Abstract
Background: Rising incidence of inflammatory bowel disease (IBD) is an increasing concern among patients of young age worldwide and its most important complication is colitis-associated cancer (CAC). Vitamin D (VD) deficiency is common in IBD and inversely associated with disease activity; meanwhile, vitamin D receptor (VDR) signaling in the gut protects the mucosal epithelial barrier and inhibits inflammation in the colon. This study aims to investigate the connection between VDR expression and IBD in human colorectal tissues. Research design and methods: Using a cross-sectional analysis, this study investigated VDR nuclear immunohistochemistry expression in 35 subjects. The expression level was measured in patients with IBD, and compared with healthy controls (cut off 36.29%). Results: VDR nuclear expression was significantly down-regulated in colorectal tissues of patients with IBD, compared with controls (p = 0.025). Under-expression of VDR was more remarkable in colon cells of patients with UC (p = 0.023). These results confirm the protective role of VD for colonic mucosa in human colon as well, and suggest a benefit from VD supplementation in IBD patients. Conclusions: Our findings add to the body of evidence regarding the positive effect of VD in colorectal mucosal integrity. This study contributes in establishing one of the proposed markers related to disease activity, which can also predict the risk for complications.
1. Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic relapsing and remitting inflammatory condition with a multifactorial etiology. It involves an interplay between the innate immune system, the microbiome, and the environment in genetically predisposed individuals [1]. The incidence of IBD is increasing worldwide and, despite several established treatment regimens, it represents a major cause of lifelong morbidity and impaired quality of life. The integrity of the bowel mucosal barrier is constantly challenged by cell turnover and the luminal antigen content. One of the most important factors in the pathogenesis of this disease is an insufficient intestinal epithelial barrier [2].
The major complication of IBD is colitis-associated cancer (CAC). With the function of cytokines produced during chronic inflammatory response and their associated signaling pathways, the susceptible cell survives apoptosis and goes from dysplasia to CRC [3].
It is well known that long-standing IBD disposes to the development of colitis-associated cancer through the inflammation-dysplasia pathway. Patients with inflammatory bowel disease have an increased risk of developing colorectal cancer, and this risk is related to disease duration, extent, and cumulative inflammation burden [4]. The concept of cumulative inflammatory burden highlights the importance of considering histologic inflammation over time as an important risk factor for CAC [5]. Since this transformation involves the process of “field cancerisation” (dysplastic precursor lesions that may arise in multiple areas of the colon), it is evident that certain mucosal processes occur prior to the manifestation of any histological changes. Multiple genetic changes in the cells of the intestinal mucosa are associated with the development of CAC from inflamed cells in patients with IBD. Mutations in the tumor-related genes of intestinal epithelial cells and organ systems can be attributed to the action of inflammatory cytokines and reactive oxygen species [6]. Several markers for IBD-related dysplasia have been proposed (Ki67, p53, CEA), but they lacked precision due to respective limitations.
Vitamin D has been linked to a wide range of biological activities, including the modulation of gut mucosal immunity and the integrity of the intestinal barrier [7]. Vitamin D receptor (VDR) signaling in the gut protects the mucosal epithelial barrier and inhibits inflammation in the colon; however, vitamin D deficiency is common in IBD and inversely associated with disease activity, higher number of relapses, more frequent postoperative recurrence, poorer quality of life, and nonresponse to biologic therapies compared with healthy individuals or IBD patients with high vitamin D levels [8].
The VDR gene is located in the chromosome 12 region, which is linked to IBD, according to genome screening techniques. Genetic variation might alter the binding affinity of the vitamin D for VDR and it was associated with immune and inflammatory disease, including IBD [9]. According to genetic epidemiological studies, a link has been suggested between polymorphisms in the VDR gene region to the development of IBD [10,11]. Vitamin D/VDR signaling affects the expression of several genes, regulates the immune system, and modulates the inflammatory response in IBD, including experimental models, as well as humans [12,13,14].
Importantly, a decreased level of VDR expression in colonic cells of patients with ulcerative colitis has been associated with an increased risk of colon cancer [15]. VDR may represent a marker of mucosal dysplasia and cancer in UC patients, and could potentially be useful in patient surveillance and tumor prevention [16]. VDR is highly expressed in the small intestine and colon, and has critical regulatory actions for proliferation and differentiation, intestinal barrier function, innate immunity, and host defence in the gut [17]. The vitamin D/VDR signaling pathway controls the epithelial permeability by regulating the expression of several components of tight junctions and adherens junctions, as well as the release of antimicrobial peptides and mucins [18]. In mice lacking VDR, the disruption of the epithelial junctions was severe, and in Caco-2 cells monolayers, 1,25(OH)2D preserved the integrity of tight junctions [19]. Animals kept on a vitamin D-depleted diet and mouse models specifically lacking VDR expression in the intestinal epithelium are more susceptible to experimental colitis, and conversely, the induction of epithelial VDR reduces disease activity [20].
When it comes to humans, the results are less clear: studies assessing VDR expression in IBD vs. non-IBD controls have reported conflicting data, as two of them reported significantly lower VDR levels in inflamed IBD biopsies [20,21], while two other studies were not able to prove significant differences [22,23]. However, only few studies were performed in human tissue, most of them evaluating only overall VDR expression.
The aim of our study is to analyze the connection between VDR expression and inflammatory bowel disease by determining the nuclear VDR expression in colorectal cells of patients with IBD, in comparison with normal mucosa.
The hypotheses of this investigation are stated as:
- VDR nuclear expression is significantly reduced in patients with IBD compared to controls;
- VDR down-regulation in patients with inflammatory bowel disease (especially patients with UC) could establish stratifying them into groups, regarding closer surveillance according to their risk for CAC;
- VDR expression could contribute in differentiating patients with IBD according to their specific diagnosis, into patients with UC and patients with CD, respectively.
The confirmation of significant results will contribute in selecting IBD patients for closer surveillance, in order to prevent the complications of this disease, including colitis-associated cancer.
