Adherence to Recommended Immunization Schedules in Patients with Inflammatory Bowel Disease on Biologics and Small Molecule Therapies
Abstract
:1. Introduction
2. Materials and Methods
Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Serrano, C.; Mirada, G.; Marsal, J.R.; Ortega, M.; Sol, J.; Solano, R.; Artigues, E.M.; Estany, P. Compliance with the guidelines on recommended immunization schedule in patients with inflammatory bowel disease: Implications on public health policies. BMC Public Health 2020, 20, 713. [Google Scholar] [CrossRef]
- Mosli, M.; Alawadhi, S.; Hasan, F.; Abou Rached, A.; Sanai, F.; Danese, S. Incidence, prevalence, and clinical epidemiology of inflammatory bowel disease in the Arab World: A systematic review and meta-analysis. Inflamm. Intest. Dis. 2021, 6, 123–131. [Google Scholar] [CrossRef]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef]
- DiPiro, J.T.; Talbert, R.L.; Yee, G.C.; Matzke, G.R.; Wells, B.G.; Posey, L.M. (Eds.) Hemstreet BAInflammatory Bowel Disease; Pharmacotherapy: A Pathophysiologic Approach, 10th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Wheeler, J.; Slack, N.; Duncan, A.; Whitehead, P.; Russell, G.; Harvey, R. The diagnosis of intra-abdominal abscesses in patients with severe Crohn’s disease. QJM Int. J. Med. 1992, 82, 159–167. [Google Scholar]
- Melmed, G.Y. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm. Bowel Dis. 2009, 15, 1410–1416. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Reich, J.; Wasan, S.; Farraye, F.A. Vaccinating patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2016, 12, 540. [Google Scholar]
- Macaluso, F.S.; Liguori, G.; Galli, M. Vaccinations in patients with inflammatory bowel disease. Dig. Liver Dis. 2021, 53, 1539–1545. [Google Scholar] [CrossRef]
- Fiorino, G.; Peyrin-Biroulet, L.; Naccarato, P.; Szabò, H.; Sociale, O.R.; Vetrano, S.; Fries, W.; Montanelli, A.; Repici, A.; Malesci, A.; et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: A prospective study. Inflamm. Bowel Dis. 2012, 18, 1042–1047. [Google Scholar] [CrossRef]
- Sánchez-Tembleque, M.D.; Corella, C.; Pérez-Calle, J.L. Vaccines and recommendations for their use in inflammatory bowel disease. World J. Gastroenterol. 2013, 19, 1354–1358. [Google Scholar] [CrossRef]
- Leung, V.S.; Nguyen, M.T.; Bush, T.M. Disseminated primary varicella after initiation of infliximab for Crohn’s disease. Off. J. Am. Coll. Gastroenterol. ACG 2004, 99, 2503–2504. [Google Scholar] [CrossRef]
- Ritz, M.A.; Jost, R. Severe pneumococcal pneumonia following treatment with infliximab for Crohn’s disease. Inflamm. Bowel Dis. 2001, 7, 327. [Google Scholar] [CrossRef]
- Foster, K.; Devitt, N.; Gallagher, P.; Abbott, R. Overwhelming pneumococcal septicaemia in a patient with ulcerative colitis and splenic atrophy. Gut 1982, 23, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.E.; Olson, A.D.; Kraker, S.; Dickinson, C.J. Overwhelming varicella pneumonia in a patient with Crohn’s disease treated with 6-mercaptopurine. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Bernal, I.; Domènech, E.; García-Planella, E.; Cabré, E.; Gassull, M.A. Infecciones oportunistas en pacientes con enfermedad inflamatoria intestinal bajo tratamiento inmunosupresor. Gastroenterol. Y Hepatol. 2003, 26, 19–22. [Google Scholar] [CrossRef]
- Esteve, M.; Saro, C.; Gonzalez-Huix, F.; Suarez, F.; Forne, M.; Viver, J. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: Need for primary prophylaxis. Gut 2004, 53, 1363–1365. [Google Scholar] [CrossRef]
