Elevated Incidence and Risk of Emergent Cirrhosis Complications in Alcoholic Cirrhosis Compared with Other Etiologies
Abstract
:1. Introduction
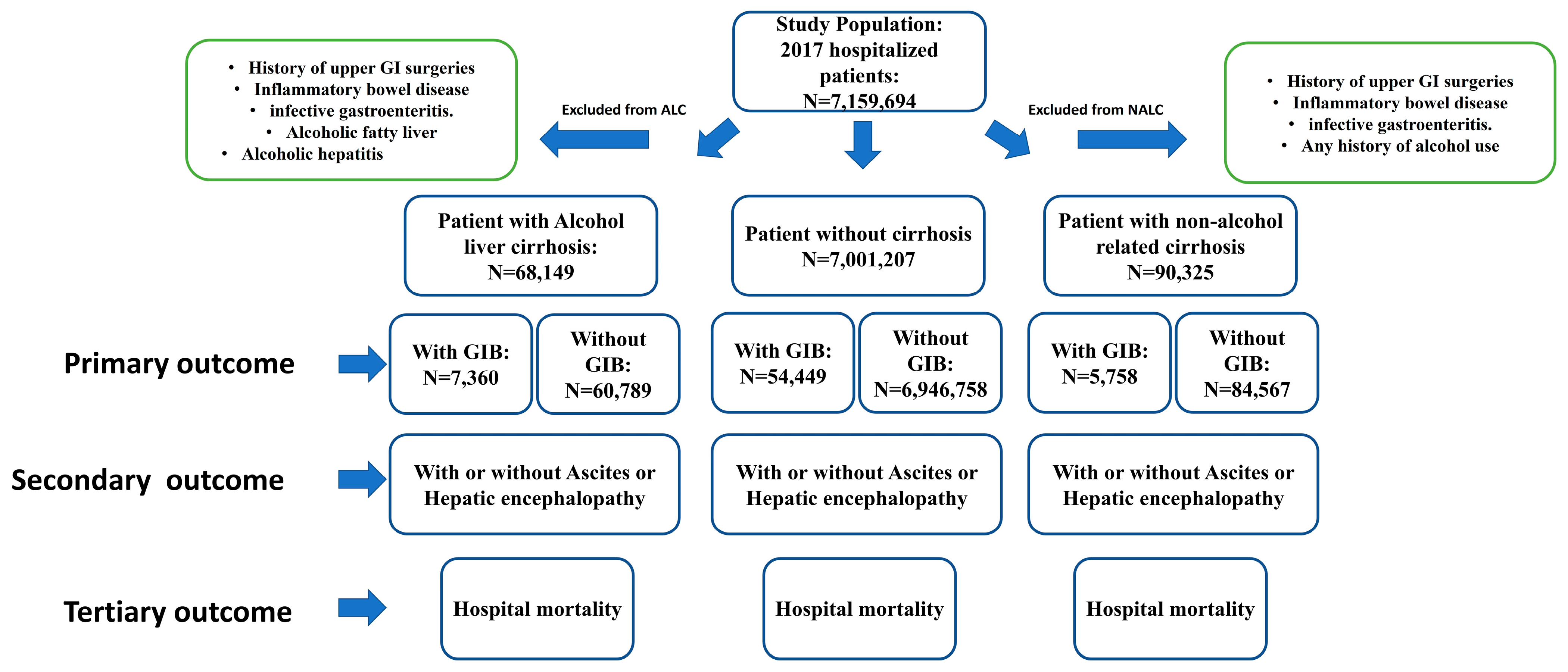
2. Materials and Methods
2.1. Database
2.2. Data Collection and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Demographic and Medical Profiles across Various Groups
3.2. Complications of Cirrhosis across Various Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuppan, D.; Afdhal, N.H. Liver Cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver Cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, S.; Kliethermes, S.; Cao, G.; Shoham, D.; Durazo, R.; Luke, A.; Volk, M.L. The Epidemiology of Cirrhosis in the United States: A Population-Based Study. J. Clin. Gastroenterol. 2015, 49, 690–696. [Google Scholar] [CrossRef]
- Termeie, O.; Fiedler, L.; Martinez, L.; Foster, J.; Perumareddi, P.; Levine, R.S.; Hennekens, C.H. Alarming Trends: Mortality from Alcoholic Cirrhosis in the United States. Am. J. Med. 2022, 135, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Prevention and Management of Gastroesophageal Varices and Variceal Hemorrhage in Cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef]
- Moledina, S.M.; Komba, E. Risk Factors for Mortality among Patients Admitted with Upper Gastrointestinal Bleeding at a Tertiary Hospital: A Prospective Cohort Study. BMC Gastroenterol. 2017, 17, 165. [Google Scholar] [CrossRef]
- Carbonell, N.; Pauwels, A.; Serfaty, L.; Fourdan, O.; Lévy, V.G.; Poupon, R. Improved Survival after Variceal Bleeding in Patients with Cirrhosis over the Past Two Decades. Hepatology 2004, 40, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Hilzenrat, N.; Sherker, A.H. Esophageal Varices: Pathophysiology, Approach, and Clinical Dilemmas. Int. J. Hepatol. 2012, 2012, 795063. [Google Scholar] [CrossRef]
