Abstract
The overall prevalence of hyponatremia in cirrhotics is around 50%. Hypovolemic hyponatremia is a result of excessive fluid loss caused mostly by diuretic treatment or diarrhea. More common is hypervolemic hyponatremia, which results from excessive activation of water and sodium-retaining mechanisms caused by effective arterial hypovolemia. This review focuses on the associations of hyponatremia with clinical outcomes and reviews the available data on its management. Hyponatremia is a strong predictor of mortality and is also associated with an increased probability of hepatorenal syndrome, disturbance of consciousness, infections, and unfavorable post-transplant outcomes. In the management of hyponatremia, it is crucial to distinguish between hypovolemic and hypervolemic hyponatremia. The treatment of hypervolemic hyponatremia should be started only in symptomatic patients. The cessation of the treatment with traditional diuretics and fluid restriction may prevent further decrease in natremia. Pharmacological treatment is directed towards cirrhosis itself, precipitating factor, or hyponatremia directly. Currently, only albumin infusions can be recommended routinely. Other possibilities, such as vaptans, splanchnic vasoconstrictors, niravoline, or osmotic diuretics, are restricted to specific use cases (e.g., imminent liver transplantation) or need more research to determine their efficacy. We tried to summarize the management of hyponatremia into a concise flowchart.
1. Introduction
The prevalence of hyponatremia among patients with liver cirrhosis ranges from 40% in outpatients to 57% in hospitalized patients [1]. Serum sodium less than 130 mmol/L occurs in 21.6% of patients. Serum sodium levels are independent of age, sex, or etiology of cirrhosis [1]. These data suggest that hyponatremia is a common complication in these patients.
There is some debate over the cut-off value of sodium used to define hyponatremia in cirrhotics. Traditionally, a cut-off of 130 mmol/L has been used [2]. It has been proven, however, that even a mild decrease in serum sodium (130–134 mmol/L) carries a worse prognosis, compared to patients with sodium in the normal range [1]. The lower limit of the normal range is used as a definition of hyponatremia in some more recent papers [3] and other medical specialties [4,5]. In the latest EASL guidelines on the management of ascites, the definition still has not been consolidated, but serum sodium levels less than 135 mmol/L should be considered [6]. There is a need for the consolidation of this discrepancy to avoid confusion and allow easier comparison of various studies.
Hyponatremia could be classified according to various viewpoints. Based on volume status hyponatremia could be hypovolemic, euvolemic and hypervolemic. Similarly, we can classify hyponatremia based on serum osmolality and antidiuretic hormone (ADH) levels [7]. Pseudohyponatremia could be also a cause of low sodium concentration in the biochemistry panel in patients with acute or chronic liver failure [8].
Hypovolemic hyponatremia in the setting of liver cirrhosis is most often the result of inadequately high doses of diuretics, particularly in a patient with a stable course of liver disease. Another possible cause is gastrointestinal fluid loss in severe diarrhea. Patients show signs of dehydration and in extreme cases even prerenal kidney failure [9].
Euvolemic hyponatremia is the most common cause of hyponatremia in the general population. Causes of euvolemic hyponatremia include (i) syndrome of inadequate antidiuretic hormone secretion (SIADH), (ii) low dietary sodium intake, (iii) and primary polydipsia, all of which are possible in compensated cirrhosis, especially with strict low sodium diet recommendations [7].
Pseudohyponatremia should be taken into account, especially in acute events in liver cirrhosis, such as acute-on-chronic liver failure (ACLF). This type of hyponatremia usually occurs in the presence of large organic molecules such as triglycerides or proteins, which effectively decrease the amount of plasma water fraction (93% in healthy people) to as low as 80%. Actual plasma sodium concentration and osmolality are unchanged; however, measured sodium concentration in total plasma volume is lower due to the lower amount of plasma water in the specimen [7]. This situation could occur in Zieve’s syndrome, where the excessive delipidation markedly increases serum triglycerides [10].
Fluid retention, which is one of the hallmarks of decompensated cirrhosis, could cause hypervolemic (dilutional) hyponatremia. This type of hyponatremia is typical in cirrhosis [9]. Detailed discussion about the pathogenesis of this type of hyponatremia is beyond the scope of this paper, but it is briefly summarized in Figure 1. It may occur either as a result of an identifiable trigger (precipitating factor) or spontaneously because of the progression of the underlying circulatory derangements. Precipitating factor is identified in about 50% of patients with hyponatremia [11]. The most common precipitating factor is infection, gastrointestinal hemorrhage, or active alcoholism [12].
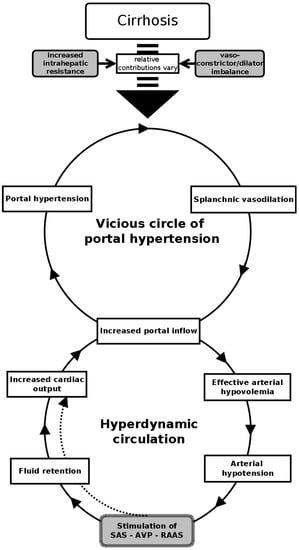
Figure 1.
Schematic representation of pathophysiology of the hypervolemic hyponatremia in cirrhotic patients. (SAS—sympathoadrenal system, AVP—arginine–vasopressine, RAAS—renin–angiotensin–aldosterone system).
This systematic review addresses the clinical significance of this hypervolemic hyponatremia, particularly as a predictor of complications and mortality of cirrhotic patients. Treatment strategies focused on the correction of dilutional hyponatremia are be discussed.
2. Search Strategy
We performed the following search in the Medline database: “((sodium[Title]) OR hyponatremia[Title]) AND (cirrhosis[Title])” in English or Slovak languages. The search was performed on 9 July 2023. The primary search identified 327 publications in the Medline database. References of found publications were also searched for any other relevant publications (n = 145). Relevant publications were screened by title and/or abstract. Historical unavailable publications (n = 40) and off-topic publications along with case reports (n = 68) were excluded. The remaining 364 publications were assessed for eligibility based on full text. Publications focused on epidemiology (n = 10) and pathophysiology (n = 160) were excluded. We included 68 publications on the clinical significance of hyponatremia (prognosis and/or quality of life), 94 publications on treatment, and 32 comprehensive reviews (Figure 2).
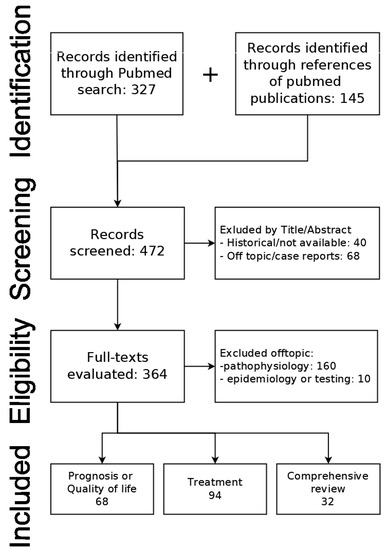
Figure 2.
PRISMA flowchart of source search and selection.
3. Clinical Significance
Hypervolemic hyponatremia can be considered as the marker of hemodynamic derangements in cirrhosis (Figure 1). The development of hyponatremia usually occurs only after the decompensation of cirrhosis, with mean sodium levels in compensated cirrhosis corresponding to the general population [13]. It has been proven in multiple studies that it correlates not only with the hepatic vein pressure gradient (HVPG) [14], the presence of spontaneous splenorenal shunt [15], the presence and the degree of ascites [16], but also with the degree of metabolic liver failure [17]. Sodium levels also directly correlate with altered sympathovagal balance in cirrhosis [18]. The association of hyponatremia and variceal bleeding is rarely mentioned in the literature and no clear association has been reported [19]. Besides that, hyponatremia contributes to cell dysfunction on its own, by causing cellular edema. Cirrhotic patients with hyponatremia have significantly reduced quality of life, mainly due to neurological symptoms, but also due to restricted fluid intake. The presence and risk of adverse clinical outcomes are summarized in Figure 3.