2. Materials and Methods
2.1. Study Participants
In total, 35 patients (18 women and 17 men, age range 19–81 years) whose diagnosis had been confirmed by endoscopy and pathology examination (biopsy/histopathology) were included in this study. Of these, 17 patients were diagnosed with ulcerative colitis, 10 with Crohn’s disease, and 8 were controls (patients who underwent surveillance colonoscopy). Their tissue specimens were obtained with consent for defining microscopic diagnosis.
2.2. Study Design
The study design was cross-sectional. Patient age and sex were recorded and colonoscopy with biopsy was performed for diagnostic determination. For patients who underwent surgery, the report was provided, as well as biopsy reports from colonic resections.
2.3. Sample Processing for IHC Examination
Patient samples consisted of biopsy samples and colon resections. Selected paraffin blocks were cut and the slides were prepared for the IHC procedure. Slides were incubated with primary VDR antibody (purified rat VDR, clone 9A7, 1:500 dilution, Thermo Scientific, Waltham, MA, USA) overnight at 4 °C. Slides were then washed with PBS + 0.05% Tween, 3 × 10 min, and then exposed to dextran polymer peroxidase envision system (HRP/rabbit and mouse DAKO Envision Kit), at 4 °C for 30 min. The slides were rinsed again in PBS + 0.05% Tween, 3 × 10 min. The chromogenic substance 3,3′-diaminobenzidine (DAKO) for 6 min was applied to visualize the staining, and nuclear counterstaining was performed with Mayer’s hematoxylin for 5 min. Slides were first washed in distilled water and then in PBS for 10 s, then washed in distilled water and mounted.
For negative control, slides were incubated with PBS without the primary antibody. Slides known to be immunostained positively were used for positive control.
2.4. Evaluation of Immunostaining
Nuclear VDR staining was evaluated at low magnification. Two pathologists examined the slides and a consensus was reached for each sample. Positive cells were expressed as a percentage (%) of the total number of epithelial cells. Mean nuclear VDR expression for all subjects (patients and control group) was 33.7%. A staining percentage of 33.7% of cells or more was considered a high expression, and below this level, it was classified as low expression.
2.5. Statistical Analysis
Analysis of variance was carried out in order to check if there was a difference between the control and patient groups (UC and CD) in VDR expression levels.
An independent sample t-test was carried out in order to check whether there were significant differences in VDR expression between the control group and patients diagnosed with UC.
For the performing of all statistical analyses, IBM SPSS Statistics version 23.0 was used.
A p value of 0.05 or less was considered statistically significant.
2.6. Ethical Considerations
The study was approved under the ethics of our Medical University Ethics Committee. Samples were selected from surgical specimens, as well as endoscopic biopsies of patients with inflammatory bowel disease.
3. Results
The clinical-pathological characteristics of the patients are summarized in Table 1.

Table 1.
Sample characteristics (n = 35).
Of the recruited patients, 17 of 35 were diagnosed (by colonoscopy examination and subsequent biopsy) as ulcerative colitis (UC), 10 of them as Crohn’s disease (CD), and 8 were controls.
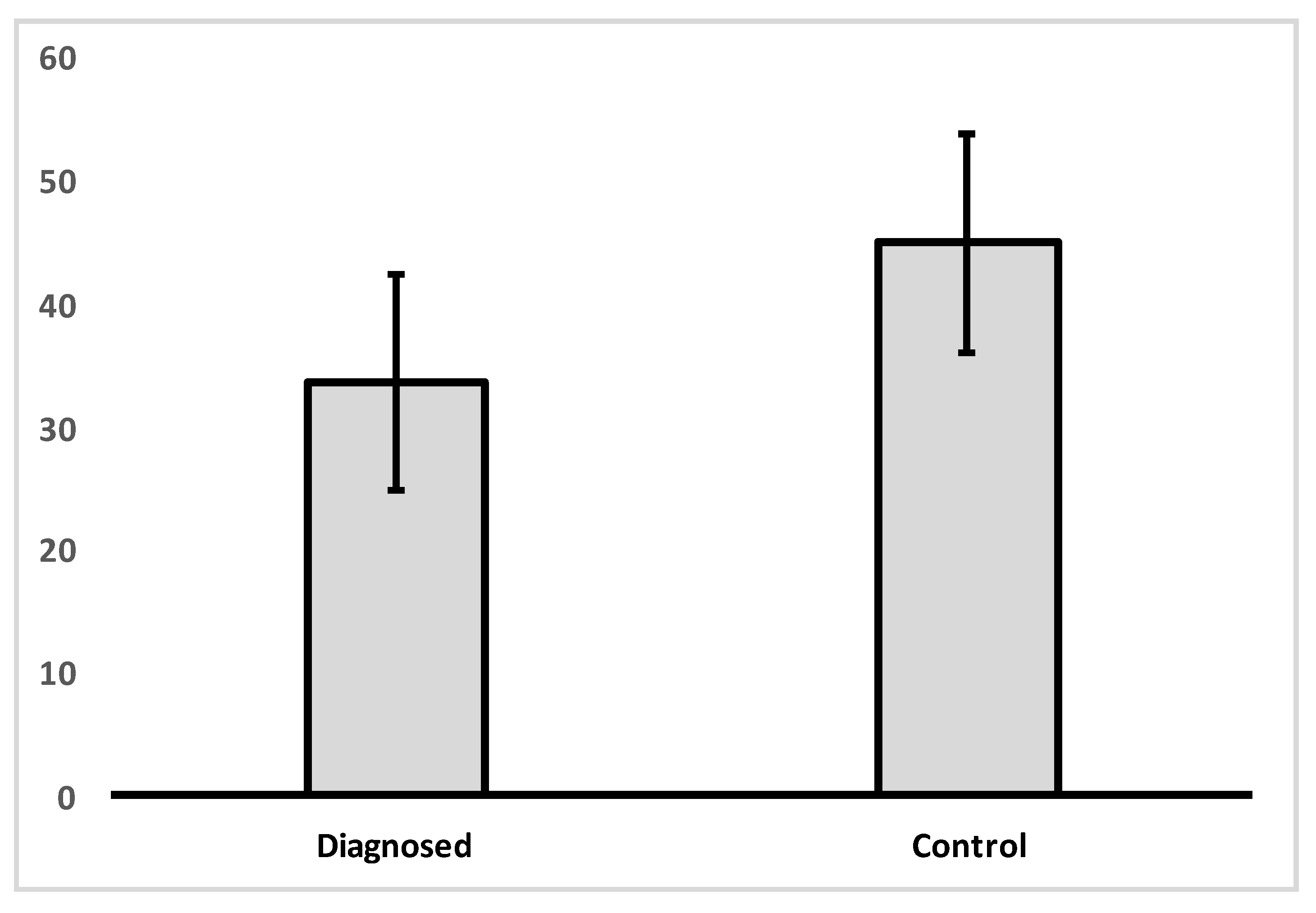
Analysis of variance was carried out in order to determine if there was a difference between the control and IBD patient groups in VDR nuclear expression levels (Figure 1, Table 2). A significant difference was found between groups in VDR levels [f(1, 33) = 5.495, p = 0.025].