- Jakab, S.S.; Garcia-Tsao, G. Screening and Surveillance of Varices in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 26–29. [Google Scholar] [CrossRef]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global Epidemiology of Cirrhosis—Aetiology, Trends and Predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic Liver Disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef]
- Sepanlou, S.G.; Safiri, S.; Bisignano, S.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The Global, Regional, and National Burden of Cirrhosis by Cause in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Hashimoto, E.; Tokushige, K. Prevalence, Gender, Ethnic Variations, and Prognosis of Nash. J. Gastroenterol. 2011, 46 (Suppl. 1), 63–69. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Gi Epidemiology: Nonalcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2007, 25, 883–889. [Google Scholar] [CrossRef]
- Zaman, A.; Becker, T.; Lapidus, J.; Benner, K. Risk Factors for the Presence of Varices in Cirrhotic Patients without a History of Variceal Hemorrhage. Arch. Intern. Med. 2001, 161, 2564–2570. [Google Scholar] [CrossRef]
- D’Amico, G.; Morabito, A. Noninvasive Markers of Esophageal Varices: Another Round, Not the Last. Hepatology 2004, 39, 30–34. [Google Scholar] [CrossRef]
- Cerqueira, R.M.; Andrade, L.; Correia, M.R.; Fernandes, C.D.; Manso, M.C. Risk Factors for in-Hospital Mortality in Cirrhotic Patients with Oesophageal Variceal Bleeding. Eur. J. Gastroenterol. Hepatol. 2012, 24, 551–557. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; De Franchis, R. Upper Digestive Bleeding in Cirrhosis. Post-Therapeutic Outcome and Prognostic Indicators. Hepatology 2003, 38, 599–612. [Google Scholar] [CrossRef]
- Garceau, A.J.; Chalmers, T.C. The Natural History of Cirrhosis. N. Engl. J. Med. 1963, 268, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Conn, H.O.; Lindenmuth, W.W.; May, C.J.; Ramsby, G.R. Prophylactic Portacaval Anastomosis. Medicine 1972, 51, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Elatty, E.A.A.; Elshayeb, E.I.; Badr, M.H.; Mousa, W.A.E.; El Mansory, M.F. Noninvasive Parameters for Assessment of Esophageal Varices. Egypt. J. Intern. Med. 2019, 31, 536–543. [Google Scholar] [CrossRef]