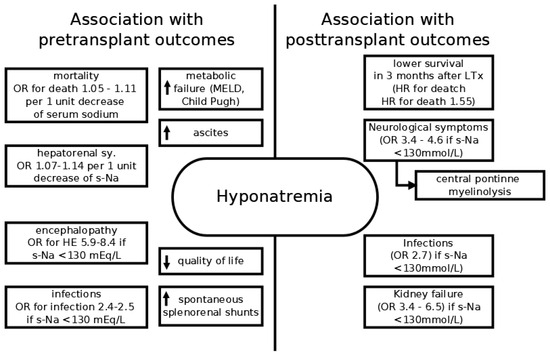
Figure 3.
Schematic representation of adverse clinical outcomes associated with hyponatremia. Arrows denote increased or decreased association with the condition.
3.1. Mortality
It is not surprising that hyponatremia is a strong predictor of mortality in cirrhotics. Multiple studies have reported that patients with hyponatremia have higher mortality, even when controlled for liver function. That is true irrespective of the sodium cut-off (130 mmol/L or 135 mmol/L) used to define hyponatremia. Indeed, there is a strong association between serum sodium and mortality with a reported increase in the likelihood of death at 3 months around 1.05–1.11 per 1 unit decrease in serum sodium [3,16,20,21]. Borroni et al. found that patients with sodium < 130 mmol/L had higher in-hospital mortality rates compared to controls (26.3% versus 8.9%) [22]. In a study by Heuman et al., sodium < 135 mmol/L was an independent predictor of mortality with OR 2.755 (95% CI 1.305–5.814) [23]. Moreover, patients with sodium in the upper half of the reference range have better survival than patients with sodium from 135 to 140 mmol/L [24]. Hyponatremia is an adverse sign even in patients with compensated cirrhosis. Umemura et al. reported, that compensated cirrhotics with sodium < 139 mmol/L had significantly worse cumulative survival than the rest of the cohort [21]. These data were so convincing that they prompted the inclusion of sodium into the MELD itself. In a study by Ruf et al., the inclusion of sodium into the MELD score increased the area under the curve for 3-month mortality from 0.894 to 0.908 [25]. Subsequently, several models, which included serum sodium through different formulas appeared, including MELDNa [26], iMELD [27], and MESO [28]. Although natremia is also an independent predictor of survival in pediatric patients with liver cirrhosis due to biliary atresia, its incorporation into pediatric end-stage liver disease score did not improve prediction accuracy, potentially signifying less impact on survival in children [29].
3.2. Hepatorenal Syndrome
Severe vasoconstriction in the kidney caused by hemodynamic changes in cirrhosis that cause dilutional hyponatremia could also lead to hepatorenal syndrome (HRS). The prevalence of HRS in a study by Angeli et al. was 17% in patients with sodium < 130 mmol/L compared to 6% in patients with sodium > 130 mmol/L [1]. Other studies found lower mean sodium levels in patients with HRS compared to patients without HRS [30,31]. Low levels of serum sodium also predict the development of HRS in patients with normal kidney function. Indeed, in studies published by Gines et al. (no OR given) [32], Ahn et al. (HR 0.93, 95% CI 0.87–0.99) [33], and our group (OR 0.87, 95% CI 0.78–0.97) [34] serum sodium was found to be a significant and independent predictor of HRS in patients with yet normal kidney function.
3.3. Neurological Symptoms
Liver failure is accompanied by the disturbance of neurological functions for various reasons. Recent pathophysiological theory of hepatic encephalopathy (HE) identifies low-grade cerebral edema, caused by the synergic effect of intracellular ammonium, glutamine, and other neurotoxins in the setting of stimulated chronic inflammatory milieu, as a direct cause of HE. In this case, hyponatremia may worsen the astrocyte swelling by changes in plasma osmolality. Clinical data confirms hyponatremia as an independent predictor of HE. In a study by Guevara et al. hyponatremia (s-Na < 130 mmol/L) had an adjusted hazard ratio of 8.36 for the development of HE, although the study included only 61 patients with multiple episodes and high incidence of HE [35]. Somewhat lower OR (5.891; 95% CI 1.49–23.3) of severe (grade III and above) HE was reported in a study by Kim et al. [17]. Gines et al. reported a significantly higher probability of HE in cirrhotics treated with diuretics who also had hyponatremia (s-Na < 135 mmol/L) [9]. The largest study to date from Denmark included 1116 patients out of which 302 developed overt HE. Authors report that for every 1 mmol/L decrease in natremia, the hazard of HE increased by 8%. The 1-year cumulative risk of HE in patients with natremia < 130 mmol/L was 57% compared to only 6% in patients with serum sodium ≥ 145 mmol/L [36]. Several studies evaluated cognitive disability resulting from the combination of HE and hyponatremia. In a study by Wunsch et al., s-Na showed linear association (regression coefficient 0.059, 95% CI 0.024–0.093) with physical, but not a mental component of the SF-36 questionnaire, that was independent of HE [37]. Ahluwalia et al. evaluated cognitive functions in addition to the quality of life. Interestingly, the cognitive abilities of patients with hyponatremia were superior to patients with HE; however, health-related quality of life was lower in these patients [38]. Improvement of hyponatremia may improve cognitive abilities even in patients without over-HE. As evident from the post-hoc analysis of two double-blind, randomized, parallel-group studies comparing satavaptan with placebo, patients who had an improvement of hyponatremia had significantly better performance on more difficult B variant of the Trail-making test (improvement of about 12% in patients with improved natremia vs. 4% in patients without natremia improvement [39].
3.4. Infections
Infections are diagnosed in up to 50% of patients with liver cirrhosis [40], and significantly (almost 4-fold) increase the mortality of cirrhotic patients [41]. Observations by Kim et al. confirm the frequent presence of hyponatremia in cirrhotics with infections (OR, 2.562; CI, 1.162–5.653) [17]. Besides spontaneous bacterial peritonitis, the presence of hyponatremia is associated also with soft tissue and skin infections. In a study by Pereira et al., patients with this type of infection had significantly lower mean values of s-Na (132 ± 6 vs. 135 ± 6) and the prevalence of s-Na < 130 mmol/L was higher in the infected patients (40% vs. 25%) [42]. Lower sodium was also associated with higher mortality in infected patients and a higher risk of subsequent kidney injury [43]. Hyponatremia not only accompanies infections but also is an additional risk factor for the development of infections in cirrhotics. In a multicentric study by Angeli et al. patients with s-Na < 130 mmol/L had OR of 2.36 (95% CI 1.41–3.93) for the development of spontaneous bacterial peritonitis during the following four weeks [1].
3.5. Posttransplant Outcomes
The success of the liver transplantation (LTx) depends in part on the pre-transplant status of the patient. Serum sodium could provide significant information about the risk of post-transplant complications. Quick correction of hyponatremia after the LTx could lead to central pontine myelinolysis. In one series of 379 adult LTx, 12 patients underwent transplantation in hyponatremic condition (s-Na < 127 mmol/L). Six patients developed neurological complications in the early postoperative period, out of which three had central pontine myelinolysis. Confusion was present in two and one patient suffered a seizure [44]. Rapid change in s-Na after LTx was an independent and significant predictor of neurological complications in a study by Lee et al. [45]. Adverse influence of hyponatremia can be followed up to 3 months post LTx. Two studies have documented worse survival, higher rates of neurologic complications (OR 3.4–4.6), and renal failure (OR 3.4–6.5) one month after LTx in patients with s-Na < 130 mmol/L [46,47]. A study by Londono et al. also showed higher odds of infections in patients with s-Na < 130 mmol/L (OR 2.7) [47]. Dawwas et al. followed patients after LTx for three years. Although the mortality of hyponatremic patients was higher even at the end of follow-up, further analysis showed that this excess mortality was limited to the early (3 months) post-transplant period (HR for death 1.55; 95% CI, 1.18–2.04). No significant difference in mortality in the remainder of the follow-up (HR 1.05, 95% CI 0.78–1.42) was found [48]. One of the largest studies that included 2733 patients undergoing LTx for cirrhosis was published in 2016. Authors demonstrated that patients with severe hyponatremia (<125 mmol/L) and hypernatremia (≥ 150 mmol/L) had the worst posttransplant outcomes, however only hypernatremia was associated with poor survival in multivariate Cox regression [49].
