First-Line Systemic Therapy Outcomes in Western Population with Locally Advanced and Metastatic Gastric Cancer—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Types of Studies
2.2. Types of Participants
2.3. Types of Interventions
2.4. Types of Outcome Measures
2.5. Search Strategy
2.6. Selection Criteria
2.7. Data Extraction and Management
2.8. Risk of Bias Assessment
2.9. Data Synthesis
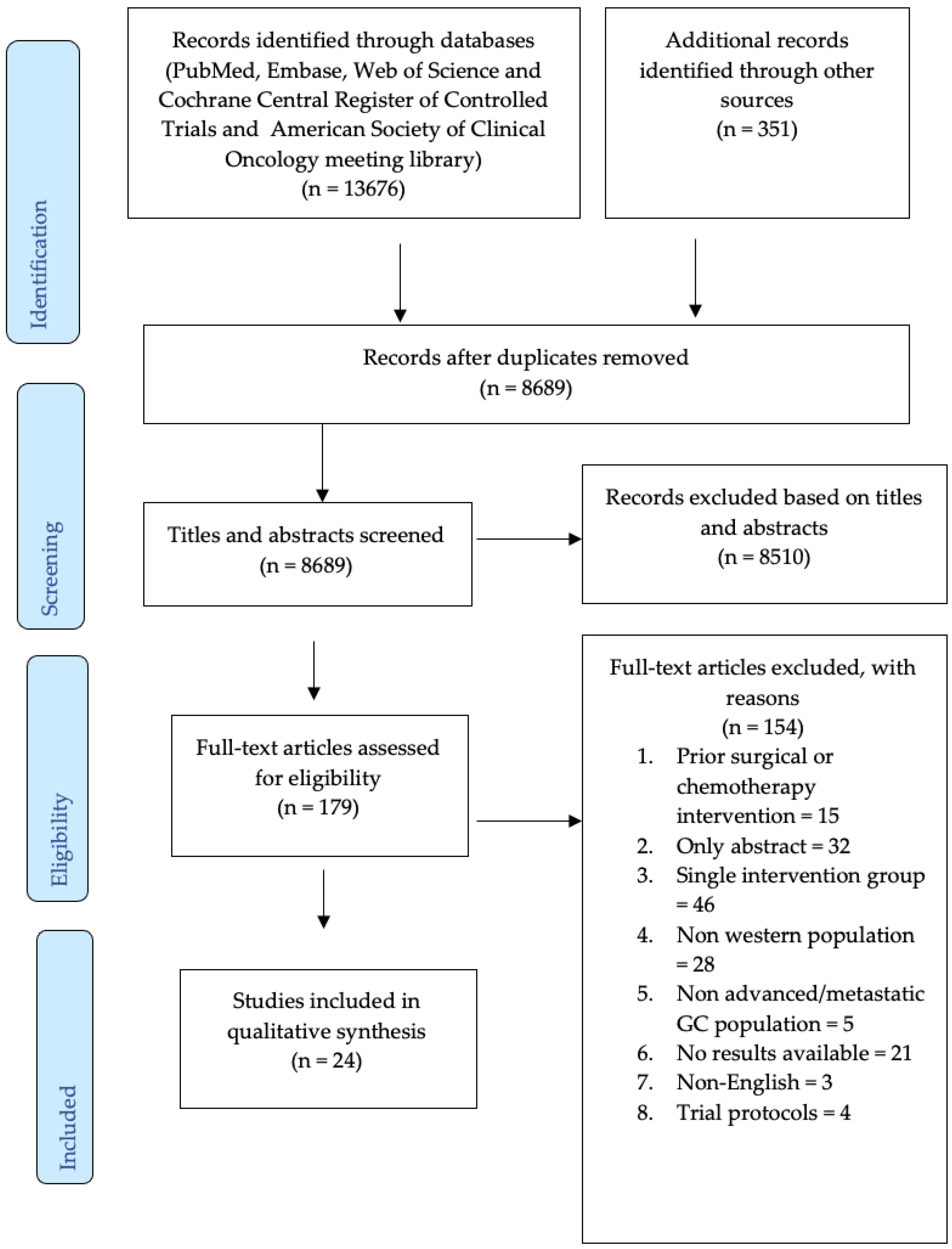
3. Results
3.1. Characteristics of Included Studies
3.2. Overall Survival
3.3. Progression-Free Survival
3.4. Objective Response Rate
3.5. Other Outcomes Reported
3.6. Adverse Events and Safety
3.7. Risk of Bias Assessment
4. Discussion
4.1. Overall Survival
4.2. Progression-Free Survival
4.3. Objective Response Rate
4.4. Other Outcomes
4.5. Limitations
4.6. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategy for Systematic Review
- Search: Clinical, economic, and/or humanistic outcomes of first-line systemic therapies in patients with unresectable locally advanced/metastatic gastric cancer—A Systematic Review
- Citation Account (if any): Endnote
- Limits: English language
- Type of publications: phase II and phase III randomized controlled trials
- Inclusions:
- P-Adults aged ≥ 18 years with stage II–IV gastric cancer
- I-First-line systemic therapies as approved by NCCN 2022 guidelines
- C-First-line systemic therapies and/or best supportive care/placebo
- O-Clinical: overall survival, progression-free survival, time to progression, and objective response rate.
- Safety: grade ≥ 3 treatment-related adverse event
- Humanistic: quality of life, utility estimates, where available
- Exclusion: non-English publications, observational, narrative reviews, systematic reviews, meta-analyses, and non-research publications will be excluded. Also, studies that focused exclusively on other lines of therapy than first-line systemic therapy as well as other interventions such as radiotherapy, surgical, and total neoadjuvant chemotherapy will be excluded.
- Limits: English language and trials conducted among western population or countries
- Search Dates: inception to May 2022
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, S.A.; Phillips, J.L.; Menck, H.R. The National Cancer Data Base report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy. Cancer 2000, 88, 921–932. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241. [Google Scholar]
- Eslick, G.D. Helicobacter pylori infection causes gastric cancer A review of the epidemiological, meta-analytic, and experimental evidence. World J. Gastroenterol. 2006, 12, 2991. [Google Scholar] [CrossRef] [PubMed]
- Murata-Kamiya, N. Pathophysiological functions of the CagA oncoprotein during infection by Helicobacter pylori. Microbes Infect. 2011, 13, 799–807. [Google Scholar] [CrossRef]
- Hu, Z.; Ajani, J.A.; Wei, Q. Molecular Epidemiology of Gastric Cancer: Current Status and Future Prospects. Gastrointest. Cancer Res. GCR 2007, 1, 12–19. [Google Scholar] [PubMed]
- Zhang, H.; Feng, M.; Feng, Y.; Bu, Z.; Li, Z.; Jia, S.; Ji, J. Germline mutations in hereditary diffuse gastric cancer. Chin. J. Cancer Res. 2018, 30, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.; Varga, M.G.; Wang, T.; Tsugane, S.; Shimazu, T.; Zheng, W.; Abnet, C.C.; Yoo, K.-Y.; Park, S.K.; Kim, J.; et al. Smoking, Helicobacter Pylori Serology, and Gastric Cancer Risk in Prospective Studies from China, Japan, and Korea. Cancer Prev. Res. 2019, 12, 667–674. [Google Scholar] [CrossRef]
- Wiseman, M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef]
- Koh, T.; Wang, T. Tumors of the Stomach. In Sleisenger & Fordtran’s Gastrointestinal and Liver Disease, 7th ed.; Saunders: Philadelphia, PA, USA, 2002; pp. 829–844. [Google Scholar]
- Xia, J.Y.; Aadam, A.A. Advances in screening and detection of gastric cancer. J. Surg. Oncol. 2022, 125, 1104–1109. [Google Scholar] [CrossRef]
- Stomach Cancer Statistics|Gastric Cancer Statistics. Available online: https://www.cancer.org/cancer/stomach-cancer/about/key-statistics.html (accessed on 29 September 2021).