- Millonig, G.; Kern, M.; Ludwiczek, O.; Nachbaur, K.; Vogel, W. Subfulminant hepatitis B after infliximab in Crohn’s disease: Need for HBV-screening? World J. Gastroenterol. WJG 2006, 12, 974. [Google Scholar] [CrossRef]
- Kotton, C.N. Nailing down the shingles in IBD. Inflamm. Bowel Dis. 2007, 13, 1178–1179. [Google Scholar] [CrossRef]
- Gupta, G.; Lautenbach, E.; Lewis, J.D. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, P.; Furst, K.; Tully, K. Tuberculosis associated with blocking agents against tumor necrosis factor-alpha-California, 2002–2003. MMWR Morb. Mortal. Wkly. Rep. 2004, 53, 683–686. [Google Scholar]
- Benchimol, E.I.; Tse, F.; Carroll, M.W.; deBruyn, J.C.; McNeil, S.A.; Pham-Huy, A.; Seow, C.H.; Barrett, L.L.; Bessissow, T.; Carman, N.; et al. Canadian Association of Gastroenterology clinical practice guideline for immunizations in patients with inflammatory bowel disease (IBD)—Part 1: Live vaccines. J. Can. Assoc. Gastroenterol. 2021, 4, e59–e71. [Google Scholar] [CrossRef]
- Manser, C.N.; Maillard, M.H.; Rogler, G.; Schreiner, P.; Rieder, F.; Bühler, S. Vaccination in patients with inflammatory bowel diseases. Digestion 2020, 101, 58–68. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision; World Health Organization: Geneva, Switzerland, 2016.
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strobe Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, M.S.; Kim, E.S.; Lee, J.; Lee, J.M.; Choi, H.S.; Keum, B.; Jeen, Y.T.; Lee, H.S.; Chun, H.J.; et al. Higher risk of tuberculosis in combination therapy for inflammatory bowel disease: A nationwide population-based cohort study in South Korea. Medicine 2020, 99, e22897. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Barrett, K.J.; Davies, R.J.; Bennett, C.; Gittens, S. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Chebli, J.M.F.; Gaburri, P.D.; de Almeida Costa, L.; Chebli, L.A.; da Rocha Ribeiro, T.C.; Aguiar, N.P.; Malaguti, C.; Furtado, M.C. Gastroenterology and Hepatology Research. J. GHR 2018, 7, 2542–2554. [Google Scholar]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; Van Assen, S.; Bijl, M.; Breedveld, F.C.; D’amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef]
- Malhi, G.; Rumman, A.; Thanabalan, R.; Croitoru, K.; Silverberg, M.S.; Hillary Steinhart, A.; Nguyen, G.C. Vaccination in Inflammatory Bowel Disease Patients: Attitudes, Knowledge, and Uptake. J. Crohn’s Colitis 2015, 9, 439–444. [Google Scholar] [CrossRef]
- Ford, T.; Danchin, M.; McMinn, A.; Perrett, K.; Alex, G.; Crawford, N.W. Immunisation status of children and adolescents with a new diagnosis of inflammatory bowel disease. BMC Infect. Dis. 2022, 22, 6. [Google Scholar] [CrossRef]
- Jamur, C.M.; Marques, J.; Kim, M.S.; Petterle, R.R.; Amarante, H.M. Immunization status of patients with inflammatory bowel disease. Arq. De Gastroenterol. 2019, 56, 124–130. [Google Scholar]
- Ryu, H.H.; Chang, K.; Kim, N.; Lee, H.-S.; Hwang, S.W.; Park, S.H.; Yang, D.-H.; Byeon, J.-S.; Myung, S.-J.; Yang, S.-K.; et al. Insufficient vaccination and inadequate immunization rates among Korean patients with inflammatory bowel diseases. Medicine 2021, 100, e27714. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cuesta, P.; González-Alayón, C.; Jurado-García, J.; Iglesias-Flores, E.M.; Barranco-Quintana, J.L.; García-García, L.; Salgueiro-Rodríguez, I.M.; Benitez-Cantero, J.M.; García-Sánchez, V. Adherence to a predefined vaccination program in patients with inflammatory bowel disease. Gastroenterol. Y Hepatol. 2016, 39, 385–392. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Epidemiological Report on Tuberculosis; WHO: Geneva, Switzerland, 2022.
