New Insights into Surgical Management of Intrahepatic Cholangiocarcinoma in the Era of “Transplant Oncology”
Abstract
:1. Introduction
2. Diagnosis and Staging
3. Prognosis
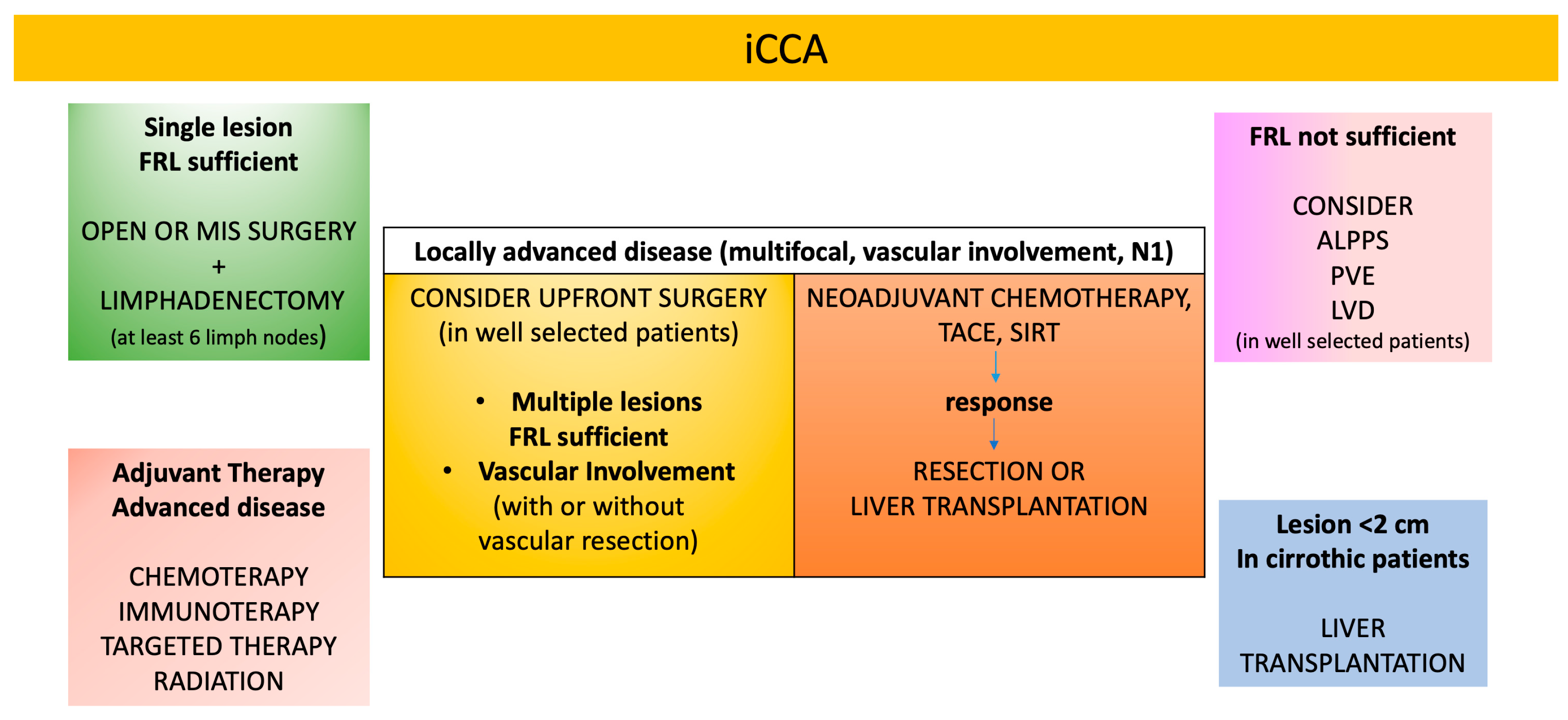
4. Surgical Management
5. Anatomical vs. Non-Anatomical Liver Resection
6. Lymphadenectomy
7. Inadequate Future Liver Remnant
8. Role Played by Liver Transplantation in Treating iCCA
9. Minimally Invasive Surgery
10. Neoadjuvant Therapy
11. Systemic Treatments in Advanced Disease
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. 1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on Intrahepatic Cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, S.; Nevi, L.; Overi, D.; Landolina, N.; Faccioli, J.; Giulitti, F.; Napoletano, C.; Oddi, A.; Marziani, A.M.; Costantini, D.; et al. Metformin exerts anti-cancerogenic effects and reverses epithelial-to-mesenchymal transition trait in primary human intrahepatic cholangiocarcinoma cells. Sci. Rep. 2021, 11, 2557. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 98–107. [Google Scholar] [CrossRef]
- Hyder, O.; Hatzaras, I.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013, 153, 811–818. [Google Scholar] [CrossRef]
- Blechacz, B.R.A.; Gores, G.J. Cholangiocarcinoma. Clin. Liver Dis. 2008, 12, 131–150. [Google Scholar] [CrossRef]
- Munugala, N.; Maithel, S.K.; Shroff, R.T. Novel biomarkers and the future of targeted therapies in cholangiocarcinoma: A narrative review. Hepatobiliary Surg. Nutr. 2022, 11, 253–266. [Google Scholar] [CrossRef]
- Rompianesi, G.; Di Martino, M.; Gordon-Weeks, A.; Montalti, R.; Troisi, R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J. Gastrointest. Oncol. 2021, 13, 332–350. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef]
- Hu, L.S.; Zhang, X.F.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; Shen, F.; et al. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023, 77, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Brunese, M.C.; Fantozzi, M.R.; Fusco, R.; De Muzio, F.; Gabelloni, M.; Danti, G.; Borgheresi, A.; Palumbo, P.; Bruno, F.; Gandolfo, N.; et al. Update on the Applications of Radiomics in Diagnosis, Staging, and Recurrence of Intrahepatic Cholangiocarcinoma. Diagnostics 2023, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Coburn, N.G.; Baxter, N.N.; Kiss, A.; Law, C.H. Surgical management of intrahepatic cholangiocarcinoma—A population-based study. Ann. Surg. Oncol. 2008, 15, 600–608. [Google Scholar] [CrossRef]
- Doussot, A.; Gonen, M.; Wiggers, J.K.; Groot-Koerkamp, B.; DeMatteo, R.P.; Fuks, D.; Allen, P.J.; Farges, O.; Kingham, T.P.; Regimbeau, J.M.; et al. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J. Am. Coll. Surg. 2016, 223, 493–505.e2. [Google Scholar] [CrossRef]
- Liu, H.; Lin, L.; Lin, Z.; Chen, Y.; Huang, Q.; Ding, L.; Lou, J.; Zheng, S.; Bi, X.; Wang, J.; et al. Impact of surgical margin width on long-term outcomes for intrahepatic cholangiocarcinoma: A multicenter study. BMC Cancer 2021, 21, 840. [Google Scholar] [CrossRef]