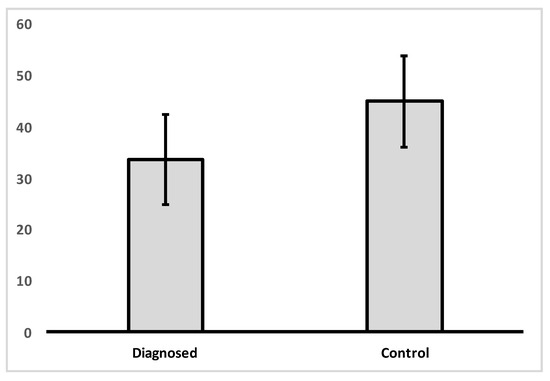
Figure 1.
Mean levels of VDR nuclear expression in colon tissue for control group and IBD diagnosed group.

Table 2.
Descriptive statistics for VDR expression for control and diagnosed groups.
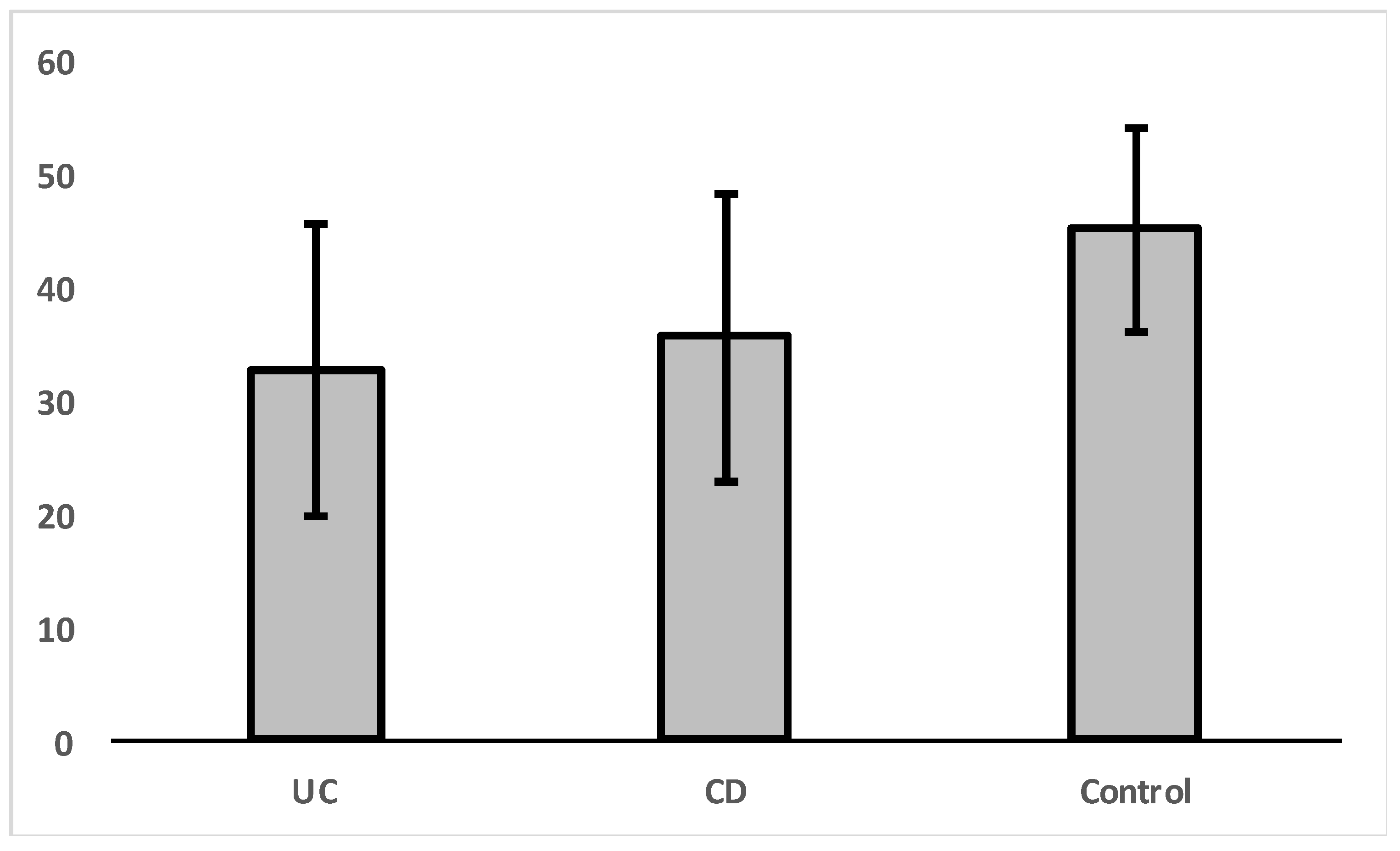
An independent sample t-test was carried out in order to check whether there were significant differences in VDR expression between the control group and patients diagnosed with ulcerative colitis (UC) or Crohn’s disease (CD) separately (Figure 2, Table 3). The results indicated that there is a significant difference in VDR expression levels for patients with ulcerative colitis [t(1,23) = 2.44, p = 0.023].