- Casamayor, M.; Morlock, R.; Maeda, H.; Anjani, J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience 2018, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 29 September 2021).
- National Cancer Institute. Gastric Cancer Treatment (PDQ®)–Health Professional Version. 2021. Available online: https://www.cancer.gov/types/stomach/hp/stomach-treatment-pdq#_81 (accessed on 29 September 2021).
- Genentech: News Features|Genentech Medicine Approved in Stomach Cancer. Available online: https://www.gene.com/media/news-features/genentech-medicine-approved-in-stomach-cancer#:~:text=On%20October%2020%2C%202010%2C%20Herceptin (accessed on 29 September 2021).
- Lao, Y.; Shen, D.; Zhang, W.; He, R.; Jiang, M. Immune Checkpoint Inhibitors in Cancer Therapy-How to Overcome Drug Resistance? Cancers 2022, 14, 3575. [Google Scholar] [CrossRef]
- Rodrigues, S.; Figueiredo, C. Recent insights into the use of immune checkpoint inhibitors in gastric cancer. Porto Biomed. J. 2022, 7, e162. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, F.; Zhou, N.; Gu, Y.-M.; Zhang, Y.-T.; He, Y.-D.; Wang, L.; Yang, L.-X.; Zhao, Y.; Li, Y.-M. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: A systematic review and meta-analysis. OncoImmunology 2019, 8, e1581547. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S.; Thuss-Patience, P.C.; Knorrenschild, J.R.; Goekkurt, E.; Dechow, T.N.; Hofheinz, R.-D.; Luley, K.B.; Ettrich, T.J.; Pink, D.; Lindig, U.; et al. FOLFOX versus FOLFOX plus nivolumab and ipilimumab administered in parallel or sequentially versus FLOT plus nivolumab administered in parallel in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction: A randomized phase 2 trial of the AIO. J. Clin. Oncol. 2022, 40, 4043. [Google Scholar] [CrossRef]
- Patrinely, J.R.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti–PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744. [Google Scholar] [CrossRef]
- Ter Veer, E.; Haj Mohammad, N.; Van Valkenhoef, G.; Ngai, L.; Mali, R.; Van Oijen, M.; Van Laarhoven, H. 2369 Efficacy and safety of first line chemotherapy in Advanced EsophagoGastric Cancer (AEGC): A network meta-analysis. Eur. J. Cancer 2015, 51, S459. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, F.; Ma, X.; Shen, G.; Ren, D.; Zheng, F.; Du, F.; Wang, Z.; Ahmad, R.; Yuan, X.; et al. A comparison between triplet and doublet chemotherapy in improving the survival of patients with advanced gastric cancer: A systematic review and meta-analysis. BMC Cancer 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, J.; Ma, S. Fluoropyrimidine-Based Chemotherapy as First-Line Treatment for Advanced Gastric Cancer: A Bayesian Network Meta-Analysis. Pathol. Oncol. Res. 2016, 22, 853–861. [Google Scholar] [CrossRef]
- Song, H.; Zhu, J.; Lu, D. Molecular-targeted first-line therapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2016, 2016, CD011461. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) V5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 29 September 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Fodor, M.B.; Tjulandin, S.A.; Moiseyenko, V.M.; Chao, Y.; Filho, S.C.; Majlis, A.; Assadourian, S.; Van Cutsem, E. Phase II Multi-Institutional Randomized Trial of Docetaxel Plus Cisplatin with or without Fluorouracil in Patients with Untreated, Advanced Gastric, or Gastroesophageal Adenocarcinoma. J. Clin. Oncol. 2005, 23, 5660–5667. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E.; et al. Quality of Life with Docetaxel Plus Cisplatin and Fluorouracil Compared with Cisplatin and Fluorouracil from a Phase III Trial for Advanced Gastric or Gastroesophageal Adenocarcinoma: The V-325 Study Group. J. Clin. Oncol. 2007, 25, 3210–3216. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Rodriguez, W.; Bodoky, G.; Moiseyenko, V.; Lichinitser, M.; Gorbunova, V.; Vynnychenko, I.; Garin, A.; Lang, I.; Falcon, S. Multicenter Phase III Comparison of Cisplatin/S-1 with Cisplatin/Infusional Fluorouracil in Advanced Gastric or Gastroesophageal Adenocarcinoma Study: The FLAGS Trial. J. Clin. Oncol. 2010, 28, 1547–1553. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Pauligk, C.; Homann, N.; Hartmann, J.T.; Moehler, M.; Probst, S.; Rethwisch, V.; Stoehlmacher-Williams, J.; Prasnikar, N.; Hollerbach, S.; et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: A randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur. J. Cancer 2013, 49, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Bouché, O.; Raoul, J.L.; Bonnetain, F.; Giovannini, M.; Etienne, P.L.; Lledo, G.; Arsène, D.; Paitel, J.F.; Guérin-Meyer, V.; Mitry, E.; et al. Randomized Multicenter Phase II Trial of a Biweekly Regimen of Fluorouracil and Leucovorin (LV5FU2), LV5FU2 Plus Cisplatin, or LV5FU2 Plus Irinotecan in Patients with Previously Untreated Metastatic Gastric Cancer: A Fédération Francophone de Cancérologie Digestive Group Study—FFCD 9803. J. Clin. Oncol. 2004, 22, 4319–4328. [Google Scholar] [CrossRef]
- Cascinu, S.; Galizia, E.; Labianca, R.; Ferraù, F.; Pucci, F.; Silva, R.R.; Luppi, G.; Beretta, G.D.; Berardi, R.; Scartozzi, M. Pegylated liposomal doxorubicin, 5-fluorouracil and cisplatin versus mitomycin-C, 5-fluorouracil and cisplatin for advanced gastric cancer: A randomized phase II trial. Cancer Chemother. Pharmacol. 2010, 68, 37–43. [Google Scholar] [CrossRef][Green Version]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef]