- Dai, Y.S.; Hu, H.J.; Lv, T.R.; Hu, Y.F.; Zou, R.Q.; Li, F.Y. The influence of resection margin width in patients with intrahepatic cholangiocarcinoma: A meta-analysis. World J. Surg. Oncol. 2023, 21, 16. [Google Scholar] [CrossRef]
- Jiang, J.H.; Fang, D.Z.; Hu, Y.T. Influence of surgical margin width on survival rate after resection of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. BMJ Open 2023, 13, e067222. [Google Scholar] [CrossRef]
- Watanabe, Y.; Matsuyama, Y.; Izumi, N.; Kubo, S.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Nakashima, O.; Kudo, M. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: A nationwide survey of the Liver Cancer Study Group of Japan. Surgery 2020, 167, 793–802. [Google Scholar] [CrossRef]
- Van Keulen, A.M.; Büttner, S.; Erdmann, J.I.; Hagendoorn, J.; Hoogwater, F.J.H.; Ijzermans, J.N.M.; Neumann, U.P.; Polak, W.G.; De Jonge, J.; Olthof, P.B.; et al. Major complications and mortality after resection of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Surgery 2023, 173, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Moazzam, Z.; Woldesenbet, S.; Araujo Lima, H.; Alaimo, L.; Munir, M.M.; Shaikh, C.F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; et al. Predictors and Prognostic Significance of Postoperative Complications for Patients with Intrahepatic Cholangiocarcinoma. World J. Surg. 2023, 47, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, Z.; Alaimo, L.; Endo, Y.; Lima, H.A.; Ruzzenente, A.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; et al. Combined Tumor Burden Score and Carbohydrate Antigen 19-9 Grading System to Predict Outcomes Among Patients with Intrahepatic Cholangiocarcinoma. J. Am. Coll. Surg. 2023, 236, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, L.; Moazzam, Z.; Endo, Y.; Lima, H.A.; Ruzzenente, A.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; et al. Long-Term Recurrence-Free and Overall Survival Differ Based on Common, Proliferative, and Inflammatory Subtypes After Resection of Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2023, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhao, S.; Zhu, H.; Ji, G.; Zhang, X. Primary tumor resection improves survival in patients with multifocal intrahepatic cholangiocarcinoma based on a population study. Sci. Rep. 2021, 11, 12166. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Franssen, S.; Soares, K.C.; Jolissaint, J.S.; Tsilimigras, D.I.; Buettner, S.; Alexandrescu, S.; Marques, H.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; et al. Comparison of Hepatic Arterial Infusion Pump Chemotherapy vs Resection for Patients With Multifocal Intrahepatic Cholangiocarcinoma. JAMA Surg. 2022, 157, 590–596. [Google Scholar] [CrossRef]
- Bartsch, F.; Heuft, L.K.; Baumgart, J.; Hoppe-Lotichius, M.; Margies, R.; Gerber, T.S.; Foerster, F.; Weinmann, A.; Straub, B.K.; Mittler, J.; et al. Influence of Lymphangio (L), Vascular (V), and Perineural (Pn) Invasion on Recurrence and Survival of Resected Intrahepatic Cholangiocarcinoma. J. Clin. Med. 2021, 10, 2426. [Google Scholar] [CrossRef]
- Angelico, R.; Sensi, B.; Parente, A.; Siragusa, L.; Gazia, C.; Tisone, G.; Manzia, T.M. Vascular Involvements in Cholangiocarcinoma: Tips and Tricks. Cancers 2021, 13, 3735. [Google Scholar] [CrossRef]