- Cherian, J.V.; Deepak, N.; Ponnusamy, R.P.; Somasundaram, A.; Jayanthi, V. Non-Invasive Predictors of Esophageal Varices. Saudi J. Gastroenterol. 2011, 17, 64–68. [Google Scholar]
- Zhao, Y.; Ren, M.; Lu, G.; Lu, X.; Yin, Y.; Zhang, D.; Wang, X.; Ma, W.; Li, Y.; Cai, G.; et al. The Prognosis Analysis of Liver Cirrhosis with Acute Variceal Bleeding and Validation of Current Prognostic Models: A Large Scale Retrospective Cohort Study. BioMed Res. Int. 2020, 2020, 7372868. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Fontana, R.J.; Di Bisceglie, A.M.; Everhart, J.E.; Doherty, M.C.; Everson, G.T.; Donovan, J.A.; Malet, P.F.; Mehta, S.; Sheikh, M.Y.; et al. The Prevalence and Risk Factors Associated with Esophageal Varices in Subjects with Hepatitis C and Advanced Fibrosis. Gastrointest. Endosc. 2006, 64, 855–864. [Google Scholar] [CrossRef]
- Beazell, J.M.; Ivy, A.C. The Influence of Alcohol on the Digestive Tract; a Review. Q. J. Stud. Alcohol 1940, 1, 45–73. [Google Scholar] [CrossRef]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol Res. 2017, 38, 163–171. [Google Scholar]
- Pan, J.; Li, C.; Chen, W.; Yu, C.; Li, Y.; Shen, Z. Alcohol Consumption and the Risk of Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. Alcohol Alcohol. 2018, 54, 62–69. [Google Scholar] [CrossRef]
- Tanaka, A.; Cui, R.; Kitamura, A.; Liu, K.; Imano, H.; Yamagishi, K.; Kiyama, M.; Okada, T.; Iso, H. Heavy Alcohol Consumption Is Associated with Impaired Endothelial Function. J. Atheroscler. Thromb. 2016, 23, 1047–1054. [Google Scholar] [CrossRef]
- Brizzolara, A.L.; Morris, D.G.; Burnstock, G. Ethanol Affects Sympathetic Cotransmission and Endothelium-Dependent Relaxation in the Rat. Eur. J. Pharmacol. 1994, 254, 175–181. [Google Scholar] [CrossRef]