- Cocconi, G.; Bella, M.; Zironi, S.; Algeri, R.; Di Costanzo, F.; De Lisi, V.; Luppi, G.; Mazzocchi, B.; Rodinò, C.; Soldani, M. Fluorouracil, doxorubicin, and mitomycin combination versus PELF chemotherapy in advanced gastric cancer: A prospective randomized trial of the Italian Oncology Group for Clinical Research. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994, 12, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Chilvers, C.E.D.; Amadori, D.; Medi, F.; Fountzilas, G.; Rauschecker, H.; Vassilopoulos, P.; Ferreira, E.P.; Vannozzi, G.; Bliss, J.M.; et al. Randomised trial of epirubicin versus fluorouracil in advanced gastric cancer. Ann. Oncol. 1994, 5, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.; Pozzo, C.; Zaluski, J.; Dank, M.; Barone, C.; Valvere, V.; Yalcin, S.; Peschel, C.; Wenczl, M.; Goker, E.; et al. Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: Results of a randomised phase III trial. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2009, 18, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab as First-Line Therapy in Combination with Cisplatin and Fluoropyrimidine in Patients with Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (RAINFALL): A Global, Randomised, Double-Blinded, Placebo-Controlled, Phase 3 Trial. SSRN Electron. J. 2018. [Google Scholar] [CrossRef]
- Gubanski, M.; Johnsson, A.; Fernebro, E.; Kadar, L.; Karlberg, I.; Flygare, P.; Berglund, A.; Glimelius, B.; Lind, P.A.; Gastric Cancer Taxotere vs. Campto Trial (GATAC) Study Group. Randomized phase II study of sequential docetaxel and irinotecan with 5-fluorouracil/folinic acid (leucovorin) in patients with advanced gastric cancer: The GATAC trial. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2010, 13, 155–161. [Google Scholar] [CrossRef][Green Version]
- Högner, A.; Al-Batran, S.; Siveke, J.T.; Lorenz, M.; Bartels, P.; Breithaupt, K.; Malfertheiner, P.; Homann, N.; Stein, A.; Gläser, D.; et al. Pazopanib with 5-FU and oxaliplatin as first line therapy in advanced gastric cancer: A randomized phase-II study-The PaFLO trial. A study of the Arbeitsgemeinschaft Internistische Onkologie AIO-STO-0510. Int. J. Cancer 2022, 150, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- İçli, F.; Çelik, İ.; Aykan, F.; Üner, A.; Demirkazik, A.; Özet, A.; Özgüroǧlu, M.; Taş, F.; Akbulut, H.; Firat, D. A randomized Phase III trial of etoposide, epirubicin, and cisplatin versus 5-fluorouracil, epirubicin, and cisplatin in the treatment of patients with advanced gastric carcinoma. Turkish Oncology Group. Cancer 1998, 83, 2475–2480. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Kang, W.-K.; Shin, D.-B.; Chen, J.; Xiong, J.; Wang, J.; Lichinitser, M.; Guan, Z.; Khasanov, R.; Zheng, L.; et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann. Oncol. 2009, 20, 666–673. [Google Scholar] [CrossRef]
- Ochenduszko, S.; Puskulluoglu, M.; Konopka, K.; Fijorek, K.; Urbanczyk, K.; Budzynski, A.; Matlok, M.; Lazar, A.; Sinczak-Kuta, A.; Pedziwiatr, M.; et al. Comparison of efficacy and safety of first-line palliative chemotherapy with EOX and mDCF regimens in patients with locally advanced inoperable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma: A randomized phase 3 trial. Med. Oncol. 2015, 32, 242. [Google Scholar] [CrossRef]
- Petersen, P.C.; Petersen, L.N.; Vogelius, I.; Bjerregaard, J.K.; Baeksgaard, L. A randomized phase 2 trial of first-line docetaxel, carboplatin, capecitabine (CTX) and epirubicin, oxaliplatin, capecitabine (EOX) in advanced esophagogastric adenocarcinoma. Acta Oncol. 2021, 60, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.D.; Fazio, N.; Stupp, R.; Falk, S.; Bernhard, J.; Saletti, P.; Köberle, D.; Borner, M.M.; Rufibach, K.; Maibach, R.; et al. Docetaxel, Cisplatin, and Fluorouracil; Docetaxel and Cisplatin; and Epirubicin, Cisplatin, and Fluorouracil as Systemic Treatment for Advanced Gastric Carcinoma: A Randomized Phase II Trial of the Swiss Group for Clinical Cancer Research. J. Clin. Oncol. 2007, 25, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Van Cutsem, E.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.-W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs. Chemotherapy Alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Doi, T.; Hosaka, H.; Thuss-Patience, P.; Santoro, A.; Longo, F.; Ozyilkan, O.; Cicin, I.; Park, D.; Zaanan, A.; et al. Efficacy and safety of trifluridine/tipiracil in older and younger patients with metastatic gastric or gastroesophageal junction cancer: Subgroup analysis of a randomized phase 3 study (TAGS). Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2022, 25, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E.; et al. Phase III Study of Docetaxel and Cisplatin Plus Fluorouracil Compared with Cisplatin and Fluorouracil as First-Line Therapy for Advanced Gastric Cancer: A Report of the V325 Study Group. J. Clin. Oncol. 2006, 24, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.; Cunningham, D.; Scarffe, J.H.; Harper, P.; Norman, A.; Joffe, J.K.; Hughes, M.; Mansi, J.; Findlay, M.; Hill, A.; et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cai, M.; Shuai, X.; Gao, J.; Wang, G.; Tao, K. First-Line Systemic Therapy for Advanced Gastric Cancer: A Systematic Review and Network Meta-Analysis of 118 Randomized Controlled Trials. SSRN Electron. J. 2018. [Google Scholar] [CrossRef]
- Wagner, A.D.; Grothe, W.; Haerting, J.; Kleber, G.; Grothey, A.; Fleig, W.E. Chemotherapy in Advanced Gastric Cancer: A Systematic Review and Meta-Analysis Based on Aggregate Data. J. Clin. Oncol. 2006, 24, 2903–2909. [Google Scholar] [CrossRef]
- CheckMate 649: Long-Term Data Support Nivolumab Plus Chemotherapy but Not Nivolumab Plus Ipilimumab in Gastric Cancer—The ASCO Post. 2021. Available online: https://ascopost.com/issues/october-25-2021/checkmate-649-long-term-data-support-nivolumab-plus-chemotherapy-but-not-nivolumab-plus-ipilimumab-in-gastric-cancer/#:~:text=Objective%20response%20rates%20were%2060 (accessed on 10 June 2022).
- Takashima, A.; Iizumi, S.; Boku, N. Survival after failure of first-line chemotherapy in advanced gastric cancer patients: Differences between Japan and the rest of the world. Jpn. J. Clin. Oncol. 2017, 47, 583–589. [Google Scholar] [CrossRef]