- Luo, S.; Wu, L.; Li, M.; Wang, J.; Wang, C.; Yang, J.; Zhang, L.; Ge, J.; Sun, C.; Li, E.; et al. Validation of the Prognostic Role for Surgical Treatment in Stage II Intrahepatic Cholangiocarcinoma: A SEER Population-Based Study. J. Clin. Med. 2023, 12, 675. [Google Scholar] [CrossRef]
- Fábrega-Foster, K.; Ghasabeh, M.A.; Pawlik, T.M.; Kamel, I.R. Multimodality imaging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, A.; Brandi, G.; Greco, F.; Prosperi, E.; Palloni, A.; Serenari, M.; Frega, G.; Ravaioli, M.; Rizzo, A.; Cescon, M. Can repeated surgical resection offer a chance of cure for recurrent cholangiocarcinoma? Langenbecks Arch. Surg. 2023, 408, 102. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, F.; Eberhard, J.; Rückert, F.; Schmelzle, M.; Lehwald-Tywuschik, N.; Fichtner-Feigl, S.; Gaedcke, J.; Oldhafer, K.J.; Oldhafer, F.; Diener, M.; et al. Repeated resection for recurrent intrahepatic cholangiocarcinoma: A retrospective German multicentre study. Liver Int. 2021, 41, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Si, A.; Li, J.; Yang, Z.; Xia, Y.; Yang, T.; Lei, Z.; Cheng, Z.; Pawlik, T.M.; Lau, W.Y.; Shen, F. Impact of Anatomical Versus Non-anatomical Liver Resection on Short- and Long-Term Outcomes for Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 1841–1850. [Google Scholar] [CrossRef]
- Wang, C.; Ciren, P.; Danzeng, A.; Li, Y.; Zeng, C.L.; Zhang, Z.W.; Huang, Z.Y.; Chen, Y.F.; Zhang, W.G.; Zhang, B.X.; et al. Anatomical Resection Improved the Outcome of Intrahepatic Cholangiocarcinoma: A Propensity Score Matching Analysis of a Retrospective Cohort. J. Oncol. 2022, 25, 2022. [Google Scholar] [CrossRef]
- Li, B.; Song, J.L.; Aierken, Y.; Chen, Y.; Zheng, J.L.; Yang, J.Y. Nonanatomic resection is not inferior to anatomic resection for primary intrahepatic cholangiocarcinoma: A propensity score analysis. Sci. Rep. 2018, 8, 17799. [Google Scholar] [CrossRef]
- Sposito, C.; Droz Dit Busset, M.; Virdis, M.; Citterio, D.; Flores, M.; Bongini, M.; Niger, M.; Mazzaferro, V. The role of lymphadenectomy in the surgical treatment of intrahepatic cholangiocarcinoma: A review. Eur. J. Surg. Oncol. 2022, 48, 150–159. [Google Scholar] [CrossRef]
- Navarro, J.G.; Lee, J.H.; Kang, I.; Rho, S.Y.; Choi, G.H.; Han, D.H.; Kim, K.S.; Choi, J.S. Prognostic significance of and risk prediction model for lymph node metastasis in resectable intrahepatic cholangiocarcinoma: Do all require lymph node dissection? HPB 2020, 22, 1411–1419. [Google Scholar] [CrossRef]
- Huang, T.; Liu, H.; Lin, Z.; Kong, J.; Lin, K.; Lin, Z.; Chen, Y.; Lin, Q.; Zhou, W.; Li, J.; et al. Preoperative prediction of intrahepatic cholangiocarcinoma lymph node metastasis by means of machine learning: A multicenter study in China. BMC Cancer 2022, 22, 931. [Google Scholar] [CrossRef]