4. Management of Hyponatremia in Cirrhosis
The choice of treatment method depends on two principal factors. The first is the pathophysiology of hyponatremia and the second is the rate of sodium decline. A different approach is needed for acute hypovolemic hyponatremia caused by excessive diuretic treatment and different for chronic hypervolemic (dilutional) hyponatremia in patients with sodium and fluid retention. In the first case the immediate rehydration with the replenishment of body sodium by normal saline infusion is required, along with the cessation of diuretics [7]. Hypervolemic hyponatremia in cirrhosis is more a result than a cause of hemodynamic changes in cirrhosis. Therefore, the treatment should be aimed at the cause of liver disease (e.g., hepatitis B treatment improves portal hypertension [50]). The secondary option is the symptomatic treatment, which should be focused at the amelioration of splanchnic vasodilation and the increase in the solute-free water excretion together with the preservation of body sodium [9]. We have currently no good data that this approach has any beneficial effect on hard outcomes, such as clinical complications or mortality [51,52].
The initiation of the treatment should follow the development of symptoms. Although hyponatremia significantly lowers the quality of life of cirrhotic patients [37], many of the patients are asymptomatic or present with very mild symptoms. No specific s-Na cut-off value for the start of the treatment has been defined and both major guidelines (AASLD and EASL) recommend treatment only after the development of symptoms [53,54]. The rate of chronic hyponatremia correction should never exceed 12 mmol/L in 24 h because of the risk of central pontine myelinolysis [55].
The remainder of this section focuses on the management of dilutional hyponatremia in cirrhosis. Real-life analysis of treatment practices from a US registry with data accumulated in 2010 through 2013 shows that treatment of hypervolemic hyponatremia of cirrhosis still proves difficult in real-life practice and the adherence to guidelines is low. Majority of patients received normal saline (36%), fluid restriction, which should be the first line choice, was initiated in 33% of patients, followed by no specific therapy (20%), tolvaptan (5%), and hypertonic saline (2%). First-line treatment choice was associated with the degree of hyponatremia (highest in no specific therapy group, followed by fluid restriction and normal saline group. The lowest natremia was observed in the hypertonic saline and tolvaptan group [56].
4.1. Diet and Lifestyle Changes
Fluid restriction to 1000 mL/day is currently the first-line treatment for hypervolemic hyponatremia. Despite relatively low efficacy, it is considered a standard of care in current EASL and AASLD guidelines [53,54]. Although no placebo-controlled studies are possible, some efficacy data come from the multiple Vaptans trials. In the study by Gines et al. 1.5 L/24 h fluid restriction regimen increased the s-Na by 1.3 ± 4.2 mmol/L at day 5 and the improvement of s-Na of more than 5 mmol/L occurred in 18% of patients [57]. Even more strict fluid restriction (1.0 L/24 h) did not result in an increase in s-Na in a control arm of the study for lixivaptan [58]. Fluid restriction prevented further decrease in s-Na in both studies. In the registry data reported by Sigal et al., fluid restriction led to an increase in s-Na ≥ 5 mmol/L in 39% of patients [56].
Expansion of effective blood volume by physical methods could also have some positive effects on hyponatremia. Patients who were treated by head-out water immersion showed a significant decrease in AVP levels after acute water load (20 mL/kg) compared to the control group without water immersion. These lower levels of AVP were also preserved after the water load had been excreted. Unfortunately, sodium levels were not reported in this study [59]. A similar effect could theoretically be achieved by horizontal position or passive leg raising [60]. The effect of these interventions on s-Na directly has not been described in the available literature.
4.2. Transjugular Intrahepatic Portosystemic Shunt (TIPS)
Hypervolemic hyponatremia in cirrhosis is usually accompanied by severe ascites. The ascites are refractory in about 10% from the onset and become diuretic-resistant in a further 20% of patients during the course of the disease [61]. The insertion of TIPS is a valid treatment option in these patients [62] and it could also improve hyponatremia. Multiple studies documented the increase in serum sodium approximately 30 days after the insertion of TIPS [63,64,65,66]; however, some studies did not [67,68], and no clear recommendation can be given.
4.3. Pharmacological Therapy
Symptomatic pharmacological therapy could either directly antagonize the effect of AVP or attempt to increase effective arterial blood volume by ameliorating vasodilation (inhibition of NO synthase), inducing vasoconstriction, or plasma expansion by albumin.
The first step should be the evaluation of medical treatment the patient is currently taking. Known medications that decrease s-Na concentration include traditional diuretics (furosemide, spironolactone, thiazides), vasopressin analogs, and potentially non-selective beta-blockers (NSBB). According to older EASL guidelines, traditional diuretics should be discontinued if there is severe hyponatremia (serum sodium concentration < 120 mmol/L) (Level B1) [54]; however, this recommendation is no longer present in the current guideline [6].
Several reports about non-selective beta-blockers and carvedilol leading to worsening of arterial hypovolemia and higher risk of kidney injury caused confusion about their use in patients with severely decompensated cirrhosis and infections [69]. However, current reports, reflected also in the most recent guidelines, show, that NSBB has the potential to reduce mortality in severely decompensated cirrhotic patients without worsening hyponatremia [53].
Hypertonic saline solution with loop diuretic has been used in clinical practice for the management of SIADH [70]. Its efficacy in cirrhosis is limited and, most of all, short-lived. Furthermore, it worsens ascites and edema, therefore this treatment cannot be recommended universally in the management of hypervolemic hyponatremia [9], but it should be restricted to symptomatic life-threatening hyponatremia (e.g., patients with cardiorespiratory distress or severely disturbed consciousness, or can be considered in severe hyponatremia immediately before liver transplantation [6].
There is mounting evidence about albumin in the treatment of hypervolemic hyponatremia in cirrhosis. Initial case reports were reported in 1990 by McCormic et al. [71], followed by RCT published by Jalan et al. One-week treatment with albumin not only significantly increased s-Na, but also reduced the risk for infections, renal failure, hepatic encephalopathy, and mortality during the treatment period [72]. These results were confirmed in two retrospective observations in rather large cohorts of cirrhotic patients [73,74]. A recent meta-analysis, that included 25 RCTs and five cohort studies confirmed the effect of human albumin on the resolution of hyponatremia (OR = 1.50, 95% CI = 1.17–1.92, p = 0.001) and the prevention of hyponatremia (OR = 0.55, 95% CI = 0.38–0.80, p = 0.001) [75]. The quality of evidence in the meta-analysis was unfortunately low. The largest randomized controlled study focused on the efficacy of long-term albumin administration in decompensated cirrhosis was the ANSWER study, which randomized 440 patients into the standard-of-care plus albumin versus standard-of-care only groups. Improvement of hyponatremia was one of the secondary outcomes. Hyponatremia normalization was observed in 45% of the albumin group patients versus 28% of the standard medical therapy-only group, p = 0.042, regular albumin infusions also prevented the development of hyponatremia in previously normonatremic patients (incidence risk ratio 0.539 [CI 0.338–0.859, p = 0.008]) during the 18-month duration of the study. No follow-up after treatment conclusion was reported [76]. The persistence of this effect after the discontinuation of the albumin seems unlikely. Therefore, this treatment should be considered in the short term for patients awaiting transplantation.
Another interesting notion is the induction of free water excretion by osmotic diuresis. One small study evaluated the effect of urea on ascites and hyponatremia and found that the administration of 30–90 g of urea daily significantly increased diuresis, s-Na level, and sodium output. The average diuresis increased from 1.05 ± 0.10 to 2.24 ± 0.24 L/day, the change being so big that one patient suffered from dehydration and subsequent prerenal kidney failure [77]. A similar effect on the diuresis has been described for mannitol infusion as well [78]. Treatment with osmotic diuretics is regarded as outdated and thus frequently overlooked; however, it can be of value in the management of patients with ascites and hyponatremia.
4.3.1. Vasoconstriction Therapy
Terlipressin is a vasoconstrictive drug used often in patients with bleeding esophageal varices. It is an analog of AVP that works predominantly on V1 receptors in blood vessels; however, it also retains some affinity for V2 receptors [79]. Therefore, it has a mixed effect on the s-Na level. As described by Sola et al. terlipressin, but not somatostatin, led to a significant decrease in s-Na in patients with bleeding esophageal varices [80]. This decrease is most apparent in patients with the highest baseline sodium (i.e., patients with low baseline SAS, RAAS, and AVP activation) and is not as evident in patients with baseline hyponatremia [80,81]. On the other hand, the treatment of hepatorenal syndrome with terlipressin in combination with albumin could increase the s-Na [81], although it is difficult to ascertain if it is predominantly the effect of vasoconstrictor or albumin.