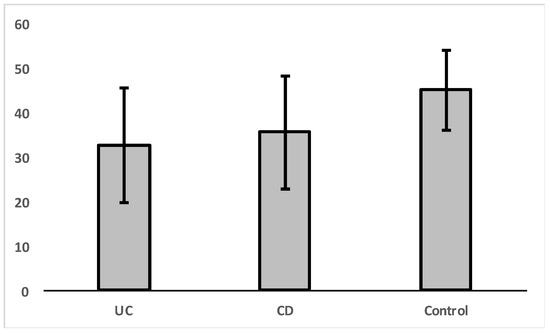
Figure 2.
Mean levels of VDR nuclear expression in colon cells for diagnosed patients with UC, CD, and control group.

Table 3.
Descriptive statistics of VDR expression by groups.
There was no significant difference in VDR expression levels between CD and UC groups [t(1, 25) = 0.557, p = 0.582].
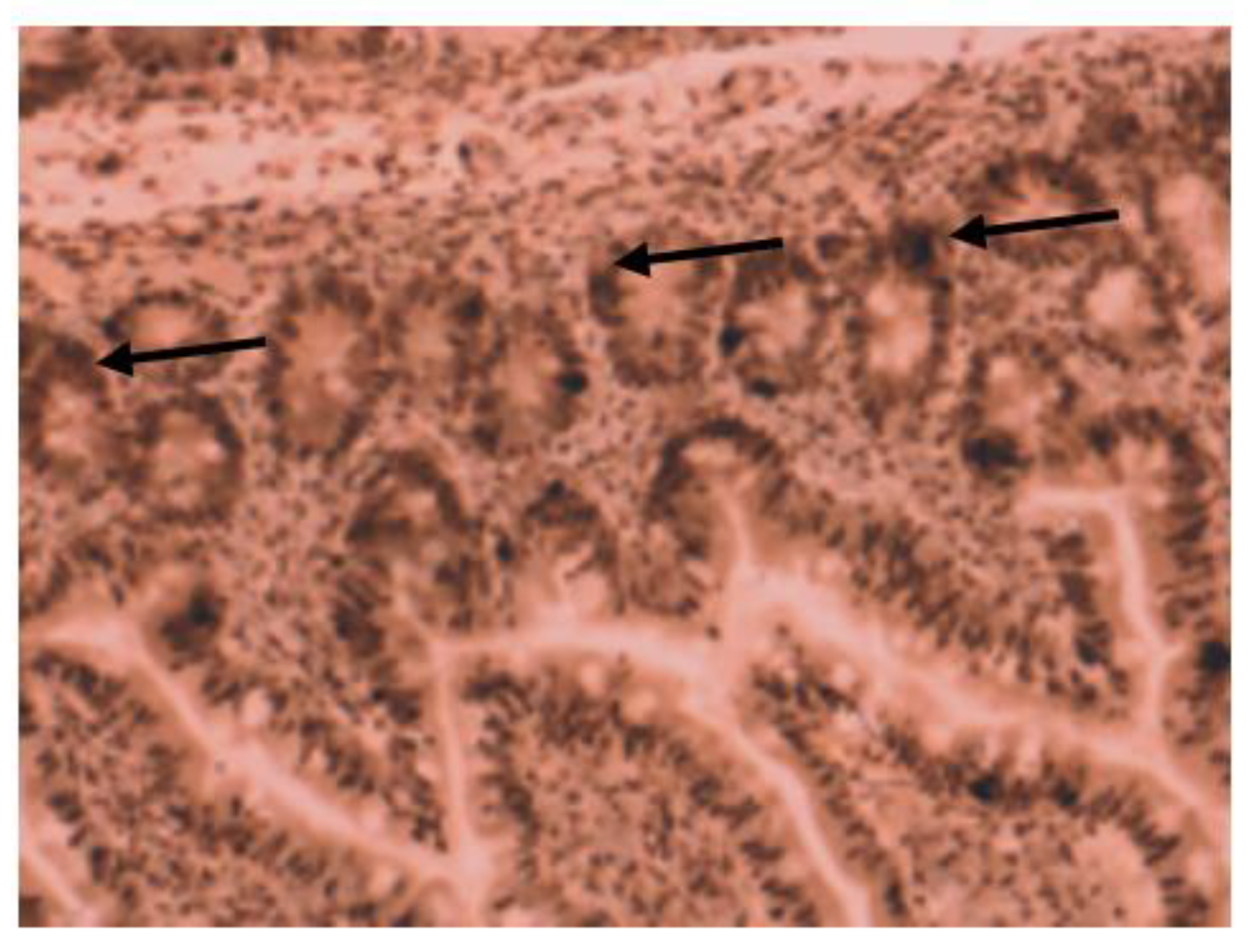