- Benson III, A.B.; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Saenz, D.A.; Are, C.; Brown, D.B.; Chang, D.T.; Covey, A.M.; et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 563–573. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, D.H.; Choi, G.H.; Choi, J.S.; Kim, K.S. Oncologic Impact of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma: A Propensity Score-Matched Study. J. Gastrointest. Surg. 2019, 23, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Xue, F.; Dong, D.H.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann. Surg. 2021, 274, e1187–e1195. [Google Scholar] [CrossRef]
- Moazzam, Z.; Alaimo, L.; Endo, Y.; Lima, H.A..; Pawlik, T.M. Predictors, Patterns, and Impact of Adequate Lymphadenectomy in Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2023, 9, 1966–1977. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, C.; Li, H.; Ren, H.; Cai, Y.; Lan, T.; Wu, H. Adequate lymph node dissection is essential for accurate nodal staging in intrahepatic cholangiocarcinoma: A population-based study. Cancer Med. 2023, 12, 8184–8198. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, D.; Li, W.; Tan, W.; Zhu, S.; Chen, X.; Min, J.; Shang, C.; Chen, Y. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB 2019, 21, 784–792. [Google Scholar] [CrossRef]
- Yoh, T.; Cauchy, F.; Le Roy, B.; Seo, S.; Taura, K.; Hobeika, C.; Dokmak, S.; Farges, O.; Gelli, M.; Sa Cunha, A.; et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery 2019, 166, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Sposito, C.; Ratti, F.; Cucchetti, A.; Ardito, F.; Ruzzenente, A.; Di Sandro, S.; Maspero, M.; Ercolani, G.; Di Benedetto, F.; Guglielmi, A.; et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J. Hepatol. 2023, 78, 356–363. [Google Scholar] [CrossRef]
- Kang, C.M.; Suh, K.S.; Yi, N.J.; Hong, T.H.; Park, S.J.; Ahn, K.S.; Hayashi, H.; Choi, S.B.; Jeong, C.Y.; Takahara, T.; et al. Should Lymph Nodes Be Retrieved in Patients with Intrahepatic Cholangiocarcinoma? A Collaborative Korea-Japan Study. Cancers 2021, 13, 445. [Google Scholar] [CrossRef]
- Endo, Y.; Moazzam, Z.; Lima, H.A.; Alaimo, L.; Munir, M.M.; Shaikh, C.F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; et al. The impact of tumor location on the value of lymphadenectomy for intrahepatic cholangiocarcinoma. HPB 2023, 25, 25650–25658. [Google Scholar] [CrossRef]
- Vitale, A.; Moustafa, M.; Spolverato, G.; Gani, F.; Cillo, U.; Pawlik, T.M. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2016, 113, 685–691. [Google Scholar] [CrossRef]
- Bagante, F.; Spolverato, G.; Weiss, M.; Alexandrescu, S.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; Shen, F.; et al. Surgical Management of Intrahepatic Cholangiocarcinoma in Patients with Cirrhosis: Impact of Lymphadenectomy on Peri-Operative Outcomes. World J. Surg. 2018, 42, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Tokidis, E.; Basourakos, S.P.; Ntzani, E.E.; Lianos, G.D.; Pentheroudakis, G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur. J. Surg. Oncol. 2017, 43, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Serenari, M.; Ratti, F.; Guglielmo, N.; Zanello, M.; Mocchegiani, F.; Lenzi, J.; Colledan, M.; Mazzaferro, V.; Cillo, U.; Ferrero, A.; et al. Evolution of minimally invasive techniques and surgical outcomes of ALPPS in Italy: A comprehensive trend analysis over 10 years from a national prospective registry. Surg. Endosc. 