoican, Danusia Adriana Pușcașu, Simin Ayatollahi, Emanuela Mihălțeanu, Raluca Simona Costache, Florentina Ioniță-Radu, and Mariana Jinga. 2023. "Glycemic Abnormalities in Pancreatic Cystic Lesions—A Single-Center Retrospective Analysis" Gastroenterology Insights 14, no. 2: 191-203. https://doi.org/10.3390/gastroent14020015
APA StyleBalaban, D. V., Coman, L., Balaban, M., Zoican, A., Pușcașu, D. A., Ayatollahi, S., Mihălțeanu, E., Costache, R. S., Ioniță-Radu, F., & Jinga, M. (2023). Glycemic Abnormalities in Pancreatic Cystic Lesions—A Single-Center Retrospective Analysis. Gastroenterology Insights, 14(2), 191-203. https://doi.org/10.3390/gastroent14020015