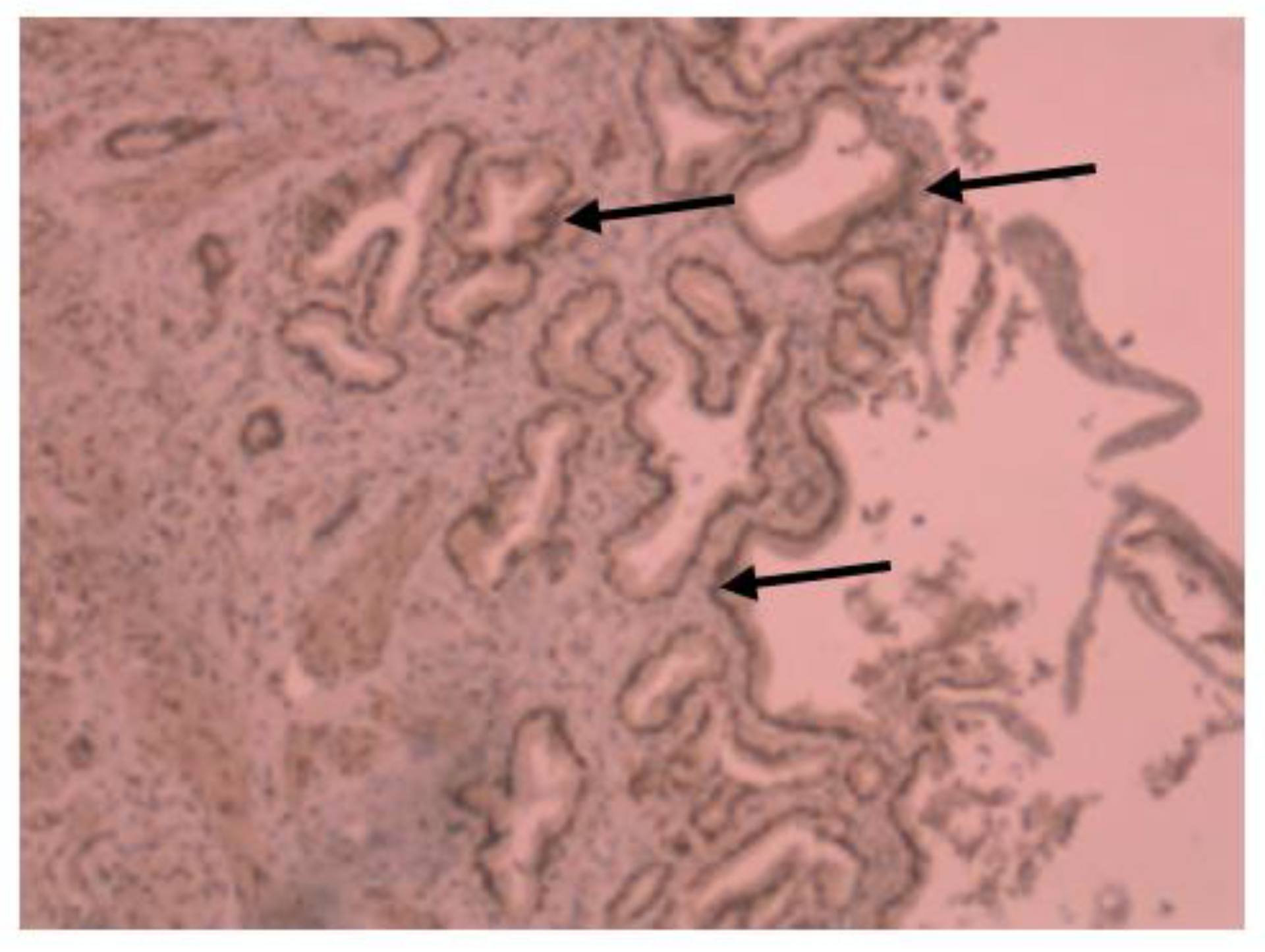
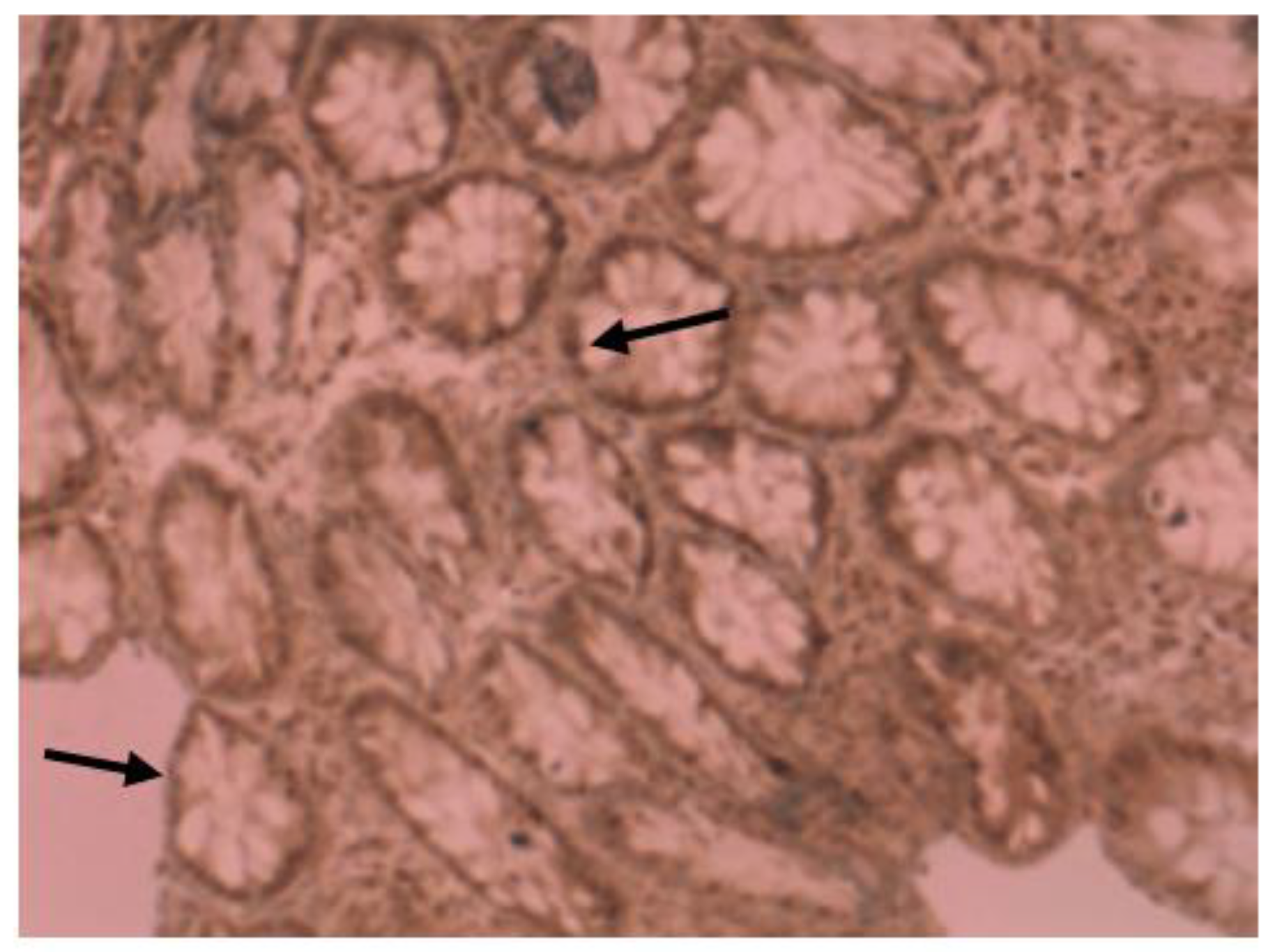
Medium VDR nuclear expression was high in normal colon (45%), while it was low in colorectal tissues of patients with IBD (33.7%). In colon tissue of patients with Crohn’s disease (Figure 3), medium VDR nuclear expression was 35.5%, and in colon cells of patients with ulcerative colitis (Figure 4), it was 32.65%.