Duvoux et al. reported a significant increase in s-Na (123.0 ± 6.0 vs. 131.2 ± 4.1, p = 0.01) after ten days of hepatorenal syndrome treatment with noradrenaline and albumin [82]. The tendency toward s-Na increase was also reported in the study by Tavakkoli et al., who examined the effect of solo noradrenaline compared to midodrine + octreotide on hepatorenal syndrome. Although the rise of s-Na was relatively steep (noradrenaline group 118.72 ± 34.82 to 131.63 ± 5.04 and midodrine + octreotide group 121.35 ± 34.4 to 128.58 ± 21.08), it did not reach statistical significance, probably because the study was not designed for this outcome [83]. Also, a small retrospective observation of 10 patients reported significant increase in s-Na after Midodrine and Octreotide treatment [84]
Midodrine was evaluated in multiple conditions associated with liver cirrhosis, because of its convenient oral formulation, no need for vital functions monitoring, and relatively few side effects. Singh et al. tried midodrine in the management of refractory ascites, but also reported the changes of s-Na. Sodium in the standard medical therapy group decreased significantly (after 1 month and after 3 months as well), however, no significant change was observed in the midodrine + standard medical therapy group [85]. Mid-sized randomized controlled trial explored the effect of midodrine with albumin vs. placebo on the incidence of complications of cirrhosis among patients on the liver transplant waiting list, where hyponatremia was one of them. Although no significant effects on the overall incidence of complications or mortality were found, patients in the active arm had less severe hyponatremia episodes with the lowest s-Na levels 134 ± 3 vs. 129 ± 5 mEq/L in the placebo group, p = 0.04 [86].
Nitric oxide is a major contributor to splanchnic vasodilation in cirrhosis. Therefore, the therapeutic possibilities of Nitric oxide synthase inhibitors have been explored in multiple trials. The results, however, are controversial. It seems that longer infusions (>3 h) administered repeatedly have a positive effect on sodium excretion and renal functions, compared to a single dose [87], but no data on the s-Na or AVP concentrations have been reported.
4.3.2. Antidiuretic Hormone Antagonists
Increased concentrations of AVP may well be the most important mechanism of hypervolemic hyponatremia in cirrhosis. It is not surprising that the antagonists of AVP have been tried in multiple clinical studies. Demeclocycline, a tetracycline derivate, reduces the sensitivity to AVP thus increasing the free water excretion. Clinical usefulness is limited due to frequent nephrotoxicity [88]. Centrally acting κ-opioid receptor agonist Niravoline has the ability to decrease the synthesis and release of AVP. Doses between 0.5–1 mg/day have been shown to significantly increase free water excretion and s-Na concentration in patients with cirrhosis. Higher doses also induced reversible confusion and personality changes [89]. Further development of this drug has been abandoned and it is not clinically available.
Vassopresin V2 receptor blockers (vaptans) have successfully passed clinical testing and have been approved for the treatment of hyponatremia in SIADH. Further testing evaluated their effectiveness in congestive heart failure and cirrhosis. Multiple studies have documented the increase in free water excretion and increase in s-Na following the administration of vaptans.
Based on the efficient increase in s-Na in SALT-1 and SALT-2 trials in predominantly cardiac patients [90], several subsequent studies evaluated the efficacy specifically in hypervolemic hyponatremia associated with liver cirrhosis. A subanalysis of SALT 1 and 2, which contained only cirrhotic patients, was published in 2012 by Cardenas et al. and revealed that tolvaptan caused a mean increase in the area under the curve of s-Na level over the study period by 4.2 ± 3.4 mmol/L compared to 1.2 ± 3.5 mmol/L in the placebo group. Furthermore, the treatment showed the improvement of symptoms measured by the mental component of the SF-36 questionnaire (47.7 vs. 43.3 points in the placebo group). However, after the cessation of the therapy hyponatremia recurred [91]. Okita et al. reported similar results, with additional improvement of the ascites and edema [92]. This finding has been confirmed in another study from Japan that evaluated tolvaptan with concomitant standard diuretic treatment [93]. Tolvaptan remained effective also when administered for more than 50 days [94]. Recent data question the efficacy of tolvaptan, particularly in patients with severe hyponatremia. Real-life observations showed response rate (increase in s-Na > 130 mEq/L) in only 20–22% of patients with baseline hyponatremia ≤ 125 or 130 mmol/L [95,96].
Conivaptan, currently the only FDA-approved short-term parenteral treatment for hypervolemic hyponatremia, unfortunately, does not have available robust clinical data in patients with liver cirrhosis. One RCT with conivaptan included 84 patients with hypervolemic and euvolemic hyponatremia (various causes); however, the proportion of cirrhotics was low (possibly from 1 to 8%) and not precisely reported. Conivaptan significantly increased the s-Na area under the curve over 4 days compared to the placebo. The treatment needed to be discontinued in 4 patients due to serious injection site reactions [97]. The only other available publication is a retrospective observation of 24 cirrhotic patients with hyponatremia evaluated for a liver transplant. Conivaptan raised serum sodium in patients on but also off diuretic treatment [98].
Lixivaptan was the first of the vaptans that was tried in patients with cirrhosis. Two small RCTs with a short treatment period (7 days) demonstrated dose-related free water excretion and an increase in the s-Na during the treatment period [58,99]. A larger BALANCE trial, that included 652 patients, was performed in hypervolemic hyponatremia with congestive heart failure. In the treatment group, the change of s-Na at day 7 was 2.5 mmol/L compared to 1.3 mmol/L in the placebo group [100]. Based on this modest efficacy, the FDA, in 2012, recommended the use of lixivaptan in hypervolemic hyponatremia [101].
Satavaptan has been also evaluated in the setting of cirrhotic hyponatremia in three large studies by Wong et al. Patients were followed for 52 weeks in all three studies. The first study involved patients with uncomplicated ascites that were managed with satavaptan or placebo, both with diuretic treatment. The second and third studies were focused on the refractory ascites that needed repeated large volume paracenteses and patients received satavaptan in combination with diuretics or as a solo treatment. Satavaptan was more effective in the correction of hyponatremia. However, at week 12, no significant difference was observed in the incidence of worsening ascites (study 1) or in the cumulative number of required large-volume paracenteses (studies 2 and 3) between the satavaptan and placebo groups [102].
The primary outcomes of most of the referenced studies were the changes in s-Na or symptoms. A meta-analysis published by Dahl et al. tried to summarize the effect of tolvaptan, satavaptan, and lixivaptan on mortality. Twelve studies that included 2266 patients were included. The meta-analysis confirmed the strong effect of vaptans on hyponatremia and partially also the effect on water retention. However, no effect of the vaptan treatment on mortality (RR = 1.06, 95% CI = 0.90–1.26), hepatic encephalopathy (RR = 0.87, 95% CI = 0.73–1.03), variceal bleeding (RR = 1.04, 95% CI = 0.56–1.92), spontaneous bacterial peritonitis (RR = 0.73, 95% CI = 0.53–1.01) or renal impairment (RR = 1.16, 95% CI = 0.88–1.53) was found [103].
Adverse effects of vaptans consistently reported in all trials are dry mouth and thirst sensations. More detrimental adverse effects also reported with higher frequency than placebo were dehydration, acute prerenal kidney injury, and hypotension [56]. Tolvaptan could also lead to liver injury on its own [104], which led the FDA to limit the duration of the treatment to 30 days and recommend the avoidance of this treatment in patients with liver disease, including cirrhosis [105,106].
Currently, FDA approves only short-term i.v. administration of conivaptan for the treatment of hypervolemic hyponatremia, with special precautions in patients with hepatic impairment. In Europe, only tolvaptan from the vaptan group has been approved by EMEA and only for the treatment of SIADH. The use of vaptans to treat hypervolemic hyponatremia in cirrhosis is limited to clinical trials in Europe (EASL), also there is no clear recommendation in the AASLD guidelines, only acknowledgment, that for short-term (≤30 days) treatment may raise serum sodium level [53,54].