- Wasan, S.K.; Calderwood, A.H.; Long, M.D.; Kappelman, M.D.; Sandler, R.S.; Farraye, F.A. Immunization Rates and Vaccine Beliefs Among Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 246–250. [Google Scholar] [CrossRef]
- Dai, C.; Jiang, M.; Huang, Y.-H. COVID-19 Vaccination in Patients with Inflammatory Bowel Disease: A Survey from China. Dig. Dis. Sci. 2022, 67, 3468–3470. [Google Scholar] [CrossRef]
- Narula, N.; Dhillon, A.S.; Chauhan, U.; Marshall, J.K. An audit of influenza vaccination status in adults with inflammatory bowel disease. Can. J. Gastroenterol. 2012, 26, 593–596. [Google Scholar] [CrossRef]
- Selby, L.; Hoellein, A.; Wilson, J.F. Are primary care providers uncomfortable providing routine preventive care for inflammatory bowel disease patients? Dig. Dis. Sci. 2011, 56, 819–824. [Google Scholar] [CrossRef]
- Kim, S.B.; Park, S.J.; Chung, S.H.; Hahn, K.Y.; Moon, D.C.; Hong, S.P.; Cheon, J.H.; Kim, T.I.; Kim, W.H. Vaccination and complementary and alternative medicine in patients with inflammatory bowel disease. Intest. Res. 2014, 12, 124–130. [Google Scholar] [CrossRef]
- Langhorst, J.; Anthonisen, I.B.; Steder-Neukamm, U.; Luedtke, R.; Spahn, G.; Michalsen, A.; Dobos, G.J. Patterns of complementary and alternative medicine (CAM) use in patients with inflammatory bowel disease: Perceived stress is a potential indicator for CAM use. Complement. Ther. Med. 2007, 15, 30–37. [Google Scholar] [CrossRef]
- Karr, J.R.; Lu, J.J.; Smith, R.B.; Thomas, A.C. Using computerized physician order entry to ensure appropriate vaccination of patients with inflammatory bowel disease. Ochsner J. 2016, 16, 90–95. [Google Scholar]
- Alsanafi, M.; Salim, N.A.; Sallam, M. Willingness to get HPV vaccination among female university students in Kuwait and its relation to vaccine conspiracy beliefs. Hum. Vaccin. Immunother. 2023, 19, 2194772. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; Zurba, Y.; Al Abdulsalam, A.; Alfadhli, A.; Elouali, S. COVID-19 vaccine hesitancy among patients with inflammatory bowel disease receiving biologic therapies in Kuwait: A cross-sectional study. Vaccines 2021, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; Alrashed, F.; Alfadhli, A. COVID-19 Vaccine Booster Dose Willingness among Patients with Inflammatory Bowel Disease on Infliximab and Vedolizumab: A Cross-Sectional Study. Vaccines 2022, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Panagos, K.; Alizadeh, M.; Bell, M.; Bourmaf, M.; Zisman, E.; Paul, P.; Sibel, L.; Wong, U. Patients with inflammatory bowel disease are more hesitant about Coronavirus disease 2019 vaccination. Front. Med. 2022, 9, 1005121. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Abdullah, I.; Alfadhli, A.; Ali, H.; Abu-Farha, M.; Channanath, A.M.; Abubaker, J.A.; Al-Mulla, F. Impact of BNT162b2 mRNA Vaccination on the Development of Short and Long-Term Vaccine-Related Adverse Events in Inflammatory Bowel Disease: A Multi-Center Prospective Study. Front. Med. 2022, 9, 881027. [Google Scholar] [CrossRef]
| Baseline Characteristics of Patients | |
|---|---|
| Total sample (n) | 394 |
| Age (mean) | 31.4 |
| N (%) | |
| Gender | |
| Female | 159(40.3) |
| Male | 235 (59.7) |
| Type of IBD | |
| Crohn’s disease | 285 (72.3) |
| Ulcerative colitis | 109 (27.7) |
| Nationality | |
| Kuwaiti | 297 (75.4) |
| Non-Kuwaiti | 97 (24.6) |
| Smoking status | |
| Smoker | 73 (18.5) |
| Non-smoker | 321 (81.5) |
| Comorbidities | |
| (Asthma, COPD, HTN, DM, CHD, CAD, CKD) | |
| Yes | 108 (27.4) |
| No | 286 (72.6) |
| Biologic use | N (%) |
| Infliximab | 117 (29.7) |
| Ustekinumab | 111 (28.2) |
| Adalimumab | 75 (19.1) |
| Vedolizumab | 60 (15.2) |
| Golimumab | 21 (5.3) |
| Upadacitinib | 10 (2.5) |
| Concurrent Immunomodulators use | 102 (25.9) |
| Current Corticosteroids use | 25 (6.3) |
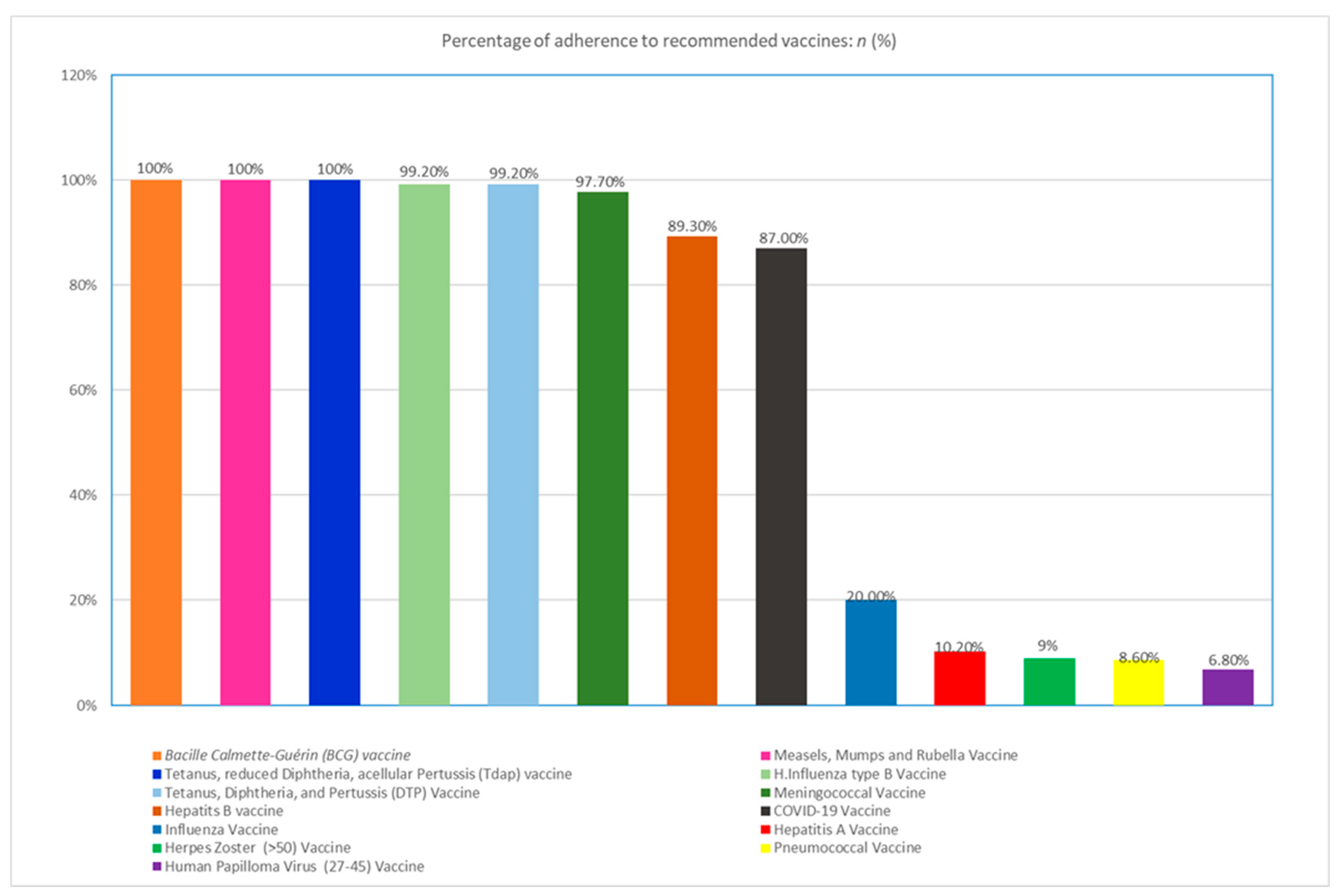
| Percentage of Adherence to Recommended Vaccines: | n (%). |
|---|---|
| Measles, Mumps and Rubella Vaccine | 394 (100) |