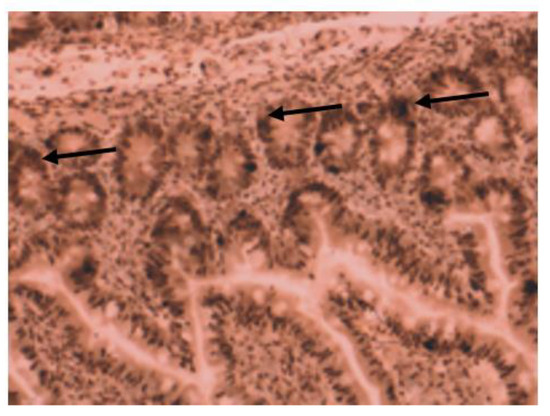
Figure 3.
Immunohistochemical image of VDR expression in colon tissue of a patient with Crohn’s disease.
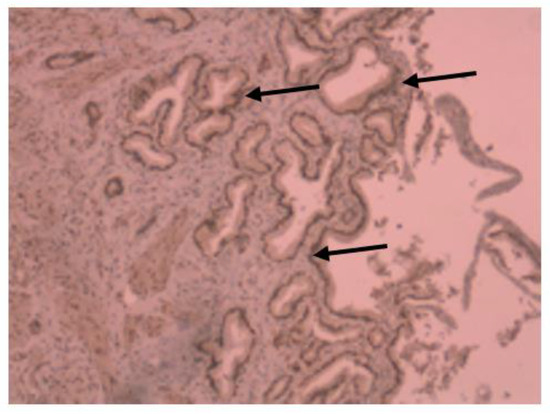
Figure 4.
Immunohistochemical image of VDR expression in colon tissue of a patient with ulcerative colitis.
All patients from the control group had high VDR nuclear expression (Figure 5); however, in the IBD group, only half of them (14 of 27 patients) had high VDR expression. The expression was analyzed separately by diagnosis as well (for patients with Crohn’s disease and ulcerative colitis).
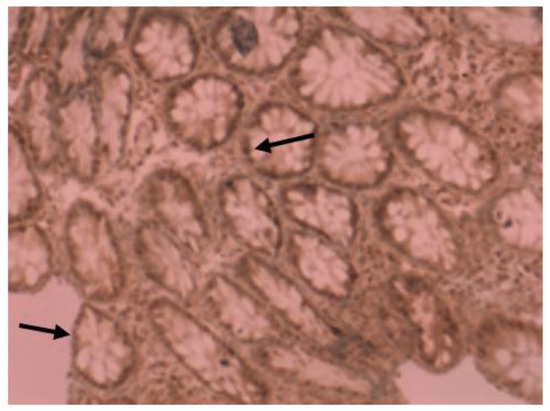
Figure 5.
Immunohistochemical image of VDR expression in normal tissue of a patient from control group.
VDR nuclear expression correlated inversely with the degree of inflammation (systemic inflammation markers: ESR, CRP, white cell count, platelet count, and albumin; marker of intestinal inflammation: fecal calprotectin).
VDR expression was higher in regions with no apparent inflammation (apparently healthy mucosa) of IBD patients, in comparison to the expression in the mucosa with visible inflammation (higher degree of inflammation): 45% vs. 35.5% for patients with Crohn’s disease, and 41.6% vs. 32.65% for patients with ulcerative colitis.
Apparently healthy mucosa of patients with IBD showed less VDR expression, compared with the mucosa of subjects without IBD (45% vs. 50 for subjects with ulcerative colitis and 41.6% vs. 50 for subjects with ulcerative colitis).
VDR expression correlated negatively with disease activity of IBD patients (both ulcerative colitis and Crohn’s disease).
4. Discussion
In this study, we found a significant difference in VDR nuclear IHC expression between patients with IBD and the control group. Different from previous studies, where overall VDR expression was analyzed, we evaluated nuclear VDR expression in particular, in order to obtain maximally reliable results.
These results support our first hypothesis and they build on existing evidence (mostly gained from preclinical studies, in which overall VDR expression was analyzed) regarding down-regulation of VDR in inflammatory bowel disease.
Our results are in line with [20,22,24]. Liu et al. concluded that epithelial VDR signaling inhibits colitis by protecting the mucosal epithelial barrier, independently of VDR actions in the non-epithelial immune system [20]. According to Garg et al., VDR may be up-regulated as a compensatory mechanism, and a deficiency to up-regulate may predispose to intestinal inflammation [22].
A research study published by Abrey-Delgado Y et al. (2016) could not confirm statistically significant differences in VDR immunohistochemistry scores in comparisons between control mucosa, normal appearing mucosa from IBD patients, and visually diseased mucosa from IBD patients [23]. However, in their previous research study (2015), the authors had found a significant decrease in colonic VDR expression in sporadic dysplasia and CRC, but not in CAC or in IBD when compared to normal tissue.