5. Conclusions
Hyponatremia in cirrhosis is, according to the current understanding of pathophysiology, a result of profound hemodynamic changes in advanced cirrhosis. It is not only a marker of poor prognosis, but it also adds detrimental effects by worsening the quality of life and contributing to the disturbance of consciousness in patients with hepatic encephalopathy. Concise visualization of the associations between hyponatremia and clinical outcomes is provided in Figure 3 and the general approach to the management of hyponatremia in cirrhosis is summarized in Figure 4. In previously eunatremic patients, one can try to identify and treat the precipitating factor that has worsened the splanchnic vasodilation, such as infection or alcoholism relapse. In patients without obvious triggers, the development of hyponatremia should warrant the evaluation of a liver transplant. Symptomatic treatment by hypertonic saline with or without loop diuretic is not routinely recommended; however, it can be considered in very serious hyponatremia with serious disturbance of consciousness [9]. Albumin administration increases s-Na; moreover, it reduces the incidence of other major complications such as hepatorenal syndrome or infections, without adverse effects [72]. Albumin infusions are currently recommended by AASLD guidelines in symptomatic hyponatremia with serum sodium < 120 mEq/L [53]. Initial optimism for the routine use of vaptans, direct antagonists of antidiuretic hormone, is, for the most part, gone. Although treatment with vaptans has a very strong effect on the correction of hyponatremia, the effect on symptoms (ascites, edema as well as consciousness and quality of life) is modest and the effect on the mortality or complications of cirrhosis is absent completely.
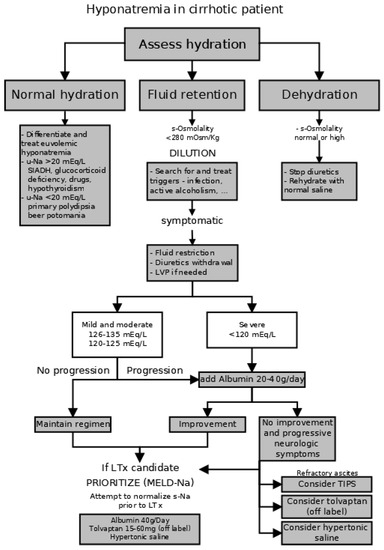
Figure 4.
Proposed algorithm for the management of hyponatremia in cirrhotic patients. (SIADH—syndrome of inadequate antidiuretic hormone secretion, LVP—large-volume paracentesis with plasma expansion, LTx—liver transplant, TIPS—transjugular intrahepatic portosystemic shunt.
Since the definitive correction of hemodynamic derangements in cirrhosis could be only achieved by liver transplantation, no other causal treatment of hypervolemic hyponatremia seems to be feasible. Future research should therefore focus on the optimization of symptomatic treatment—defining the roles of vasoconstrictors (such as noradrenaline, midodrine, or NO synthase inhibitors), albumin, hypertonic saline, osmotic diuretics, and vaptans in the symptomatic management of hyponatremia. There are also contradictions about the effect of TIPS on hyponatremia stemming probably from different TIPS indications in the published studies (variceal bleeding or ascites control), that should be resolved as TIPS is becoming widely available.
It is important to acknowledge several potential limitations in this review. The quality and heterogeneity of the included studies could impact the overall robustness of our findings. Particularly the clinical observations in cirrhosis tended to be mostly single-center studies, commonly from tertiary or referral hospitals with a low number of participants which suffer from selection bias. Also, it is difficult to evaluate any causality in the observed associations with clinical outcomes. All the reported associations were the results of observational clinical studies that cannot possibly allow us to infer the causative contribution of hyponatremia to the clinical outcomes. Most of the interventions have been explored either in observational studies, or limited-size RCTs. More robust RCTs are available only for albumin and vaptans; however, in the case of vaptans, improvement of hyponatremia in cirrhotics was commonly only a secondary outcome.
Moreover, conflicting results among the included studies may lead to uncertainties in the overall conclusions of this review, particularly regarding the optimal approach to the correction of hyponatremia. These limitations underscore the need for cautious interpretation of our findings and emphasize the importance of further research to address these issues and provide more comprehensive insights.
Author Contributions
All authors have significantly contributed to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The preparation of this manuscript required no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Angeli, P.; Wong, F.; Watson, H.; Gines, P. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology 2006, 44, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Gines, P.; Berl, T.; Bernardi, M.; Bichet, D.G.; Hamon, G.; Jimenez, W.; Liard, J.F.; Martin, P.Y.; Schrier, R.W. Hyponatremia in cirrhosis: From pathogenesis to treatment. Hepatology 1998, 28, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Biggins, S.W.; Kremers, W.K.; Wiesner, R.H.; Kamath, P.S.; Benson, J.T.; Edwards, E.; Therneau, T.M. Hyponatremia and mortality among patients on the liver-transplant waiting list. N. Engl. J. Med. 2008, 359, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Steels, P.; Grieten, L.; Nijst, P.; Tang, W.H.; Mullens, W. Hyponatremia in acute decompensated heart failure: Depletion versus dilution. J. Am. Coll. Cardiol. 2015, 65, 480–492. [Google Scholar] [CrossRef]
- Adrogue, H.J.; Madias, N.E. The challenge of hyponatremia. J. Am. Soc. Nephrol. JASN 2012, 23, 1140–1148. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Sahay, M.; Sahay, R. Hyponatremia: A practical approach. Indian. J. Endocrinol. Metab. 2014, 18, 760–771. [Google Scholar] [CrossRef]
- Vo, H.; Gosmanov, A.R.; Garcia-Rosell, M.; Wall, B.M. Pseudohyponatremia in acute liver disease. Am. J. Med. Sci. 2013, 345, 62–64. [Google Scholar] [CrossRef]
- Gines, P.; Guevara, M. Hyponatremia in cirrhosis: Pathogenesis, clinical significance, and management. Hepatology 2008, 48, 1002–1010. [Google Scholar] [CrossRef]
- Melrose, W.D.; Bell, P.A.; Jupe, D.M.; Baikie, M.J. Alcohol-associated haemolysis in Zieve’s syndrome: A clinical and laboratory study of five cases. Clin. Lab. Haematol. 1990, 12, 159–167. [Google Scholar] [CrossRef]
- Porcel, A.; Diaz, F.; Rendon, P.; Macias, M.; Martin-Herrera, L.; Giron-Gonzalez, J.A. Dilutional hyponatremia in patients with cirrhosis and ascites. Arch. Intern. Med. 2002, 162, 323–328. [Google Scholar] [CrossRef]
- Cárdenas, A.; Solà, E.; Rodríguez, E.; Barreto, R.; Graupera, I.; Pavesi, M.; Saliba, F.; Welzel, T.M.; Martinez-Gonzalez, J.; Gustot, T.; et al. Hyponatremia influences the outcome of patients with acute-on-chronic liver failure: An analysis of the CANONIC study. Crit. Care 2014, 18, 700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, D.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.; Paternostro, R.; Schwabl, P.; Scheiner, B.; Stättermayer, A.F.; Pinter, M.; et al. Systemic Inflammation Increases across Distinct Stages of Advanced Chronic Liver Disease and Correlates with Decompensation and Mortality. J. Hepatol. 2021, 74, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Huo, T.I.; Yang, Y.Y.; Hou, M.C.; Lee, P.C.; Lin, H.C.; Lee, F.Y.; Chi, C.W.; Lee, S.D. Correlation and comparison of the model for end-stage liver disease, portal pressure, and serum sodium for outcome prediction in patients with liver cirrhosis. J. Clin. Gastroenterol. 2007, 41, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Kondo, T.; Kiyono, S.; Sekimoto, T.; Takahashi, M.; Okugawa, H.; Yokosuka, O. Relationship and interaction between serum sodium concentration and portal hemodynamics in patients with cirrhosis. J. Gastroenterol. Hepatol. 2015, 30, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Mal, G.; Khalid, S.; Baloch, G.H.; Akbar, Y. Frequency of hyponatraemia and its influence on liver cirrhosis-related complications. J. Pak. Med. Assoc. 2010, 60, 116–120. [Google Scholar]