2023, 37, 5285–5294. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moustafa, M.; Linecker, M.; Lurje, G.; Capobianco, I.; Baumgart, J.; Ratti, F.; Rauchfuss, F.; Balci, D.; Fernandes, E.; et al. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-center Study. Ann. Surg. Oncol. 2020, 27, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Czigany, Z.; Lurje, I.; Strnad, P.; Bruners, P.; Ulmer, T.F.; den Dulk, M.; Lurje, G.; Neumann, U.P. The role of ALPPS in intrahepatic cholangiocarcinoma. Langenbecks Arch. Surg. 2019, 404, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidis, P.; Marangoni, G.; Ahmad, J.; Azoulay, D. Simultaneous portal and hepatic vein embolization is better than portal embolization or ALPPS for hypertrophy of future liver remnant before major hepatectomy: A systematic review and network meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 221–227. [Google Scholar] [CrossRef]
- Marino, R.; Ratti, F.; Della Corte, A.; Santangelo, D.; Clocchiatti, L.; Canevari, C.; Magnani, P.; Pedica, F.; Casadei-Gardini, A.; De Cobelli, F.; et al. Comparing Liver Venous Deprivation and Portal Vein Embolization for Perihilar Cholangiocarcinoma: Is It Time to Shift the Focus to Hepatic Functional Reserve Rather than Hypertrophy? Cancers 2023, 15, 4363. [Google Scholar] [CrossRef]
- O’Grady, J.G.; Polson, R.J.; Rolles, K.; Calne, R.Y.; Williams, R. Liver Transplantation for Malignant Disease Results in 93 Consecutive Patients. Ann. Surg. 1988, 207, 373–379. [Google Scholar] [CrossRef]
- Quaresima, S.; Melandro, F.; Giovanardi, F.; Shah, K.; De Peppo, V.; Mennini, G.; Ghinolfi, D.; Limkemann, A.; Pawlik, T.M.; Lai, Q. New Insights in the Setting of Transplant Oncology. Medicina 2023, 59, 568. [Google Scholar] [CrossRef]
- Sapisochín, G.; Fernández de Sevilla, E.; Echeverri, J.; Charco, R. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: Should liver transplantation be reconsidered in these patients? Am. J. Transplant. 2014, 14, 660–667. [Google Scholar] [CrossRef]
- Facciuto, M.E.; Singh, M.K.; Lubezky, N.; Selim, M.A.; Robinson, D.; Kim-Schluger, L.; Florman, S.; Ward, S.C.; Thung, S.N.; Fiel, M.; et al. Tumors with intrahepatic bile duct differentiation in cirrhosis: Implications on outcomes after liver transplantation. Transplantation 2015, 99, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Croome, K.P.; Musto, K.R.; Melendez, J.; Tranesh, G.; Nakhleh, R.; Taner, C.B.; Nguyen, J.H.; Patel, T.; Harnois, D.M. Liver transplantation for intrahepatic cholangiocarcinoma. Liver Transpl. 2018, 24, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, K.E.; Javle, M.; Heyne, K.; Shroff, R.T.; Abdel-Wahab, R.; Gupta, N.; Mobley, C.M.; Saharia, A.; Victor, D.W.; Nguyen, D.T.; et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: A prospective case-series. Lancet Gastroenterol. Hepatol. 2018, 3, 337–348. [Google Scholar]