- Koizumi, W. Chemotherapy for advanced gastric cancer: Review of global and Japanese status. Gastrointest. Cancer Res. GCR 2007, 1, 197–203. [Google Scholar]
- Elimova, E.; Wyrwicz, L.; Blum, S.I.; Xiao, H.; Li, M.; Kondo, K.; Davenport, E.; Wang, J.; Hunter, S.; Moehler, M.H. Health-related quality of life (HRQOL) in patients (pts) with advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC) or esophageal adenocarcinoma (EAC): Results of nivolumab plus chemotherapy (NIVO+chemo) versus chemo from CheckMate 649. J. Clin. Oncol. 2021, 39, 167. [Google Scholar] [CrossRef]
- Van Amelsfoort, R.M.; van der Sluis, K.; Schats, W.; Jansen, E.P.M.; van Sandick, J.W.; Verheij, M.; Walraven, I. Health-Related Quality of Life in Locally Advanced Gastric Cancer: A Systematic Review. Cancers 2021, 13, 5934. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Da Silva, L.L.; Saeed, A. Immunotherapy Predictive Molecular Markers in Advanced Gastroesophageal Cancer: MSI and Beyond. Cancers 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.L.; He, Y.; Xu, R.H. Gastric cancer treatment: Recent progress and future perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Korfer, J.; Lordick, F.; Hacker, U.T. Molecular targets for gastric cancer treatment and future perspectives from a clinical and translational point of view. Cancers 2021, 13, 5216. [Google Scholar] [CrossRef]
| ("Stomach"[Mesh] OR "stomach"[ALL] OR "Gastric"[ALL] OR "Esophagogastric"[ALL] OR "oesophagogastric"[ALL] OR "Gastroesophageal"[ALL] OR "gastrooesophageal"[ALL]) AND ("Neoplasms"[Mesh] OR "Carcinoma"[Mesh] OR "Stomach Neoplasms"[Mesh] OR "Adenocarcinoma"[Mesh] OR "Cancer*"[ALL] OR "Neoplasm*"[ALL] OR "Adenocarcinoma*"[ALL] OR "Carcinoma*"[ALL] OR "Tumor*"[ALL] OR "Neoplasm, Stomach"[ALL] OR "Stomach Neoplasm"[ALL] OR "Neoplasms, Stomach"[ALL] OR "Gastric Neoplasms"[ALL] OR "Gastric Neoplasm"[ALL] OR "Neoplasm, Gastric"[ALL] OR "Neoplasms, Gastric"[ALL] OR "Cancer of Stomach"[ALL] OR "Stomach Cancers"[ALL] OR "Gastric Cancer"[ALL] OR "Cancer, Gastric"[ALL] OR "Cancers, Gastric"[ALL] OR "Gastric Cancers"[ALL] OR "Stomach Cancer"[ALL] OR "Cancer, Stomach"[ALL] OR "Cancers, Stomach"[ALL]) AND ("Neoplasm Metastasis"[Mesh] OR "secondary" [Subheading] OR "Advanced"[ALL] OR "Metastatic"[ALL] OR "Metastasis"[ALL] OR "Recurrent"[ALL] OR "Unresectable"[ALL] OR "Inoperable"[ALL] OR "Incurable"[ALL] OR "Neoplasm metastasis"[ALL] OR "Secondary neoplasm*"[ALL] OR "Secondary cancer*"[ALL] OR "Secondary tumor*"[ALL] OR "Stage III"[ALL] OR "Stage IV"[ALL]) AND (("Controlled Clinical Trial" [Publication Type] OR "Clinical Trials as Topic"[Mesh] OR "Clinical Trial" [Publication Type] OR "Randomized Controlled Trials as Topic"[Mesh] OR "Randomised"[ALL] OR "Randomized"[ALL] OR "Randomly"[ALL] OR "Random"[ALL] OR "Randomized controlled trial*"[ALL] OR "Controlled clinical trial*"[ALL] OR "Trial*"[ALL] OR "Clinical trial*"[ALL] OR "placebo"[ALL])) AND ("Drug Therapy"[Mesh] OR "Antineoplastic Agents"[Mesh] OR "Chemotherapy, Adjuvant"[Mesh] OR "Combined Modality Therapy"[Mesh] OR "Antineoplastic Combined Chemotherapy Protocols"[Mesh] OR "Palliative Care"[Mesh] OR "Antineoplastic Agents"[ALL] OR "Drug therapy"[ALL] OR "Chemotherapy"[ALL] OR "Adjuvant chemotherapy"[ALL] OR "Antineoplastic combined chemotherapy"[ALL] OR "Palliative care"[ALL] OR "First-line therapy"[ALL] OR "first line"[ALL] OR "Supportive care"[ALL] OR "Antineoplastic Combined Chemotherapy Protocols"[ALL] OR "Systemic therapy"[ALL]) |
| Author, Year | Country | Phase | Study Design | Total N | Treatment Arms | Age Median (Range) | Males, n (%) |
|---|---|---|---|---|---|---|---|
| Ajani, 2005 [29] | Multiple | II | Open label | 155 | Docetaxel-cisplatin | 57 (21–83) | 114 (74) |
| Docetaxel-cisplatin-fluorouracil | |||||||
| Ajani, 2007 [30] | Multiple | III | Open label | 445 | Docetaxel-cisplatin-fluorouracil | 55 (26–79) | (71.9) |
| Cisplatin-fluorouracil | 55 (25–76) | (70.5) | |||||
| Ajani, 2010 [31] | Multiple | III | Open label | 1029 | Cisplatin-S1 | 59.0 (18.85) + | 729 (70.8) |
| Cisplatin-fluorouracil | |||||||
| Al-Batran, 2008 [32] | Germany + Switzerland | III | NR | 220 | Fluorouracil-leucovorin-oxaliplatin | 64 (33–86) | 64 (57) |
| Fluorouracil-leucovorin-cisplatin | 64 (27–85) | 81 (75) | |||||
| Al-Batran, 2013 [33] | Germany | III | Open label | 143 | Fluorouracil-leucovorin-oxaliplatin-docetaxel | 69 | 51 (71) |
| Fluorouracil-leucovorin-oxaliplatin | 70 | 45 (63) | |||||
| Bouche, 2004 [34] | France | III | Open label | 136 | Leucovorin–5-flurouracil | 64 (45–75) | (82) |
| Leucovorin–5-flurouracil–cisplatin | 64 (43–76) | (80) | |||||
| Leucovorin–5-flurouracil-irinotecan | 65 (37–76) | (84) | |||||
| Cascinu, 2010 [35] | Italy | II | NR | 78 | 5-flurouracil-cisplatin-doxorubicin | 63 (33–75) | 50 (64) |
| 5-flurouracil-cisplatin-mitomycin | |||||||
| Catenacci, 2017 [36] | Multiple | III | double-blind, placebo | 609 | Rilotumumab-epirubicin-cisplatin-capecitabine | 61 (28–84) | (67) |
| Placebo-epirubicin-cisplatin-capecitabine | 59 (26–81) | (72) | |||||
| Cocconi, 1994 [37] | Italy | III | NR | 130 | Cisplatin-epirubicin-leucovorin-flurouracil | 62 (28–74) | 60 (71) |
| Fluorouracil-doxorubicin-mitomycin | 65 (40–75) | 42 (81) | |||||
| Coombes, 1994 [38] | United Kingdom | NR | NR | 69 | Epirubicin | 59.9 (9.3) * | 27 (7.5) * |
| Fluorouracil | 55.6 (9.2) * | 24 (7.3) * | |||||
| Curran, 2009 [39] | Multiple | III | NR | 333 | Irinotecan-fluorouracil | NR | NR |
| Cisplatin-fluorouracil | |||||||
| Fuchs, 2019 [40] | Multiple | III | double-blind, placebo | 645 | Ramucirumab-flurouracil-cisplatin | 60 (51–68) | 214 (66) |
| Placebo-flurouracil-cisplatin | 62 (54–68) | 215 (67) | |||||
| Gubanski, 2010 [41] | Sweden | II | Crossover | 80 | Irinotecan-5-fluorouracil-leucovorin | 63 (39–79) | (67) |