- Kim, J.H.; Lee, J.S.; Lee, S.H.; Bae, W.K.; Kim, N.H.; Kim, K.A.; Moon, Y.S. The association between the serum sodium level and the severity of complications in liver cirrhosis. Korean J. Intern. Med. 2009, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.; Calvaruso, V.; Casuccio, A.; Pennisi, G.; Licata, M.; Pintus, C.; Basso, M.G.; Velardo, M.; Daidone, M.; Amodio, E.; et al. Heart Rate Variability Is Associated with Disease Severity and Portal Hypertension in Cirrhosis. Hepatol. Commun. 2023, 7, e0050. [Google Scholar] [CrossRef]
- Mathur, S.; Gane, E.J.; McCall, J.L.; Plank, L.D. Serum sodium and hydration status predict transplant-free survival independent of MELD score in patients with cirrhosis. J. Gastroenterol. Hepatol. 2008, 23, 239–243. [Google Scholar] [CrossRef]
- Londono, M.C.; Cardenas, A.; Guevara, M.; Quinto, L.; de Las Heras, D.; Navasa, M.; Rimola, A.; Garcia-Valdecasas, J.C.; Arroyo, V.; Gines, P. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut 2007, 56, 1283–1290. [Google Scholar] [CrossRef]
- Umemura, T.; Shibata, S.; Sekiguchi, T.; Kitabatake, H.; Nozawa, Y.; Okuhara, S.; Kimura, T.; Morita, S.; Komatsu, M.; Matsumoto, A.; et al. Serum sodium concentration is associated with increased risk of mortality in patients with compensated liver cirrhosis. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2015, 45, 739–744. [Google Scholar] [CrossRef]
- Borroni, G.; Maggi, A.; Sangiovanni, A.; Cazzaniga, M.; Salerno, F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2000, 32, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Heuman, D.M.; Abou-Assi, S.G.; Habib, A.; Williams, L.M.; Stravitz, R.T.; Sanyal, A.J.; Fisher, R.A.; Mihas, A.A. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004, 40, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Bernasovská, G.; Hrušovský, S.; Štugelová, M.; Demeš, M.; Smetanová, V.; Žigrai, M. Hyponatremia-predictive mortality factor in hospitalized patients with hepatic cirrhosis. Lek. Obz. 2011, 60, 7–13. [Google Scholar]
- Ruf, A.E.; Kremers, W.K.; Chavez, L.L.; Descalzi, V.I.; Podesta, L.G.; Villamil, F.G. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2005, 11, 336–343. [Google Scholar] [CrossRef]
- Biggins, S.W.; Kim, W.R.; Terrault, N.A.; Saab, S.; Balan, V.; Schiano, T.; Benson, J.; Therneau, T.; Kremers, W.; Wiesner, R.; et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006, 130, 1652–1660. [Google Scholar] [CrossRef]
- Luca, A.; Angermayr, B.; Bertolini, G.; Koenig, F.; Vizzini, G.; Ploner, M.; Peck-Radosavljevic, M.; Gridelli, B.; Bosch, J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2007, 13, 1174–1180. [Google Scholar] [CrossRef]
- Huo, T.I.; Wang, Y.W.; Yang, Y.Y.; Lin, H.C.; Lee, P.C.; Hou, M.C.; Lee, F.Y.; Lee, S.D. Model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor and its correlation with portal pressure in patients with liver cirrhosis. Liver Int. Off. J. Int. Assoc. Study Liver 2007, 27, 498–506. [Google Scholar] [CrossRef]
- Silva Duarte Dos Santos, R.; Kieling, C.O.; Adami, M.R.; Guedes, R.R.; Vieira, S.M.G. Hypervolemic hyponatremia and transplant-free survival in children with cirrhosis due to biliary atresia. Pediatr. Transplant. 2020, 24, e13687. [Google Scholar] [CrossRef]
- Sharawey, M.A.; Shawky, E.M.; Ali, L.H.; Mohammed, A.A.; Hassan, H.A.; Fouad, Y.M. Cystatin C: A predictor of hepatorenal syndrome in patients with liver cirrhosis. Hepatol. Int. 2011, 5, 927–933. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, P.; Sharma, B.C.; Puri, A.S.; Kumar, A.; Sarin, S.K. Prevention of hepatorenal syndrome in patients with cirrhosis and ascites: A pilot randomized control trial between pentoxifylline and placebo. Eur. J. Gastroenterol. Hepatol. 2011, 23, 210–217. [Google Scholar] [CrossRef]
- Gines, A.; Escorsell, A.; Gines, P.; Salo, J.; Jimenez, W.; Inglada, L.; Navasa, M.; Claria, J.; Rimola, A.; Arroyo, V.; et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993, 105, 229–236. [Google Scholar]
- Ahn, H.S.; Kim, Y.S.; Kim, S.G.; Kim, H.K.; Min, S.K.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; Kim, B.S.; et al. Cystatin C is a good predictor of hepatorenal syndrome and survival in patients with cirrhosis who have normal serum creatinine levels. Hepato-Gastroenterol. 2012, 59, 1168–1173. [Google Scholar] [CrossRef]
- Janicko, M.; Veseliny, E.; Abraldes, J.G.; Jarcuska, P. Serum sodium identifies patients with cirrhosis at high risk of hepatorenal syndrome. Z. Fur Gastroenterol. 2013, 51, 628–634. [Google Scholar] [CrossRef]
- Guevara, M.; Baccaro, M.E.; Torre, A.; Gomez-Anson, B.; Rios, J.; Torres, F.; Rami, L.; Monte-Rubio, G.C.; Martin-Llahi, M.; Arroyo, V.; et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: A prospective study with time-dependent analysis. Am. J. Gastroenterol. 2009, 104, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Bossen, L.; Gines, P.; Vilstrup, H.; Watson, H.; Jepsen, P. Serum sodium as a risk factor for hepatic encephalopathy in patients with cirrhosis and ascites. J. Gastroenterol. Hepatol. 2019, 34, 914–920. [Google Scholar] [CrossRef]
- Wunsch, E.; Naprawa, G.; Koziarska, D.; Milkiewicz, M.; Nowacki, P.; Milkiewicz, P. Serum natremia affects health-related quality of life in patients with liver cirrhosis: A prospective, single centre study. Ann. Hepatol. 2013, 12, 448–455. [Google Scholar] [PubMed]
- Ahluwalia, V.; Wade, J.B.; Thacker, L.; Kraft, K.A.; Sterling, R.K.; Stravitz, R.T.; Fuchs, M.; Bouneva, I.; Puri, P.; Luketic, V.; et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J. Hepatol. 2013, 59, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Guevara, M.; Vilstrup, H.; Gines, P. Improvement of hyponatremia in cirrhosis is associated with improved complex information processing. J. Gastroenterol. Hepatol. 2019, 34, 1999–2003. [Google Scholar] [CrossRef]
- Bruns, T.; Zimmermann, H.W.; Stallmach, A. Risk factors and outcome of bacterial infections in cirrhosis. World J. Gastroenterol. WJG 2014, 20, 2542–2554. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, V.; D’Amico, G.; Fede, G.; Manousou, P.; Tsochatzis, E.; Pleguezuelo, M.; Burroughs, A.K. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010, 139, 1246–1256.e5. [Google Scholar] [CrossRef]
- Pereira, G.; Guevara, M.; Fagundes, C.; Sola, E.; Rodriguez, E.; Fernandez, J.; Pavesi, M.; Arroyo, V.; Gines, P. Renal failure and hyponatremia in patients with cirrhosis and skin and soft tissue infection. A retrospective study. J. Hepatol. 2012, 56, 1040–1046. [Google Scholar] [CrossRef]