| Hepatitis B Vaccine | 352 (89.3) |
| H.Influenza type B Vaccine | 391 (99.2) |
| Human Papilloma Virus (27–45) Vaccine + | 7 (6.8) |
| Herpes Zoster (>50) Vaccine * | 9 (9) |
| Hepatitis A Vaccine | 40 (10.2) |
| Influenza Vaccine | 79 (20) |
| Meningococcal Vaccine | 385 (97.7) |
| Tetanus, reduced Diphtheria, acellular Pertussis (Tdap)∙ Vaccine | 394 (100) |
| Tetanus, Diphtheria, and Pertussis (DTP) Vaccine | 391(99.2) |
| Pneumococcal Vaccine | 34(8.6) |
| COVID-19 Vaccine | 343 (87) |
| Bacille Calmette-Guérin (BCG) vaccine | 394(100) |
| Totally Vaccinated | Partially Vaccinated | p Value | |
|---|---|---|---|
| Patients above 50 years old | 43(69.4%) | 19(30.6%) | 0.6 |
| Patients below 50 years old | 193(58.1%) | 139(41.9%) | |
| Ulcerative colitis | 70(64.2%) | 39(35.8%) | 0.5 |
| Crohn’s disease | 166(58.2%) | 119(41.8%) | |
| Female patients | 127(79.9%) | 32(20.1%) | 0.001 |
| Male patients | 109(46.4%) | 126(53.6%) | |
| Kuwaiti patients | 180(60.6%) | 117(39.4%) | 0.8 |
| Non-Kuwaiti patients | 56(57.7%) | 41(42.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehab, M.; Almatar, R.; Almohammad, R.; Alfadhli, A. Adherence to Recommended Immunization Schedules in Patients with Inflammatory Bowel Disease on Biologics and Small Molecule Therapies. Gastroenterol. Insights 2023, 14, 383-393. https://doi.org/10.3390/gastroent14030028
Shehab M, Almatar R, Almohammad R, Alfadhli A. Adherence to Recommended Immunization Schedules in Patients with Inflammatory Bowel Disease on Biologics and Small Molecule Therapies. Gastroenterology Insights. 2023; 14(3):383-393. https://doi.org/10.3390/gastroent14030028
Chicago/Turabian StyleShehab, Mohammad, Ranim Almatar, Rawan Almohammad, and Ahmad Alfadhli. 2023. "Adherence to Recommended Immunization Schedules in Patients with Inflammatory Bowel Disease on Biologics and Small Molecule Therapies" Gastroenterology Insights 14, no. 3: 383-393. https://doi.org/10.3390/gastroent14030028