| Docetaxel-leucovorin | 64 (42–75) | (87) | |||||
| Högner, 2021 [42] | Germany | II | Open label | 87 | Pazopanib-5-fluorouracil-oxaliplatin | 65 | 37 (72) |
| Fluorouracil-leucovorin-oxaliplatin | 60 | 17 (63) | |||||
| Icli, 1998 [43] | Turkey | III | NR | 131 | Etoposide-epirubicin-cisplatin | 52.7 (9.2) * | (68.8) |
| 5-fluorouracil-epirubicin-cisplatin | 52.7 (9.4) * | (59.7) | |||||
| Janjigian, 2021 [44] | Multiple | III | Open label | 1581 | Nivolumab-capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 62 (54–69) | 540 (68) |
| Capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 61 (53–68) | 560 (71) | |||||
| Kang, 2009 [45] | Multiple | III | Open label | 316 | Cisplatin-capecitabine | 56 (26–74) | 103 (64) |
| Cisplatin-fluorouracil | 56 (22–73) | 108 (69) | |||||
| Ochend- uszko, 2015 [46] | Poland | III | NR | 56 | Epirubicin-oxaliplatin-capecitabine | 57.9 (10.8) | 16 (55) |
| Docetaxel-cisplatin-5-fluorouracil-leucovorin | 60.3 (9.11) | 13 (48) | |||||
| Petersen, 2021 [47] | Denmark | II | Open label | 110 | Docetaxel-carboplatin-capecitabine | 64 (36–79) | 79 (81) |
| Epirubicin-oxaliplatin-capecitabine | |||||||
| Roth, 2007 [48] | Multiple | II | 3-arm | 119 | Epirubicin-cisplatin-flurouracil | 59 (32–71) | 75 |
| Docetaxel-cisplatin | 58 (40–70) | 76 | |||||
| Docetaxel-cisplatin-fluorouracil | 61 (35–78) | 61 | |||||
| Shitara, 2020 [49] | Multiple | II | Open label | 763 | Pembrolizumab | 61 (20–83) | 180 (70.3) |
| Pembrolizumab-cisplatin-capecitabine-fluorouracil | 62 (22–83) | 195 (75.9) | |||||
| Placebo-cisplatin-capecitabine-fluorouracil | 62.5 (23–87) | 179 (71.6) | |||||
| Shitara, 2022 [50] | Multiple | III | Open label | 813 | Nivolumab- ipilimumab | 62 (22–84) | 278 (68) |
| Capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 61 (23–90) | 280 (69) | |||||
| Van Cutsem, 2006 [51] | Multiple | III | Open label | 457 | Docetaxel-cisplatin-fluorouracil Cisplatin-fluorouracil | 55 (25–79) | 317 (71) |
| Webb, 1997 [52] | United Kingdom | II | Open label | 256 | Epirubicin-cisplatin-flurouracil | 59 (35–79) | 99 |
| Fluorouracil-doxorubicin-methotrexate | 60 (29–78) | 110 |
| Author, Year | Tumor Location—Stomach Gastric, n (%) | Disease Stage, n (%) | No. of Organs Involved in Metastases, n (%) | ECOG Status n (%) | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Locally Advanced | Metastatic | 1–2 | >2 | 0–1 | ≥2 | |||
| Ajani, 2005 [29] | 106 (68) | 5 (3) | 147 (95) | 84 (61) | 61 (39) | 66 (43) | NR | Complete response, Objective response rate, Overall survival, partial response, Time to progression |
| Ajani, 2007 [30] | NR | 12 (3) | 430 (97) | (53.8) | (45.2) | 80 (36) | NR | Time to 5% deterioration of global health status from baseline |
| (54.9) | (44.6) | 81 (36) | NR | |||||
| Ajani, 2010 [31] | 855 (83.1) | 43 (4.2) | 1085 (95.7) | NR | NR | 1029 (58.6) | NR | Overall survival, Progression-free survival, Response rate |
| Al-Batran, 2008 [32] | 92 (82) | 3 (2.7) | 109 (97.3) | 59 (52.7) | 53 (47.4) | 103 (92) | 9 (8) | Overall survival, Progression-free survival, Response rate |
| 10 (9.3) | 98 (90.7) | 63 (58.3) | 45 (41.7) | 97 (90) | 11 (10) | |||
| Al-Batran, 2013 [33] | 45 (63) | 22 (31) | 50 (69) | Median = 2 | 67 (93) | 5 (7) | Objective response rate | |
| 22 (32) | 49 (68) | 65 (92) | 6 (9) | |||||
| Bouche, 2004 [34] | (71) | NR | NR | (80) | (20) | (73) | (27) | Overall survival, Progression-free survival, Response rate |
| (70) | (85) | (15) | (75) | (25) | ||||
| (67) | (83) | (17) | (78) | (22) | ||||
| Cascinu, 2011 [35] | 69 (89) | 8 (10) | 70 (89) | NR | NR | 73 (93.7) | 5 (6.3) | Objective response rate, Overall survival, Time to progression |
| Catenacci, 2017 [36] | 227 (75) | NR | 284 (93) | NR | NR | 304 (100) | 0 (0) | Duration of response, Overall survival, Progression-free survival, Time to progression |
| 195 (64) | NR | 283 (93) | 304 (100) | 1 (<1) | ||||
| Cocconi, 1994 [37] | NR | NR | NR | NR | NR | NR | 5 (6) | Duration of response, Objective response rate, Overall survival, Time to progression |
| NR | 5 (10) | |||||||
| Coombes, 1994 [38] | NR | NR | NR | NR | NR | NR | NR | Overall survival |
| Curran, 2009 [39] | NR | NR | NR | NR | NR | NR | NR | Quality of life, Time to progression |
| Fuchs, 2019 [40] | 242 (74) | NR | NR | 243 (75) | 81 (25) | 326 (100) | NR | Objective response rate, Overall survival, Progression-free survival, Time to progression |
| 239 (75) | 242 (76) | 76 (24) | 319 (100) | NR | ||||
| Gubanski, 2010 [41] | NR | NR | NR | NR | NR | (88) | (1) | Overall survival, Progression-free survival |
| NR | NR | NR | NR | NR | (99) | (18) | ||
| Högner, 2021 [42] | 21 (41) | NR | NR | 15 (27) | 6 (71) | NR | NR | Objective response rate, Overall survival, Progression-free survival |
| 19 (70) | 13 (48) | 14 (51) | ||||||
| Icli, 1998 [43] | NR | 17.2 | 82.8 | NR | NR | (65.6) | (34.4) | Objective response rate, Overall survival |
| 20.9 | 79.1 | (68.6) | (31.4) | |||||
| Janjigian, 2021 [44] | 544 (70) | 27 (3) | 757 (96) | 164 (21) | 602 (76) | NR | NR | Overall survival, Progression-free survival |
| 556 (70) | 34 (4) | 756 (95) | 183 (23) | 583 (74) | ||||
| Kang, 2009 [45] | NR | NR | NR | 127 (80) | 32 (20) | Median = 1 (0–1) | NR | Duration of response, Objective response rate, Overall survival, Progression-free survival |
| 126 (80) | 27 (20) | |||||||
| Ochend-uszko, 2015 [46] | NR | 1 (4) | 28 (97) | 27 (91) | 2 (7) | 26 (89) | 3 (10) | Overall survival, Progression-free survival |
| 3 (11) | 24 (89) | 23 (85) | 4 (15) | 25 (93) | 2 (7) | |||
| Petersen, 2021 [47] | 25 (26) | 10 (10) | 88 (89) | 54 (55) | 44 (45) | 98 (100) | NR | Overall survival, Progression-free survival |
| Roth, 2007 [48] | NR | NR | (83) | (90) | (9) | NR | NR | Overall survival, Quality of life, Time to progression |