- Follo, A.; Llovet, J.M.; Navasa, M.; Planas, R.; Forns, X.; Francitorra, A.; Rimola, A.; Gassull, M.A.; Arroyo, V.; Rodes, J. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: Incidence, clinical course, predictive factors and prognosis. Hepatology 1994, 20, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Abbasoglu, O.; Goldstein, R.M.; Vodapally, M.S.; Jennings, L.W.; Levy, M.F.; Husberg, B.S.; Klintmalm, G.B. Liver transplantation in hyponatremic patients with emphasis on central pontine myelinolysis. Clin. Transplant. 1998, 12, 263–269. [Google Scholar] [PubMed]
- Lee, J.; Kim, D.K.; Lee, J.W.; Oh, K.H.; Oh, Y.K.; Na, K.Y.; Kim, Y.S.; Han, J.S.; Suh, K.S.; Joo, K.W. Rapid correction rate of hyponatremia as an independent risk factor for neurological complication following liver transplantation. Tohoku J. Exp. Med. 2013, 229, 97–105. [Google Scholar] [PubMed]
- Karapanagiotou, A.; Kydona, C.; Papadopoulos, S.; Dimitriadis, C.; Giasnetsova, T.; Rempelakos, G.; Passakiotou, M.; Fouzas, I.; Papanikolaou, V.; Gritsi-Gerogianni, N. The effect of hyponatremia on the outcome of patients after orthotopic liver transplantation. Transplant. Proc. 2012, 44, 2724–2726. [Google Scholar] [CrossRef]
- Londono, M.C.; Guevara, M.; Rimola, A.; Navasa, M.; Taura, P.; Mas, A.; Garcia-Valdecasas, J.C.; Arroyo, V.; Gines, P. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology 2006, 130, 1135–1143. [Google Scholar] [CrossRef]
- Dawwas, M.F.; Lewsey, J.D.; Neuberger, J.M.; Gimson, A.E. The impact of serum sodium concentration on mortality after liver transplantation: A cohort multicenter study. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2007, 13, 1115–1124. [Google Scholar] [CrossRef]
- Wang, P.; Huang, G.; Tam, N.; Wu, C.; Fu, S.; Hughes, B.P.; Wu, L.; He, X. Influence of preoperative sodium concentration on outcome of patients with hepatitis B virus cirrhosis after liver transplantation. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Manolakopoulos, S.; Triantos, C.; Theodoropoulos, J.; Vlachogiannakos, J.; Kougioumtzan, A.; Papatheodoridis, G.; Tzourmakliotis, D.; Karamanolis, D.; Burroughs, A.K.; Archimandritis, A.; et al. Antiviral therapy reduces portal pressure in patients with cirrhosis due to HBeAg-negative chronic hepatitis B and significant portal hypertension. J. Hepatol. 2009, 51, 468–474. [Google Scholar] [CrossRef]
- Alukal, J.J.; John, S.; Thuluvath, P.J. Hyponatremia in Cirrhosis: An Update. Am. J. Gastroenterol. 2020, 115, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Riggio, O. Correction of hyponatraemia in cirrhosis: Treating more than a number! J. Hepatol. 2015, 62, 13–14. [Google Scholar] [CrossRef][Green Version]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Ginès, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010, 53, 397–417. [Google Scholar] [CrossRef]
- Yu, C.; Sharma, N.; Saab, S. Hyponatremia: Clinical associations, prognosis, and treatment in cirrhosis. Exp. Clin. Transplant. Off. J. Middle East. Soc. Organ. Transplant. 2013, 11, 3–11. [Google Scholar]
- Sigal, S.H.; Amin, A.; Chiodo, J.A., 3rd; Sanyal, A. Management Strategies and Outcomes for Hyponatremia in Cirrhosis in the Hyponatremia Registry. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1579508. [Google Scholar] [CrossRef] [PubMed]
- Gines, P.; Wong, F.; Watson, H.; Milutinovic, S.; del Arbol, L.R.; Olteanu, D.; Hypo, C.A.T.S.I. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: A randomized trial. Hepatology 2008, 48, 204–213. [Google Scholar] [CrossRef]
- Gerbes, A.L.; Gulberg, V.; Gines, P.; Decaux, G.; Gross, P.; Gandjini, H.; Djian, J. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: A randomized double-blind multicenter trial. Gastroenterology 2003, 124, 933–939. [Google Scholar] [CrossRef]
- Bichet, D.G.; Groves, B.M.; Schrier, R.W. Mechanisms of improvement of water and sodium excretion by immersion in decompensated cirrhotic patients. Kidney Int. 1983, 24, 788–794. [Google Scholar]
- Schrier, R.W. Renin-angiotensin in preascitic cirrhosis: Evidence for primary peripheral arterial vasodilatation. Gastroenterology 1998, 115, 489–491. [Google Scholar] [CrossRef]
- Senousy, B.-E. Evaluation and management of patients with refractory ascites. World J. Gastroenterol. 2009, 15, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Rossle, M.; Gerbes, A.L. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: A critical update. Gut 2010, 59, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Redhead, D.N.; Thomas, H.W.; Henderson, N.; O’Rourke, K.; Dillon, J.F.; Williams, B.C.; Hayes, P.C. Mechanisms of changes in renal handling of sodium following transjugular intrahepatic portal systemic stent-shunt (TIPSS). Eur. J. Gastroenterol. Hepatol. 1996, 8, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Sangro, B.; Nunez, M.; Bilbao, I.; Longo, J.; Garcia-Villarreal, L.; Zozaya, J.M.; Betes, M.; Herrero, J.I.; Prieto, J. Transjugular intrahepatic portal-systemic shunt in the treatment of refractory ascites: Effect on clinical, renal, humoral, and hemodynamic parameters. Hepatology 1995, 21, 986–994. [Google Scholar] [PubMed]
- Wong, F.; Blendis, L. Transjugular intrahepatic portosystemic shunt for refractory ascites: Tipping the sodium balance. Hepatology 1995, 22, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Sniderman, K.; Liu, P.; Allidina, Y.; Sherman, M.; Blendis, L. Transjugular intrahepatic portosystemic stent shunt: Effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann. Intern. Med. 1995, 122, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, A.S.; Ortiz, G.; Cui, J.; Wenger, J.; Bhan, I.; Chung, R.T.; Thadhani, R.I.; Irani, Z. Changes in Kidney Function After Transjugular Intrahepatic Portosystemic Shunts Versus Large-Volume Paracentesis in Cirrhosis: A Matched Cohort Analysis. Am. J. Kidney Dis. 2016, 68, 381–391. [Google Scholar] [CrossRef]
- Ginès, P.; Uriz, J.; Calahorra, B.; Garcia–Tsao, G.; Kamath, P.S.; Del Arbol, L.R.; Planas, R.; Bosch, J.; Arroyo, V.; Rodés, J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology 2002, 123, 1839–1847. [Google Scholar] [CrossRef]
- Mandorfer, M.; Bota, S.; Schwabl, P.; Bucsics, T.; Pfisterer, N.; Kruzik, M.; Hagmann, M.; Blacky, A.; Ferlitsch, A.; Sieghart, W.; et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014, 146, 1680–1690.e1. [Google Scholar] [CrossRef]
- Gross, P. Clinical management of SIADH. Ther. Adv. Endocrinol. Metab. 2012, 3, 61–73. [Google Scholar] [CrossRef]
- McCormick, P.A.; Mistry, P.; Kaye, G.; Burroughs, A.K.; McIntyre, N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut 1990, 31, 204–207. [Google Scholar] [CrossRef]
- Jalan, R.; Mookerjee, R.; Cheshire, L.; Williams, R.; Davies, N. [232] Albumin Infusion for Severe Hyponatremia in Patients with Refractory Ascites: A Randomized Clinical Trial. J. Hepatol. 2007, 46, S95. [Google Scholar] [CrossRef]
- Bai, Z.; Xu, W.; Chai, L.; Zheng, X.; Mendez-Sanchez, N.; Philips, C.A.; Cheng, G.; Qi, X. Effects of Short-Term Human Albumin Infusion for the Prevention and Treatment of Hyponatremia in Patients with Liver Cirrhosis. J. Clin. Med. 2022, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Tandon, P.; O’Leary, J.G.; Biggins, S.W.; Wong, F.; Kamath, P.S.; Garcia-Tsao, G.; Maliakkal, B.; Lai, J.C.; Fallon, M.; et al. The Impact of Albumin Use on Resolution of Hyponatremia in Hospitalized Patients With Cirrhosis. Am. J. Gastroenterol. 2018, 113, 1339. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wang, L.; Lin, H.; Tacke, F.; Cheng, G.; Qi, X. Use of Human Albumin Administration for the Prevention and Treatment of Hyponatremia in Patients with Liver Cirrhosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5928. [Google Scholar] [CrossRef] [PubMed]