- Gruttadauria, S.; Barbara, M.; Liotta, R. Liver transplantation for unresectable intrahepatic cholangiocarcinoma: An Italian experience. Updates Surg. 2021, 73, 1587–1588. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Song, W.; Zhang, Y.; Yu, J.; Lv, Y.; Liu, K. Liver transplantation for intrahepatic cholangiocarcinoma: A propensity score-matched analysis. Sci. Rep. 2023, 13, 10630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, F.W.; Jiang, K.Y.; Yang, J.; Xie, Q.Y.; Gong, J.; Yang, M.Y.; Mao, T.Y.; Lei, Z.H. Laparoscopic or open liver resection for intrahepatic cholangiocarcinoma: A meta-analysis and systematic review. Front. Oncol. 2023, 13, 1096714. [Google Scholar] [CrossRef] [PubMed]
- Pery, R.; Gudmundsdottir, H.; Nagorney, D.M.; Pencovich, N.; Smoot, R.L.; Thiels, C.A.; Truty, M.J.; Vierkant, R.A.; Warner, S.G.; Kendrick, M.L. Laparoscopic versus open liver resections for intrahepatic cholangiocarcinoma and gallbladder cancer: The Mayo clinic experience. HPB 2022, 25, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Chen, X.; Ma, D.; Du, G.; Xia, T.; Jiang, Z.; Jin, B. Laparoscopic versus open hepatectomy for intrahepatic cholangiocarcinoma in patients aged 60 and older: A retrospective cohort study. World J. Surg. Oncol. 2022, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, D.; Du, G.; An, B.; Xia, T.; Zhou, T.; Sun, Q.; Liu, F.; Wang, Y.; Sui, D.; et al. Laparoscopic vs. open anatomical hepatectomy for intrahepatic cholangiocarcinoma: A retrospective cohort study. Front. Surg. 2022, 9, 1003948. [Google Scholar] [CrossRef] [PubMed]
- Aliseda, D.; Sapisochin, G.; Martí-Cruchaga, P.; Zozaya, G.; Blanco, N.; Goh, B.K.P.; Rotellar, F. Association of Laparoscopic Surgery with Improved Perioperative and Survival Outcomes in Patients with Resectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis from Propensity-Score Matched Studies. Ann. Surg. Oncol. 2023, 30, 4888–4901. [Google Scholar] [CrossRef]
- Brustia, R.; Laurent, A.; Goumard, C.; Langella, S.; Cherqui, D.; Kawai, T.; Soubrane, O.; Cauchy, F.; Farges, O.; Menahem, B.; et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: Report of an international multicenter cohort study with propensity score matching. Surgery 2022, 171, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Ratti, F.; Casadei-Gardini, A.; Cipriani, F.; Fiorentini, G.; Pedica, F.; Burgio, V.; Cascinu, S.; Aldrighetti, L. Laparoscopic Surgery for Intrahepatic Cholangiocarcinoma: A Focus on Oncological Outcomes. J. Clin. Med. 2021, 10, 2828. [Google Scholar] [CrossRef] [PubMed]
- Magistri, P.; Assirati, G.; Ballarin, R.; Di Sandro, S.; Di Benedetto, F. Major robotic hepatectomies: Technical considerations. Updates Surg. 2021, 73, 989–997. [Google Scholar] [CrossRef]
- Hamad, A.; Ansari, A.; Li, Y.; Shen, C.; Cloyd, J.; Pawlik, T.M.; Ejaz, A. Short- and long-term outcomes following robotic and open resection for intrahepatic cholangiocarcinoma: A national cohort study. Surg. Oncol. 2022, 43, 101790. [Google Scholar] [CrossRef] [PubMed]
- Ratti, F.; Cipriani, F.; Ingallinella, S.; Tudisco, A.; Catena, M.; Aldrighetti, L. Robotic Approach for Lymphadenectomy in Biliary Tumours: The Missing Ring Between the Benefits of Laparoscopic and Reproducibility of Open Approach? Ann. Surg. 2022, 278, e780–e788. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, X. Efficacy and safety comparison of neoadjuvant chemotherapy followed by surgery and upfront surgery for treating intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2023, 23, 122. [Google Scholar] [CrossRef]