- Kelm, M.; Preik, M.; Hafner, D.J.; Strauer, B.E. Evidence for a Multifactorial Process Involved in the Impaired Flow Response to Nitric Oxide in Hypertensive Patients with Endothelial Dysfunction. Hypertension 1996, 27, 346–353. [Google Scholar] [CrossRef]
- Moore, C.M.; Van Thiel, D.H. Cirrhotic Ascites Review: Pathophysiology, Diagnosis and Management. World J. Hepatol. 2013, 5, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V. Pathophysiology, Diagnosis and Treatment of Ascites in Cirrhosis. Ann. Hepatol. 2002, 1, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Kamath, P.S.; Jalan, R.; Ginès, P.; Nevens, F.; Fernández, J.; To, U.; García-Tsao, G.; Schnabl, B. Acute-on-Chronic Liver Failure in Cirrhosis. Nat. Rev. Dis. Prim. 2016, 2, 16041. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Bode, J.C. Alcohol’s Role in Gastrointestinal Tract Disorders. Alcohol Health Res. World 1997, 21, 76–83. [Google Scholar] [PubMed]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.A.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, Intestinal Bacterial Growth, Intestinal Permeability to Endotoxin, and Medical Consequences: Summary of a Symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of Alcohol on Intestinal Epithelial Barrier Permeability and Expression of Tight Junction-Associated Proteins. Mol. Med. Rep. 2014, 9, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Elwir, S.; Rahimi, R.S. Hepatic Encephalopathy: An Update on the Pathophysiology and Therapeutic Options. J. Clin. Transl. Hepatol. 2017, 5, 142–151. [Google Scholar] [CrossRef]
- Ferenci, P. Hepatic Encephalopathy. Gastroenterol. Rep. 2017, 5, 138–147. [Google Scholar] [CrossRef]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol Res. 2015, 37, 223–236. [Google Scholar]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic Microbiome Is Altered in Alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Keshavarzian, A.; Engen, P.; Forsyth, C.B.; Sikaroodi, M.; Gillevet, P. Intestinal Dysbiosis: A Possible Mechanism of Alcohol-Induced Endotoxemia and Alcoholic Steatohepatitis in Rats. Alcohol Clin. Exp. Res. 2009, 33, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
| Alcohol Liver Cirrhosis | Nonalcohol Liver Cirrhosis | p-Value | |
|---|---|---|---|
| Age | 55.8 ± 0.04 | 62.7 ± 0.08 | <0.05 |
| Sex | |||
| Female | 20,045 (29.4%) | 44,746 (49.5%) | <0.01 |
| Male | 48,104 (70.6%) | 45,583 (50.5%) | <0.01 |
| Race | |||
| White | 44,039 (66.6%) | 58,611 (66.6%) | >0.05 |
| Black | 6410 (9.7%) | 9669 (11.0%) | <0.05 |
| Hispanic | 11,415 (17.3%) | 13,922 (15.8%) | <0.05 |
| Asian | 816 (1.2%) | 2239 (2.5%) | <0.05 |
| Hospital event | |||
| LOS (days) | 6.28 ± 0.03 | 5.89 ± 0.03 | <0.05 |
| TC (USD) | USD 68,743 ± 427 | 67,380 ± 385 | <0.05 |
| ER visit (%) | 79.6% | 74.7% | <0.05 |
| EGD/CASE | 9.2% | 5.4% | <0.01 |
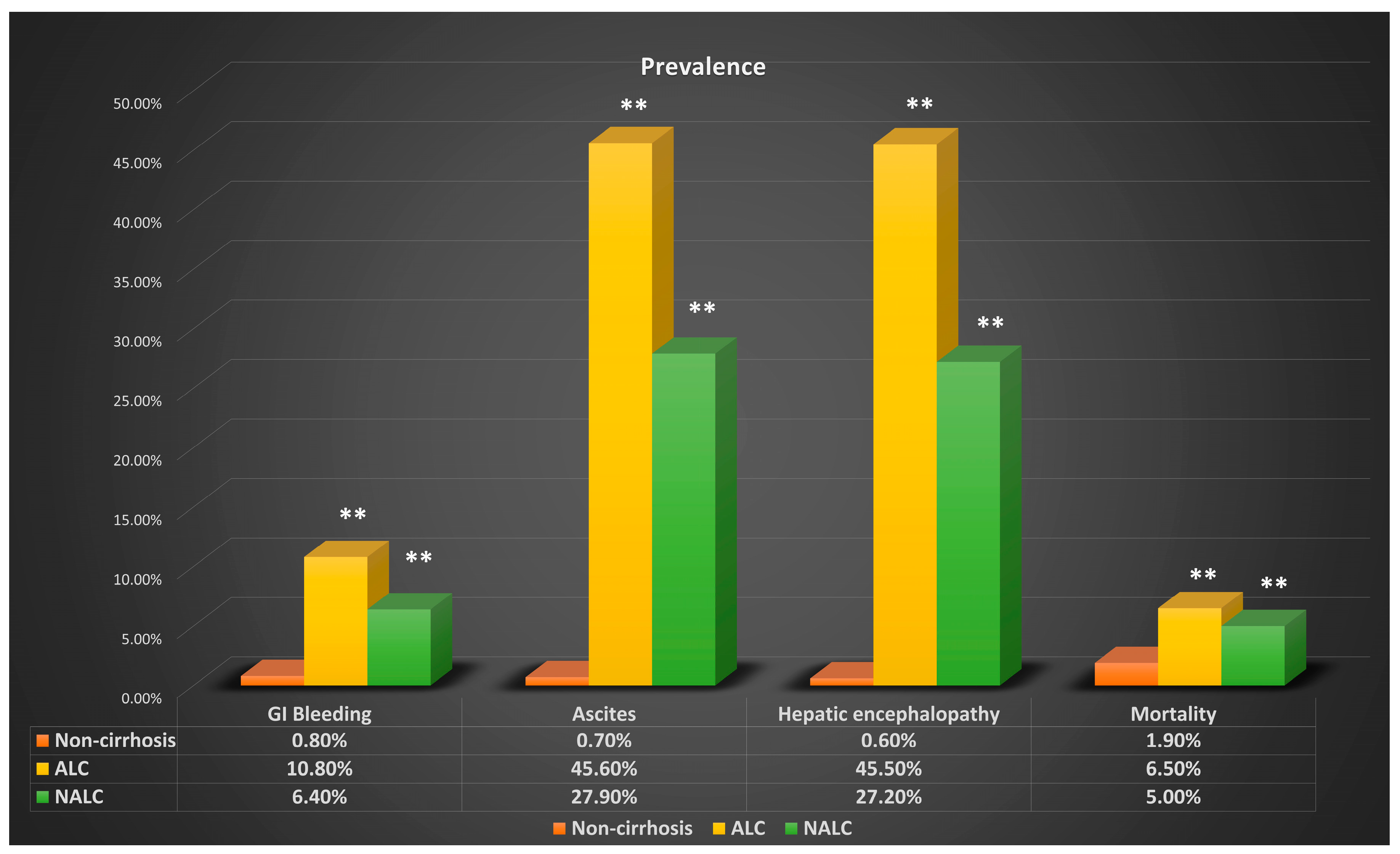
| Gastrointestinal Bleeding | ||||||
| Case | Prevalence | OR | p-Value | Adjusted OR | p-Value | |
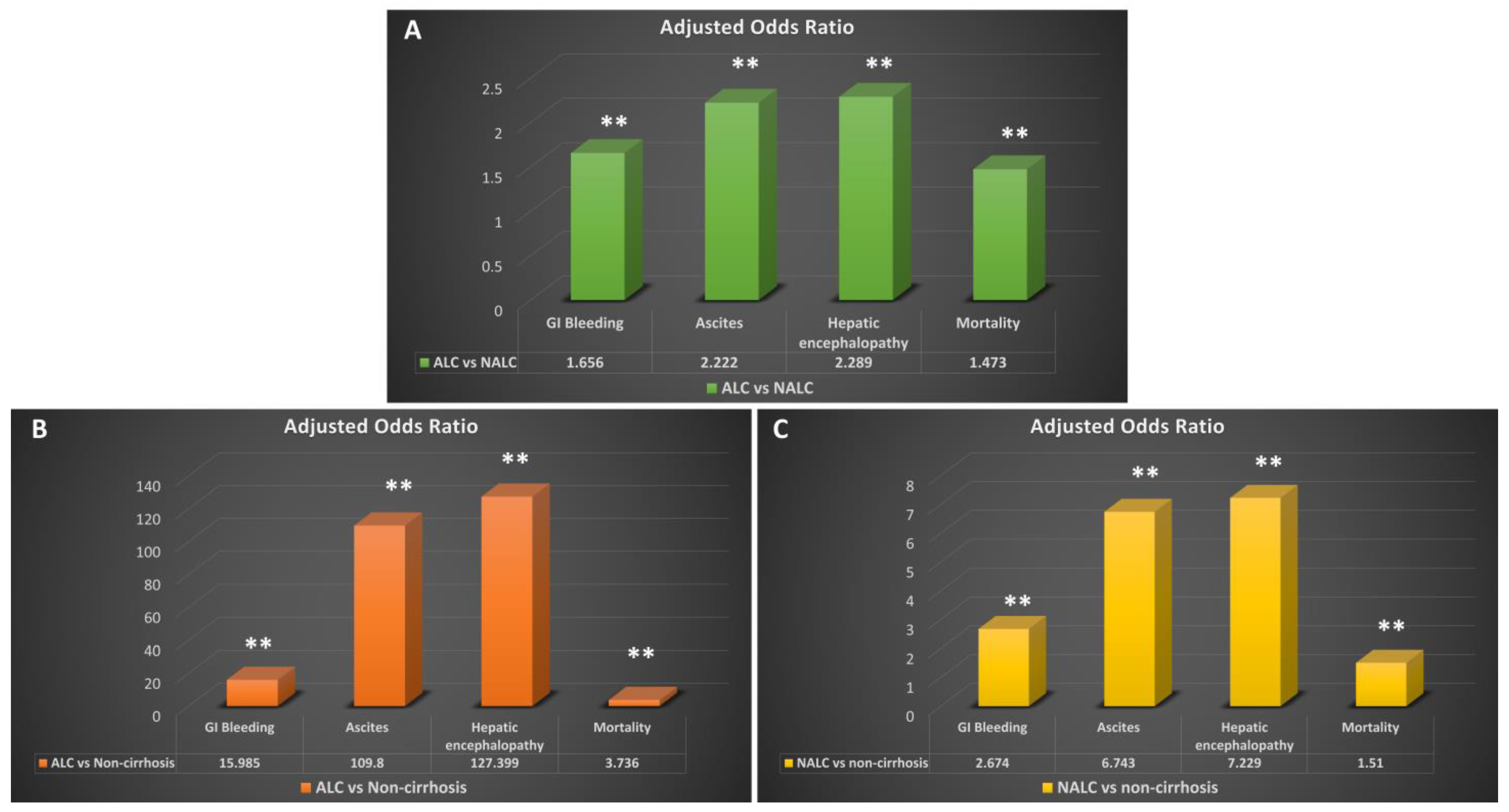
| ALC | 7360 | 10.80% | 15.446 | <0.01 | 15.985 | <0.01 |
| NALC | 5758 | 6.40% | 2.947 | <0.01 | 2.674 | <0.01 |
| NC | 54,449 | 0.80% | ||||
| ALC vs. NALC | 1.779 | <0.01 | 1.656 | <0.01 | ||
| Hepatic Encephalopathy | ||||||
| Case | Prevalence | OR | p-Value | Adjusted OR | p-Value | |
| ALC | 31,040 | 45.50% | 135.817 | <0.01 | 127.399 | <0.01 |
| NALC | 24,569 | 27.20% | 7.789 | <0.01 | 7.229 | <0.01 |
| NC | 42,851 | 0.60% | ||||
| ALC vs. NALC | 2.237 | <0.01 | 2.289 | <0.01 | ||
| Ascites | ||||||
| Case | Prevalence | OR | p-Value | Adjusted OR | p-Value | |
| ALC | 31,089 | 45.60% | 114.7 | <0.01 | 109.8 | <0.01 |
| NALC | 25,200 | 27.90% | 7.247 | <0.01 | 6.743 | <0.01 |
| NC | 50,828 | 0.70% | ||||
| ALC vs. NALC | 2.169 | <0.01 | 2.222 | <0.01 | ||