Considering ulcerative colitis alone, our results correlated with Tanaka H et al. [25], who found a considerably decreased expression of VDR in UC patients, although their results did not reach significance. However, their results confirmed that the VDR positive rate in colorectal tissues of patients with UC was significantly higher than CRC in non-UC. Based on these findings, as well as on the finding that long-term affected UC patients (>10 years, who were at high risk of developing CRC) showed significantly lower VDR expression than short-standing patients, the authors suggested that there might exist a correlation between the level of VDR expression and carcinogenesis in UC. They proposed VDR as one of the possible markers for detecting dysplasia and cancer in UC. VDR is known to exhibit lower expression in advanced cancer cells, which was confirmed by Tanaka et al. as well. The authors also showed that in most UC patients, the expression of VDR was considerably decreased compared to normal mucosa, whereas the VDR positive rate was significantly higher than CRC in non-UC. Their findings suggested that, according to the correlation between the level of VDR expression and carcinogenesis in UC, VDR could be one of the possible markers for predicting dysplasia and cancer in UC.
Wada K et al. had also confirmed a considerable decrease of VDR expression in patients with UC, compared to normal mucosa, as well as a lower VDR expression rate in UC patients with dysplasia and colon cancer than non-cancer UC [15]. Accordingly, they concluded that VDR expression in mucosa might be a possible marker to estimate the development of colon cancer in ulcerative colitis.
The significance of our results was even higher considering patients with ulcerative colitis, in comparison with patients who were diagnosed with Crohn’s disease, which is in line with our second hypothesis. However, regarding discriminating Crohn’s disease from ulcerative colitis according to VDR expression (our third hypothesis), our results are contrary to [26]. This was an unexpected finding, since we hypothesized that there is a significant difference in VDR expression between patients with UC and CD. The reason for different conclusions concerning VDR expression might be in performing different methods (IHC and RT-PCR, respectively).
Based on our findings of progressively higher VDR nuclear expression in normal mucosa of IBD patients, and non-IBD subjects, comparing to the inflamed colorectal mucosa of patients with IBD, we can propose VDR expression to serve as a marker of inflammation grade, and, accordingly, as a marker of dysplasia risk as well
According to Liu et al. (2013), reduced mucosal epithelial VDR expression is an intrinsic characteristic of IBD that is independent of serum vitamin D status, and local inflammation is a critical factor in this VDR down-regulation [20]. They provided strong evidence that epithelial VDR signaling inhibits colitis by protecting the mucosal epithelial barrier and suggested that targeting VDR expression in epithelial cells might be a useful strategy for the treatment of IBD.
One of the strengths of our study is that we performed IHC analysis in human tissues (colorectal cells of patients with IBD). Only few studies concerning the evaluation of VDR expression were performed in human cells. We evaluated nuclear expression of VDR, while to the best of our knowledge, most of the previous studies in this field have evaluated overall VDR expression.
Not taking in account (consideration) the treatment regimens of the certain patients could be considered as a limitation of our study.
In this study, the sample included patients with IBD. This may limit the generalizability of the results, but this study sample was in concordance with the aims of our study. While previous research had focused on patients with manifest dysplasia or CAC, the results of our study demonstrate that suspicious regions of inflamed colon may be detected very early—before dysplasia is manifested. It is beyond the scope of this study to evaluate VDR expression in patients with CAC.
Previous studies have concluded VDR expression is even lower in patients with dysplasia and CRC, compared to patients with CAC and IBD [17,27]. Accordingly, VDR expression could provide a valuable marker for stratifying patients with IBD, according to their risk for developing dysplasia and CAC.
Colon tumors with higher expression of VDR were more responsive to 1,25(OH)2D3 treatment [17]. Similarly, our results could help in selecting IBD patients, as well as CAC patients, who could mostly benefit from combination (adjuvant) treatment with vitamin D.
Gao H et al., 2023 found that vitamin D supplementation alleviates inflammation in ulcerative colitis, and provided evidence for the use of vitamin D in the clinical treatment of ulcerative colitis [28]. This is in line with our suggestions, according to the significance of our results.
Sharifi A et al. had demonstrated that vitamin D supplementation decreases the CD40L gene expression in patients with mild-to-moderate UC; also, CD40L gene expression correlates positively with clinical inflammatory markers in patients with UC [29].
According to Baffuto M et al., vitamin D has an important role during anti-TNF treatment of patients with Crohn’s disease: higher rates of clinical response and clinical remission correlate with increased VD levels [30].
5. Conclusions
Our results confirm a significant correlation between nuclear VDR expression in colorectal cells and inflammatory bowel disease. This builds on existing evidence for the protective role of vitamin D in colorectal mucosa.
Considering our results, as well as the results of previous studies, which confirm the effects of vitamin D supplementation in suppressing inflammation [28,29,30], the analysis of VD status and vitamin D supplementation during treatment of IBD is recommended.
Additional studies with larger samples are required in order to establish the dosage and duration of vitamin D supplementation in patients with IBD. Larger subgroups (ulcerative colitis/Crohn’s disease) would also enable more reliable results according to respective correlations with VDR expression.
Additional studies are also needed regarding VDR expression as an early marker for ranking IBD patients according to their risk for dysplasia and CAC. In terms of CAC prevention among patients with IBD, further research should include a follow up of patients with the lowest expression of VDR.