- Riby, D.; Mazzotta, A.D.; Bergeat, D.; Verdure, L.; Sulpice, L.; Bourien, H.; Lièvre, A.; Rolland, Y.; Garin, E.; Boudjema, K.; et al. Downstaging with radioembolization or chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 3729–3737. [Google Scholar] [CrossRef]
- Schartz, D.A.; Porter, M.; Schartz, E.; Kallas, J.; Gupta, A.; Butani, D.; Cantos, A. Transarterial Yttrium-90 Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2022, 33, 679–686. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Becht, R.; Wasilewicz, M.P. New Options for Systemic Therapies in Intrahepatic Cholangiocarcinoma (iCCA). Medicina 2023, 59, 1174. [Google Scholar] [CrossRef] [PubMed]
| Author, Years, (Ref) | Country | N. of Patients | Outcome |
|---|---|---|---|
| Sapisochin et al., 2014, [60] | Spain | 8 (cirrhotic liver with lesion <2 cm) 21 (cirrhotic liver with lesion >2 cm or multiple) | 1-year OS: 93%; 3-year OS: 84%; 5-year OS 65% 5-year OS: 34% |
| Facciuto et al., 2015 [61] | USA | 7 (cirrhotic liver) | 1-year OS: 71%; 5-year OS: 57% |
| Lee et al., 2018 [62] | USA | 12 (cirrhotic liver with lesion <2 cm) | 1- and 5-year OS: 63.6% |
| Lunsford et al., 2018, [63] | USA | 9 (patients previously treated with chemotherapy) | 1-year OS: 100%; 3-year OS: 83.3%, 5-year OS: 83.3% |
| Gruttadauria et al., 2021, [64] | Italy | 2 (patients previously treated with SIRT) | Alive after 19 and 2 months of follow-up |
| Huang et al., 2023 [65] | China | 62 (cirrhotic liver or locally advanced disease treated with chemotherapy) | 1-year OS: 82.4%; 3-year OS: 60.3%; 5-year OS: 52.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melandro, F.; Ghinolfi, D.; Gallo, G.; Quaresima, S.; Nasto, R.A.; Rossi, M.; Mennini, G.; Lai, Q. New Insights into Surgical Management of Intrahepatic Cholangiocarcinoma in the Era of “Transplant Oncology”. Gastroenterol. Insights 2023, 14, 406-419. https://doi.org/10.3390/gastroent14030030
Melandro F, Ghinolfi D, Gallo G, Quaresima S, Nasto RA, Rossi M, Mennini G, Lai Q. New Insights into Surgical Management of Intrahepatic Cholangiocarcinoma in the Era of “Transplant Oncology”. Gastroenterology Insights. 2023; 14(3):406-419. https://doi.org/10.3390/gastroent14030030
Chicago/Turabian StyleMelandro, Fabio, Davide Ghinolfi, Gaetano Gallo, Silvia Quaresima, Riccardo Aurelio Nasto, Massimo Rossi, Gianluca Mennini, and Quirino Lai. 2023. "New Insights into Surgical Management of Intrahepatic Cholangiocarcinoma in the Era of “Transplant Oncology”" Gastroenterology Insights 14, no. 3: 406-419. https://doi.org/10.3390/gastroent14030030
APA StyleMelandro, F., Ghinolfi, D., Gallo, G., Quaresima, S., Nasto, R. A., Rossi, M., Mennini, G., & Lai, Q. (2023). New Insights into Surgical Management of Intrahepatic Cholangiocarcinoma in the Era of “Transplant Oncology”. Gastroenterology Insights, 14(3), 406-419. https://doi.org/10.3390/gastroent14030030