- Zaccherini, G.; Baldassarre, M.; Tufoni, M.; Nardelli, S.; Piano, S.; Alessandria, C.; Neri, S.; Foschi, F.G.; Levantesi, F.; Bedogni, G.; et al. Correction and Prevention of Hyponatremia in Patients With Cirrhosis and Ascites: Post Hoc Analysis of the ANSWER Study Database. Am. J. Gastroenterol. 2023, 118, 168–173. [Google Scholar] [CrossRef]
- Decaux, G.; Mols, P.; Cauchie, P.; Flamion, B.; Delwiche, F. Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea. Nephron 1986, 44, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Qureshi, U.F.; Humayoun, M.A.; Waseem, T.; Akram, J. Effect of intravenous mannitol in mobilization of resistant cirrhotic ascites. Eur. J. Gastroenterol. Hepatol. 2011, 23, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Bae, E.J.; Hwang, K.; Jeon, D.H.; Jang, H.N.; Cho, H.S.; Chang, S.H.; Park, D.J. Initial serum sodium concentration determines the decrease in sodium level after terlipressin administration in patients with liver cirrhosis. SpringerPlus 2013, 2, 519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sola, E.; Lens, S.; Guevara, M.; Martin-Llahi, M.; Fagundes, C.; Pereira, G.; Pavesi, M.; Fernandez, J.; Gonzalez-Abraldes, J.; Escorsell, A.; et al. Hyponatremia in patients treated with terlipressin for severe gastrointestinal bleeding due to portal hypertension. Hepatology 2010, 52, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Jarčuška, P.; Beňa, L.; Timková, A.; Veseliny, E.; Janičko, M. Hepatorenal syndrome in patients with acute alcoholic hepatitis. Ceska A Slov. Gastroenterol. A Hepatol. 2012, 66, 101–108. [Google Scholar]
- Duvoux, C.; Zanditenas, D.; Hezode, C.; Chauvat, A.; Monin, J.L.; Roudot-Thoraval, F.; Mallat, A.; Dhumeaux, D. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: A pilot study. Hepatology 2002, 36, 374–380. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Yazdanpanah, K.; Mansourian, M. Noradrenalin versus the combination of midodrine and octreotide in patients with hepatorenal syndrome: Randomized clinical trial. Int. J. Prev. Med. 2012, 3, 764–769. [Google Scholar] [PubMed]
- Patel, S.; Nguyen, D.S.; Rastogi, A.; Nguyen, M.K.; Nguyen, M.K. Treatment of Cirrhosis-Associated Hyponatremia with Midodrine and Octreotide. Front. Med. 2017, 4, 17. [Google Scholar] [CrossRef]
- Singh, V.; Dhungana, S.P.; Singh, B.; Vijayverghia, R.; Nain, C.K.; Sharma, N.; Bhalla, A.; Gupta, P.K. Midodrine in patients with cirrhosis and refractory or recurrent ascites: A randomized pilot study. J. Hepatol. 2012, 56, 348–354. [Google Scholar] [CrossRef]
- Solà, E.; Solé, C.; Simón-Talero, M.; Martín-Llahí, M.; Castellote, J.; Garcia-Martínez, R.; Moreira, R.; Torrens, M.; Márquez, F.; Fabrellas, N.; et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J. Hepatol. 2018, 69, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A. Nitric oxide inhibition in cirrhosis and ascites. Am. J. Gastroenterol. 2003, 98, 1666–1667. [Google Scholar] [CrossRef] [PubMed]
- Troyer, A.; Pilloy, W.; Broeckaert, I.; Demanet, J.C. Letter: Demeclocycline treatment of water retention in cirrhosis. Ann. Intern. Med. 1976, 85, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Gadano, A.; Moreau, R.; Pessione, F.; Trombino, C.; Giuily, N.; Sinnassamy, P.; Valla, D.; Lebrec, D. Aquaretic effects of niravoline, a kappa-opioid agonist, in patients with cirrhosis. J. Hepatol. 2000, 32, 38–42. [Google Scholar] [CrossRef]
- Schrier, R.W.; Gross, P.; Gheorghiade, M.; Berl, T.; Verbalis, J.G.; Czerwiec, F.S.; Orlandi, C.; Investigators, S. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N. Engl. J. Med. 2006, 355, 2099–2112. [Google Scholar] [CrossRef]
- Cardenas, A.; Gines, P.; Marotta, P.; Czerwiec, F.; Oyuang, J.; Guevara, M.; Afdhal, N.H. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J. Hepatol. 2012, 56, 571–578. [Google Scholar] [CrossRef]
- Okita, K.; Sakaida, I.; Okada, M.; Kaneko, A.; Chayama, K.; Kato, M.; Sata, M.; Yoshihara, H.; Ono, N.; Murawaki, Y. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J. Gastroenterol. 2010, 45, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, I.; Kawazoe, S.; Kajimura, K.; Saito, T.; Okuse, C.; Takaguchi, K.; Okada, M.; Okita, K.; Group, A.-D.S. Tolvaptan for improvement of hepatic edema: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2014, 44, 73–82. [Google Scholar] [CrossRef]
- Iwasa, M.; Ishihara, T.; Hasegawa, H.; Takei, Y. Cirrhosis-related hyponatremia and the role of tolvaptan. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2015, 45, E163–E165. [Google Scholar] [CrossRef][Green Version]
- Hayashi, M.; Abe, K.; Fujita, M.; Okai, K.; Takahashi, A.; Ohira, H. Association between the Serum Sodium Levels and the Response to Tolvaptan in Liver Cirrhosis Patients with Ascites and Hyponatremia. Intern. Med. 2018, 57, 2451–2458. [Google Scholar] [CrossRef]
- Pose, E.; Sola, E.; Piano, S.; Gola, E.; Graupera, I.; Guevara, M.; Cardenas, A.; Angeli, P.; Gines, P. Limited Efficacy of Tolvaptan in Patients with Cirrhosis and Severe Hyponatremia: Real-Life Experience. Am. J. Med. 2017, 130, 372–375. [Google Scholar] [CrossRef]
- Zeltser, D.; Rosansky, S.; van Rensburg, H.; Verbalis, J.G.; Smith, N.; Conivaptan Study, G. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am. J. Nephrol. 2007, 27, 447–457. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Davis, G.L. Conivaptan increases serum sodium in hyponatremic patients with end-stage liver disease. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2009, 15, 1325–1329. [Google Scholar] [CrossRef]
- Wong, F.; Blei, A.T.; Blendis, L.M.; Thuluvath, P.J. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: A multicenter, randomized, placebo-controlled trial. Hepatology 2003, 37, 182–191. [Google Scholar] [CrossRef]
- Abraham, W.T.; Aranda, J.M.; Boehmer, J.P.; Elkayam, U.; Gilbert, E.M.; Gottlieb, S.S.; Hasenfuss, G.; Kukin, M.; Lowes, B.D.; O’Connell, J.B.; et al. Rationale and design of the treatment of hyponatremia based on lixivaptan in NYHA class III/IV cardiac patient evaluation (THE BALANCE) study. Clin. Transl. Sci. 2010, 3, 249–253. [Google Scholar] [CrossRef]
- Bowman, B.T.; Rosner, M.H. Lixivaptan—An evidence-based review of its clinical potential in the treatment of hyponatremia. Core Evid. 2013, 8, 47–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, F.; Watson, H.; Gerbes, A.; Vilstrup, H.; Badalamenti, S.; Bernardi, M.; Gines, P.; Satavaptan Investigators, G. Satavaptan for the management of ascites in cirrhosis: Efficacy and safety across the spectrum of ascites severity. Gut 2012, 61, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; Gluud, L.L.; Kimer, N.; Krag, A. Meta-analysis: The safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment. Pharmacol. Ther. 2012, 36, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- SAMSCA [Package Insert]. Otsuka America Pharmaceutical, Inc.: Rockville, MD, USA, 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022275s014lbl.pdf (accessed on 1 April 2017).
- Kwo, P.Y. Management of hyponatremia in clinical hepatology practice. Curr. Gastroenterol. Rep. 2014, 16, 382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).