| (82) | (79) | (21) | ||||||
| (95) | (84) | (15) | ||||||
| Shitara, 2020 [49] | 176 (68.8) | NR | NR | NR | NR | NR | NR | NR |
| 170 (66.1) | ||||||||
| 181 (72.4) | ||||||||
| Shitara, 2022 [50] | 282 (69) | 14 (3) | 391 (96) | 83 (20) | 100 (25) | 409 (100) | NR | Duration of response, Overall survival, Progression-free survival, Quality of life |
| 282 (70) | 18 (4) | 386 (96) | 326 (80) | 304 (75) | 404 (100) | NR | ||
| Van Cutsem, 2006 [51] | NR | 12 (3) | 43 (97) | 242 (54) | 200 (45) | 161 (36) | NR | Objective response rate, Overall survival, Time to progression |
| Webb, 1997 [52] | 72 (57) | 47 (37) | 79 (63) | NR | NR | 96 (76) | 30 (24) | Objective response rate, Overall survival, Quality of life |
| 73 (56) | 51 (40) | 79 (60) | 97 (75) | 32 (25) | ||||
| Author, Year | Treatment Arms | n for Each Arm | Overall Survival, Months | Progression Free Survival, Months | Objective Response Rate, % | Other Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | HR (95% CI); p | Median | HR (95% CI); p | Median | p Value | Description | HR (95% CI); p | |||
| Ajani, 2005 [29] | Docetaxel-cisplatin | 76 | 9.6 | 1.19 (0.83–1.69) | NR | NR | 26 | NR | Time to progression: 5.9 months | 0.80 (0.52–1.22) |
| Docetaxel-cisplatin-fluorouracil | 79 | 10.5 | NR | NR | 43 | Time to progression: 5 months | ||||
| Ajani, 2007 [30] | Docetaxel-cisplatin-fluorouracil | 85 | NR | NR | NR | NR | NR | NR | Time to 5% deterioration of global health status from baseline: 6.5 months | 1.45 (1.08–1.93) p = 0.01 |
| Cisplatin-fluorouracil | 104 | NR | NR | NR | NR | NR | NR | Time to 5% deterioration of global health status from baseline: 4.2 months | ||
| Ajani, 2010 [31] | Cisplatin-S1 | 521 | 8.6 | 0.92 (0.80–1.05) 0.20 | 4.8 | 0.99 (0.86–1.14) | 29.0 | NR | Median duration of response: 6.5 months | 0.77 (0.57–1.03) |
| Cisplatin-fluorouracil | 508 | 7.9 | 5.5 | 32.0 | Median duration of response: 5.8 months | |||||
| Al-Batran, 2008 [32] | Fluorouracil-leucovorin-oxaliplatin | 112 | 10.7 (8.5–13.9) | p = 0.51 | 5.8 (4.5–6.6) | NR | 34.8 | NR | NR | NR |
| Fluorouracil-leucovorin-cisplatin | 102 | 8.8 (7.7–12.0) | 3.9 (3.1–4.8) | 24.50 | NR | NR | ||||
| Al-Batran, 2013 [33] | Fluorouracil-leucovorin-oxaliplatin-docetaxel | 72 | NR | NR | NR | NR | 48.6 (36.7–60.7) | p = 0.02 | Quality of life global health status scores at 0, 8, 16 and 24 weeks: 56.5 ± 24.4, 53.6 ± 19.9, 56.8 ± 19.5 and 53.7 ± 22.8 | No significant differences between arms |
| Fluorouracil-leucovorin-oxaliplatin | 71 | NR | NR | NR | NR | 28.2 (18.1–40.1) | Quality of life global health status scores at 0, 8, 16 and 24 weeks: 49.4 ± 24.7, 58.2 ± 19.8, 53.3 ± 21.0 and 55.5 ± 16.9 | |||
| Bouche, 2004 [34] | Leucovorin–5-flurouracil | 45 | 6.8 (2.6–11.1) | NR | 3.2 (1.8–4.6) | NR | 13 (3.4–23.3) | NR | Mean difference in global quality of life scores between Leucovorin–5-flurouracil vs Leucovorin–5-flurouracil-irinotecan = 2.2. Mean difference in global quality of life scores between Leucovorin–5-flurouracil–cisplatin vs Leucovorin–5-flurouracil-irinotecan = 0.8 | Treatment effect: 0.89; p < 0.01 |
| Leucovorin–5-flurouracil–cisplatin | 44 | 9.5 (6.9–12.2) | NR | 4.9 (3.5–6.3) | 27 (14.1–40.4) | |||||
| Leucovorin–5-flurouracil-irinotecan | 45 | 11.3 (9.3–13.3) | NR | 6.9 (5.5–8.3) | 40 (25.7–54.3) | |||||
| Cascinu, 2011 [35] | 5-flurouracil-cisplatin-doxorubicin | 39 | 12.1 | 0.63 (0.34–0.91) p = 0.02 | NR | NR | 64.1 (48–77) | p = 0.04 | Time to progression: 7.9 month | 0.62, (0.37–0.97) p = 0.04 |
| 5-flurouracil-cisplatin-mitomycin | 39 | 8.3 | NR | NR | 39 (24–54) | Time to progression: 5.1 months | ||||
| Catenacci, 2017 [36] | Rilotumumab-epirubicin-cisplatin-capecitabine | 298 | 8.8 (7.7–10.2) | 1.34 (1.10–1.63) p < 0.01) | 5.6 (5.3–5.9) | 1.26 (1.04–1.51) | 29.8 (24.3–35.7) | NR | Time to progression: 6.1 month (95% CI = 5.7–7.9) Duration of response: 2.8 months (IQR = 2.7–2.9) | 1.24 (0.96–1.59) p = 0.10 |
| Placebo-epirubicin-cisplatin-capecitabine | 299 | 10.7 (9.6–12.4) | 6.0 (5.7–7.2) | 44.6 (38.5–50.8) | Time to progression: 7.1 month (5.9–7.9) Duration of response: 2.8 months (2.6–2.9) | |||||
| Cocconi, 1994 [37] | Cisplatin-epirubicin-leucovorin-flurouracil | 85 | 8.1 (0.2–33.5) | p = 0.24 | NR | NR | 43 | p < 0.01 | Time to progression: 4.7 month (0.2–26.5) | p = 0.58 |
| Fluorouracil-doxorubicin-mitomycin | 52 | 5.6 (0.5–49.1) | NR | NR | 15 | 2.6 month (0.5–33.2) | ||||
| Coombes, 1994 [38] | Epirubicin | 36 | 88.2% died | p = 0.65 | NR | NR | NR | NR | NR | NR |
| Fluorouracil | 33 | 3.9% died | NR | NR | NR | NR | NR | NR | ||
| Curran, 2009 [39] | Irinotecan-fluorouracil | 172 | NR | NR | NR | NR | NR | NR | Time to progression: 5 month; Global health status mean 62.4 (20.1) | p = 0.088; p = 0.061 |
| Cisplatin-fluorouracil | 165 | NR | NR | NR | NR | NR | NR | Time to progression: 4.2 months; Global health status mean—56.9 (21.1) | ||
| Fuchs, 2019 [40] | Ramucirumab-flurouracil-cisplatin | 326 | 11.2 (9.9–11.9) | 0.96 (0.80–1.16) p = 0.67 | 5.7 (5.5–6.5) | 0.75, (0.61–0.94) p = 0.01 | 41.1 (35.8–46.4) | p = 0.17 | Time to progression: 6.8 month (5.9–7.7) | p = 0.70 (0.57–0.86) |
| Placebo-flurouracil-cisplatin | 319 | 10.7 (9.5–11.9) | 5.4 (4.5–5.7) | 36.4 (31.1–41.6) | Time to progression: 5.8 months (5.6–6.4) | |||||
| Gubanski, 2010 [41] | Irinotecan-5-fluorouracil-leucovorin | 39 | 11.5 | p = 0.3 | 4.9 | NR | NR | NR | NR | NR |
| Docetaxel-leucovorin | 39 | 10.6 | 5.0 | NR | NR | NR | NR | |||
| Högner, 2022 [42] | Pazopanib-5-fluorouracil-oxaliplatin | 51 | 10.2 (5.5–14.9) | 1.01 (0.62–1.65) p = 0.95 | 4.7 (2.9–6.5) | 0.96 (0.60–1.55) p = 0.88 | 25 | NR | NR | NR |
| Fluorouracil-leucovorin-oxaliplatin | 27 | 7.3 (4.9–9.7) | 4.5 (1.8–7.1) | 26 | NR | NR | ||||