| Mortality | ||||||
| Case | Prevalence | OR | p-Value | Adjusted OR | p-Value | |
| ALC | 4400 | 6.50% | 3.626 | <0.01 | 3.736 | <0.01 |
| NALC | 4524 | 5.00% | 1.664 | <0.01 | 1.51 | <0.01 |
| NC | 130,814 | 1.90% | ||||
| ALC vs. NALC | 1.308 | <0.01 | 1.473 | <0.01 |
| Gastrointestinal Bleeding | ||||||
|---|---|---|---|---|---|---|
| Case | Prevalence | OR | p-Value | Adjusted OR | p-Value | |
| NASH | 1216 | 6.3% | 2.813 | <0.01 | 2.663 | <0.01 |
| BILI C. | 164 | 5.2% | 2.449 | <0.01 | 2.275 | <0.01 |
| OTHER C. | 5451 | 6.8% | 3.031 | <0.01 | 2.727 | <0.01 |
| ALC vs. NASH | 1.818 | <0.01 | 1.589 | <0.01 | ||
| ALC vs. BILI C. | 2.267 | <0.01 | 1.821 | <0.01 | ||
| ALC vs. OTHER C. | 1.669 | <0.01 | 1.538 | <0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Collins, D.; Dague, A.; Wright, Z.; Wang, J.; Frandah, W.M. Elevated Incidence and Risk of Emergent Cirrhosis Complications in Alcoholic Cirrhosis Compared with Other Etiologies. Gastroenterol. Insights 2023, 14, 671-681. https://doi.org/10.3390/gastroent14040045
Wang X, Collins D, Dague A, Wright Z, Wang J, Frandah WM. Elevated Incidence and Risk of Emergent Cirrhosis Complications in Alcoholic Cirrhosis Compared with Other Etiologies. Gastroenterology Insights. 2023; 14(4):671-681. https://doi.org/10.3390/gastroent14040045
Chicago/Turabian StyleWang, Xiaoliang, Dominic Collins, Alex Dague, Zachary Wright, Jiayan Wang, and Wesam M. Frandah. 2023. "Elevated Incidence and Risk of Emergent Cirrhosis Complications in Alcoholic Cirrhosis Compared with Other Etiologies" Gastroenterology Insights 14, no. 4: 671-681. https://doi.org/10.3390/gastroent14040045
APA StyleWang, X., Collins, D., Dague, A., Wright, Z., Wang, J., & Frandah, W. M. (2023). Elevated Incidence and Risk of Emergent Cirrhosis Complications in Alcoholic Cirrhosis Compared with Other Etiologies. Gastroenterology Insights, 14(4), 671-681. https://doi.org/10.3390/gastroent14040045