Author Contributions
Conceptualization, A.J.-S. and S.M.-K.; methodology, A.J.-S.; software, V.H.; validation, A.J.-S., S.M.-K. and V.H.; formal analysis, M.B.; investigation, A.J.-S.; resources, S.M.-K.; data curation, V.H.; writing—original draft preparation, A.J.-S.; writing—review and editing, A.J.-S., S.M.-K. and V.H.; visualization, S.M.-K.; supervision, M.B.; project administration, A.J.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the HERAS (Higher Education Research and Applied Science) Project, Austrian Development Cooperation and Ministry of Education, Science and Technology of Republic of Kosovo, implemented by WUS-Austria, ZSI and OeaD—Austrian Agency for International Cooperation in Education and Research.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Faculty of Medicine, University of Prishtina.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank Enikoe Kallay for giving us the opportunity of carrying out part of the research in the Medical University of Vienna—Laboratory of Pathophysiology and Allergy Research. We also express our gratitude to Mytaher Haskuka for his generous help with the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. Vitamin D Therapy in Adults with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830. [Google Scholar] [CrossRef]
- Kellermann, L.; Jensen, K.B.; Bergenheim, F.; Gubatan, J.; Chou, N.D.; Moss, A.; Nielsen, O.H. Mucosal vitamin D signaling in inflammatory bowel disease. Autoimmun. Rev. 2020, 19, 102672. [Google Scholar] [CrossRef] [PubMed]
- Vaghari-Tabari, M.; Targhazeh, N.; Moein, S.; Qujeq, D.; Alemi, F.; Majidina, M.; Younesi, S.; Asemi, Z.; Yousefi, B. From inflammatory bowel disease to colorectal cancer: What’s the role of miRNAs? Cancer Cell Int. 2022, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Al Bakir, I.; Curtius, K.; Graham, T.A. From Colitis to Cancer: An Evolutionary Trajectory that Merges Maths and Biology. Front. Immunol. 2018, 9, 2368. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Hirayama, D.; Wagatsuma, K.; Yamakawa, T.; Yokoyama, Y.; Nakase, H. Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 3062. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR with New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef]
- Gallone, G.; Haerty, W.; Disanto, G.; Ramagopalan, S.V.; Ponting, C.P.; Berlanga-Taylor, A.J. Identification of genetic variants affecting vitamin D receptor binding and associations with autoimmune disease. Hum. Mol. Genet. 2017, 26, 2164–2176. [Google Scholar] [CrossRef]
- Naderi, N.; Farnood, A.; Habibi, M.; Derakhshan, F.; Balaii, H.; Motahari, Z.; Agah, M.R.; Firouzi, F.; Rad, M.G.; Aghazadeh, R.; et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2008, 23, 1816–1822. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1717S–1720S. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yan, J.; Zhi, C.; Zhou, Q.; Yuan, X. 1, 25(OH)2 D3 deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model. Gut Pathog. 2019, 11, 8. [Google Scholar] [CrossRef]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Tanaka, H.; Maeda, K.; Inoue, T.; Noda, E.; Amano, R.; Kubo, N.; Muguruma, K.; Yamada, N.; Yashiro, M.; et al. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol. Rep. 2009, 22, 1021–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nobile, S.; Tenace, M.A.; Pappa, H.M. The Role of Vitamin D in the Pathogenesis of Inflammatory Bowel Disease. Gastrointest. Disord. 2019, 1, 231–240. [Google Scholar] [CrossRef]
- Sun, J. The Role of Vitamin D and Vitamin D Receptors in Colon Cancer. Clin. Transl. Gastroenterol. 2017, 8, e103. [Google Scholar] [CrossRef]
- Domazetovic, V.; Iantomasi, T.; Bonanomi, A.G.; Stio, M. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int. J. Color. Dis. 2020, 35, 1231–1242. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.; Claud, E.C.; Sun, J. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm. Bowel Dis. 2019, 25, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Sluka, P.; Wardan, H.; Hosking, P.; Monagle, S.; Thomas, M.; Lubel, J.S.; et al. The intestinal vitamin D receptor in inflammatory bowel disease: Inverse correlation with inflammation but no relationship with circulating vitamin D status. Ther. Adv. Gastroenterol. 2019, 12, 1756284818822566. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Delgado, Y.; Isidro, R.A.; Torres, E.A.; González, A.; Cruz, M.L.; Isidro, A.A.; González-Keelan, C.I.; Medero, P.; Appleyard, C.B. Serum vitamin D and colonic vitamin D receptor in inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 3581–3591. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, T.; Liu, Y.; Lyu, L.; Li, X.; Yue, W. How would serum 25(OH) D level change in patients with inflammatory bowel disease depending on intestinal mucosa vitamin D receptor (VDR) and vitamin D1-α hydroxylase (CYP27B1)? Turk. J. Gastroenterol. 2019, 30, 132–138. [Google Scholar] [CrossRef]
- Tanaka, H.; Maeda, K.; Wada, K.; Yashiro, M.; Onoda, N.; Yamada, N.; Sawada, T.; Ohira, M.; Hirakawa, K. Correlation of vitamin D receptor expression with colorectal cancer in ulcerative colitis. Cancer Res. 2008, 68 (Suppl. 9), 5501. [Google Scholar]
- James, J.; Nielsen, B.S.; Christensen, I.; Langholz, E.; Malham, M.; Riis, L.; Høgdall, E. MUCOSAL Expression of genes PI3, ANXA1, and VDR descriminates Crohn’s disease from ulcerative colitis in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2023, 29 (Suppl. 1), S13. [Google Scholar] [CrossRef]
- Isidro, R.A.; Cruz, M.L.; Isidro, A.A.; Baez, A.; Arroyo, A.; González-Marqués, W.A.; González-Keelan, C.; Torres, E.A.; Appleyard, C.B. Immunohistochemical expression of SP-NK-1R-EGFR pathway and VDR in colonic inflammation and neoplasia. World J. Gastroenterol. 2015, 21, 1749–1758. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, H.H.; Zhang, Z.; Gao, J.; Li, J.; Li, X. Vitamin D3 alleviates inflammation in ulcerative colitis by activating the VDR-NLRP6 signaling pathway. Front. Immunol. 2023, 14, 1135930. [Google Scholar] [CrossRef]
- Sharifi, A.; Vahedi, H.; Honarvar, M.R.; Amiriani, T.; Nikniaz, Z.; Rad, E.Y.; Hosseinzadeh-Attar, M.J. Vitamin D decreases CD40L gene expression in ulcerative colitis patients: A randomized, double-blinded, placebo-controlled trial. Turk. J. Gastroenterol. 2020, 31, 99–104. [Google Scholar] [CrossRef]
- Bafutto, M.; Oliveira, E.C.; Rezende Filho, J. Use of Vitamin D with Anti-Tumor Necrosis Factor Therapy for Crohn’s Disease. Gastroenterol. Res. 2020, 13, 101–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).