| Icli, 1998 [43] | Etoposide-epirubicin-cisplatin | 64 | 6.0 | p > 0.05 | NR | NR | 20.30 | p = 0.63 | NR | NR |
| 5-fluorouracil-epirubicin-cisplatin | 67 | 5.0 | NR | NR | 15.30 | NR | NR | |||
| Janjigian, 2021 [44] | Nivolumab-capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 789 | 13.1 (6.7–19.1) | 0.71 (0.59–0.86) p < 0.01 | 7.7 (7.0–9.2) | 0.68 (0.56–0.81) p < 0.01 | NR | NR | NR | NR |
| Capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 792 | 11.1 (5.8–16.1) | 6.1 (5.6–6.9) | NR | NR | NR | NR | |||
| Kang, 2009 [45] | Cisplatin-capecitabine | 160 | 10.4 (9.1–11.0) | 0.85, (0.65–1.11) | 5.6 (4.9–7.3) | 0.81, (0.63–1.04) p < 0.01 | 46 (38–55) | 1.80 (1.11–2.94) p = 0.02 | Mean duration of response: 7.6 months | 0.88 (0.56–1.36) p = 0.55 |
| Cisplatin-fluorouracil | 156 | 8.9 (7.3–10.2) | 5.0 (4.2–6.3) | 32 (24–41) | Mean duration of response: 6.2 months | |||||
| Ochenduszko, 2015 [46] | Epirubicin-oxaliplatin-capecitabine | 29 | 9.5 | p = 0.14 | 6.4 | p = 0.44 | NR | NR | NR | NR |
| Docetaxel-cisplatin-5-fluorouracil-leucovorin | 27 | 11.9 | 6.8 | NR | NR | NR | NR | |||
| Petersen, 2021 [47] | Docetaxel-carboplatin-capecitabine | 49 | 9.8 (8.2–11.0) | NR | 6.1 (5.5–7.1) | NR | NR | NR | NR | NR |
| Epirubicin-oxaliplatin-capecitabine | 49 | 10.2 (8.0–11.9) | NR | 5.1 (4.3–7.0) | NR | NR | NR | NR | ||
| Roth, 2007 [48] | Epirubicin-cisplatin-flurouracil | 40 | 8.3 (7.2–13.0) | NR | NR | NR | NR | NR | Time to progression: 4.9 (3.2–6.1) months | NR |
| Docetaxel-cisplatin | 38 | 11 (7.8–12.5) | NR | NR | NR | NR | NR | Time to progression: 3.6 (2.8–4.5) months | ||
| Docetaxel-cisplatin-fluorouracil | 41 | 10.4 (8.3–12.0) | NR | NR | NR | NR | NR | Time to progression: 4.6 (3.5–5.6) months | ||
| Shitara, 2020 [49] | Pembrolizumab | 256 | 10.6 (7.7–13.8) | 0.91 (0.69–1.18) 0.85 (0.70–1.03) | 2.0 (1.5–2.8) | 1.66 (1.37–2.01) 0.84 (0.70–1.02) p = 0.04 | NR | NR | NR | NR |
| Pembrolizumab-cisplatin-capecitabine-fluorouracil | 257 | 12.5 (10.8–13.9) | 6.9 (5.7–7.3) | NR | NR | NR | NR | |||
| Placebo-cisplatin-capecitabine-fluorouracil | 250 | 11.1 (9.2–12.8) | 6.4 (5.7–7.0) | NR | NR | NR | NR | |||
| Shitara, 2022 [50] | Nivolumab- ipilimumab | 409 | 11.2 (9.2–13.4) | 0.89 (0.71–1.10) p = 0.23 | 2.8 (2.6–3.6) | 1.66 (1.40–1.95) | NR | NR | Duration of response: 13.8 months (9.4-17.7) | NR |
| Capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 404 | 11.6 (10.1–12.7) | 7.1 (6.9–8.2) | NR | NR | Duration of response: 6.8 months (5.6-7.2) | NR | |||
| Van Cutsem, 2006 [51] | Docetaxel-cisplatin-fluorouracil | 221 | 9.2 (8.4–10.6) | 1.29 (1.0–1.6) p = 0.02 | NR | NR | 81 (37) | p = 0.01 | Time to progression: 5.6 (4.9–5.9) months | 1.47 (1.19–1.82) |
| Cisplatin-fluorouracil | 224 | 8.6 (7.2–9.5) | NR | NR | 57 (25) | Time to progression: 3.7 (3.4-4.5) months | ||||
| Webb, 1997 [52] | Epirubicin-cisplatin-flurouracil | 111 | 8.9 | NR | NR | NR | 45 (36-54) | p < 0.01 | NR | NR |
| Fluorouracil-doxorubicin-methotrexate | 108 | 5.7 | NR | NR | NR | 21 (13–29) | NR | NR | ||
| Author, Year | Sample Size | Treatment arms | Neutropenia, n (%) | Anemia, n (%) | Thrombocytopenia, n (%) | Leukopenia, n (%) | Nausea, n (%) | Vomiting, n (%) | Diarrhea, n (%) | Fatigue, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ajani, 2005 [29] | 76 | Docetaxel-cisplatin-fluorouracil | 65 (87) | 24 (32) | 1 (1) | 49 (65) | 8 (11) | NR | 4 (5) | NR |
| 79 | Docetaxel-cisplatin | 66 (86) | 22 (29) | 9 (12) | 53 (69) | 16 (20) | NR | 16 (20) | NR | |
| Ajani, 2010 [30,31] | 521 | Cisplatin-S1 | 167 (32) | 107 (21) | 43 (8) | 71 (14) | 39 (8) | 41 (8) | NR | 64 (12) |
| 508 | Cisplatin-fluorouracil | 320 (64) | 105 (21) | 68 (14) | 167 (3) | 49 (10) | 49 (10) | NR | 67 (13) | |
| Al-Batran, 2008 [32] | 112 | Fluorouracil-leucovorin-oxaliplatin | 13 (12) | 3 (3) | 4.5 (4) | 7 (6) | 5 (5) | 3 (3) | 7 (6) | 4 (4) |
| 102 | Fluorouracil-leucovorin-cisplatin | 15 (15) | 7 (7) | 4 (4) | 12 (12) | 9 (9) | 6 (6) | 5 (5) | 7 (7) | |
| Al-Batran, 2013 [33] | 72 | Fluorouracil-leucovorin-oxaliplatin-docetaxel | 38 (53) | 8 (11) | 2 (3) | 21 (29) | 15 (21) | 3 (4) | 6 (8) | 8 (11) |
| 71 | Fluorouracil-leucovorin-oxaliplatin | 9 (13) | 3 (4) | 2 (3) | 4 (6) | 5 (7) | 1 (2) | 1 (2) | 5 (7) | |
| Bouche, 2004 [34] | 45 | Leucovorin–5-flurouracil | (11) | (16) | (2) | NR | (18) | NR | (2) | NR |
| 44 | Leucovorin–5-flurouracil-cisplatin | (61) | (30) | (2) | NR | (23) | NR | (2) | NR | |
| 45 | Leucovorin–5-flurouracil-irinotecan | (40) | (16) | (0) | NR | (9) | NR | (22) | NR | |
| Cascinu, 2011 [35] | 39 | 5-flurouracil-cisplatin-doxorubicin | 6 (15) | 0 (0) | 2 (5) | NR | 1 (3) | NR | 0 (0) | NR |
| 39 | 5-flurouracil-cisplatin-mitomycin-C | 10 (26) | 0 (0) | 8 (21) | NR | 1 (3) | NR | 1 (3) | NR | |
| Catenacci, 2017 [36] | 298 | Rilotumumab-epirubicin- cisplatin-capecitabine | 111 (37) | 97 (33) | NR | NR | 128 (43) | 100 (34) | 61 (20) | 103 (35) |
| 299 | Placebo-epirubicin-cisplatin-capecitabine | 126 (42) | 125 (42) | NR | NR | 153 (51) | 98 (33) | 81 (27) | 100 (33) | |
| Cocconi, 1994 [37] | 85 | Cisplatin-epirubicin-leucovorin-flurouracil | 8 (9) | 1 (1) | 4 | NR | 17 | 10 (37) | 3 (11) | NR |
| 52 | Fluorouracil-doxorubicin-mitomycin | 0 (0) | 1 (2) | 0 | NR | 4 | 2 (8) | 2 (8) | NR | |
| Curran, 2009 [39] | 172 | Irinotecan-fluorouracil | (24.8) | NR | (2) | NR | NR | NR | (22) | NR |
| 165 | Cisplatin-fluorouracil | (51.6) | NR | (12) | NR | NR | NR | (7) | NR | |
| Fuchs, 2019 [40] | 326 | Ramucirumab-fluorouracil-cisplatin | 85 (26) | 39 (12) | 25 (8) | NR | 22 (7) | 21 (7) | 15 (5) | 27 (8) |
| 319 | Placebo- fluorouracil-cisplatin | 85 (27) | 44 (14) | 11 (4) | NR | 26 (8) | 31 (10) | 23 (7) | 25 (9) | |
| Gubanski, 2010 [41] | 39 | Irinotecan-5-fluorouracil-leucovorin | NR | NR | NR | NR | (4) | NR | (5) | (9) |
| 39 | Docetaxel-leucovorin | NR | NR | NR | NR | (12) | NR | (2) | (3) | |
| Högner, 2022 [42] | 51 | Pazopanib-5-fluorouracil-oxaliplatin | 12 (24) | 1 (2) | 1 (2) | 3 (6) | 8 (16) | 3 (6) | 1 (2) | 4 (8) |
| 27 | Fluorouracil-leucovorin-oxaliplatin | 1 (4) | 3 (11) | 3 (11) | NR | NR | 2 (7) | 2 (7) | 1 (4) | |
| Icli, 1998 [43] | 64 | Etoposide-epirubicin-cisplatin | NR | NR | NR | NR | (6.3) | NR | (1.6) | NR |
| 67 | 5-fluorouracil-epirubicin-cisplatin | NR | NR | NR | NR | (9) | NR | (1.5) | NR | |
| Janjigian, 2021 [44] | 789 | Nivolumab-capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 31 (4) | 3 (<1) | 4 (1) | NR | 0 (0) | 2 (<1) | 2 (<1) | 0 (0) |
| 792 | Capecitabine-oxaliplatin or leucovorin-fluorouracil-oxaliplatin | 23 (3) | 1 (<1) | 1 (1) | NR | 0 (0) | 0 (0) | 0 (0) | 19 (1) | |
| Kang, 2009 [45] | 160 | Cisplatin-capecitabine | 25 (16) | NR | NR | 4 (3) | 3 (2) | 11 (7) | 8 (5) | 1 (<1) |
| 156 | Cisplatin- fluorouracil | 29 (19) | NR | NR | 6 (4) | 4 (3) | 13 (8) | 7 (5) | 4 (<3) | |
| Ochenduszko, 2015 [46] | 29 | Epirubicin-oxaliplatin-capecitabine | 21 (72) | 2 (7) | 0 (0) | 2 (7) | 1 (4) | 0 (0) | 1 (4) | 2 (7) |
| 26 | Docetaxel-cisplatin-5-fluorouracil-leucovorin | 13 (50) | 2 (7) | 0 (0) | 3 (12) | 10 (29) | 0 (0) | 1 (4) | 1 (4) | |
| Petersen, 2021 [47] | 49 | Docetaxel-carboplatin-capecitabine | 42 (82) | 3 (6) | NR | NR | 0 (0) | NR | 4 (8) | 3 (6) |
| 49 | Epirubicin-oxaliplatin-capecitabine | 42 (82) | 3 (6) | NR | NR | 0 (0) | NR | 4 (8) | 3 (6) | |
| Roth, 2007 [48] | 40 | Epirubicin-cisplatin-flurouracil | (59) | NR | (3) | NR | NR | NR | (6) | NR |
| 38 | Docetaxel-cisplatin | (76) | NR | (0) | NR | NR | NR | (3) | NR | |
| 41 | Docetaxel-cisplatin-flurouracil | (80) | NR | (3) | NR | NR | NR | (15) | NR | |
| Shitara, 2020 [49] | 254 | Pembrolizumab | 0 (0) | NR | 0 (0) | 0 (0) | 1 (0.4) | 0(0) | 1 (1) | 1 (0) |
| 250 | Pembrolizumab-cisplatin-capecitabine-fluorouracil | 63 (25) | NR | 7 (3) | 7 (3) | 19 (8) | 12(5) | 12 (5) | 19 (8) | |
| 244 | Placebo-cisplatin-capecitabine-fluorouracil | 68 (28) | NR | 6 (3) | 10 (4) | 18 (7) | 14(6) | 14 (6) | 14 (6) | |
| Shitara, 2022 [50] | 403 | Nivolumab- ipilimumab | 1 (<1) | 5 (1) | 1 (<1) | 0 (0) | 6 (1) | 4(1) | 11 (3) | 11 (3) |
| 389 | Capecitabine-oxaliplatin or Leucovorin-fluorouracil-oxaliplatin | 44 (11) | 9 (2) | 7 (2) | 9 (2) | 16 (4) | 11(3) | 15 (4) | 8 (2) | |
| Van Cutsem, 2006 [51] | 221 | Docetaxel-cisplatin-fluorouracil | 181 (82) | 40 (18) | 17 (8) | 144 (65) | 32 (14) | NR | 43 (19) | NR |
| 224 | Cisplatin- fluorouracil | 126 (57) | 57 (26) | 30 (13) | 70 (31) | 38 (17) | NR | 18 (8) | NR | |
| Webb, 1997 [52] | 126 | Epirubicin-cisplatin-flurouracil | NR | (8) | (4) | (36) | (17) | NR | (6) | NR |
| 130 | Fluorouracil-doxorubicin-methotrexate | NR | (10) | (8) | (39) | (5) | NR | (7) | NR |
| Author, Year | Randomization | Deviations from Intended Intervention | Missing Outcome Data | Measurement of Outcome | Selection of Reported Results | Overall |
|---|---|---|---|---|---|---|
| Ajani, 2005 [29] | Low | Some | Some | Low | Low | Some |
| Ajani, 2007 [30] | Low | Low | Some | Low | Low | Some |
| Ajani, 2010 [31] | Low | Low | Some | Low | Low | Some |
| Al-Batran, 2008 [32] | Some | Some | Low | Low | Low | Some |
| Al-Batran, 2013 [33] | Low | Low | Low | Low | Low | Low |
| Bouche, 2004 [34] | Low | Low | Low | Low | Low | Low |
| Cascinu, 2011 [35] | Low | Low | Low | Low | Low | Low |
| Catenacci, 2017 [36] | Low | Low | Low | Low | Low | Low |
| Cocconi, 1994 [37] | Low | Low | Some | Low | Low | Some |
| Coombes, 1994 [38] | Some | Low | Low | Low | Low | Some |
| Deng, 2013 [39] | Low | Low | Low | Low | Low | Low |
| Fuchs, 2019 [40] | Low | Low | Some | Low | Low | Some |
| Gubanski, 2010 [41] | Low | Low | Low | Low | Low | Low |
| Högner, 2022 [42] | Low | Low | Low | Low | Low | Low |
| Icli, 1998 [43] | Low | Low | Some | Low | Low | Some |
| Janjigian, 2021 [44] | Low | Low | Low | Low | Low | Low |
| Kang, 2009 [45] | Low | Some | Some | Low | Low | Some |
| Ochenduszko, 2015 [46] | Low | Low | Low | Low | Low | Low |
| Petersen, 2021 [47] | Low | Some | Some | Low | Low | Some |
| Roth, 2007 [48] | Some | Some | Low | Low | Low | Some |
| Shitara, 2020 [49] | Low | Low | Low | Low | Low | Low |
| Shitara, 2022 [50] | Low | Low | Low | Low | Low | Low |
| Van Cutsem, 2006 [51] | Low | Low | Some | Low | Low | Some |
| Webb, 1997 [52] | Some | Low | Low | Low | Some | Some |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marupuru, S.; Arku, D.; Axon, D.R.; Villa-Zapata, L.; Yaghoubi, M.; Slack, M.K.; Warholak, T. First-Line Systemic Therapy Outcomes in Western Population with Locally Advanced and Metastatic Gastric Cancer—A Systematic Review. Gastroenterol. Insights 2023, 14, 515-537. https://doi.org/10.3390/gastroent14040037
Marupuru S, Arku D, Axon DR, Villa-Zapata L, Yaghoubi M, Slack MK, Warholak T. First-Line Systemic Therapy Outcomes in Western Population with Locally Advanced and Metastatic Gastric Cancer—A Systematic Review. Gastroenterology Insights. 2023; 14(4):515-537. https://doi.org/10.3390/gastroent14040037
Chicago/Turabian StyleMarupuru, Srujitha, Daniel Arku, David R. Axon, Lorenzo Villa-Zapata, Mohsen Yaghoubi, Marion K. Slack, and Terri Warholak. 2023. "First-Line Systemic Therapy Outcomes in Western Population with Locally Advanced and Metastatic Gastric Cancer—A Systematic Review" Gastroenterology Insights 14, no. 4: 515-537. https://doi.org/10.3390/gastroent14040037
APA StyleMarupuru, S., Arku, D., Axon, D. R., Villa-Zapata, L., Yaghoubi, M., Slack, M. K., & Warholak, T. (2023). First-Line Systemic Therapy Outcomes in Western Population with Locally Advanced and Metastatic Gastric Cancer—A Systematic Review. Gastroenterology Insights, 14(4), 515-537. https://doi.org/10.3390/gastroent14040037







