The Role of Macrophage Polarization-Associated Gene Expression in the Oncological Prognosis of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Human Samples
2.2. Identification and Enrichment of DEGs
2.3. Construction and Verification of Prognostic Signature
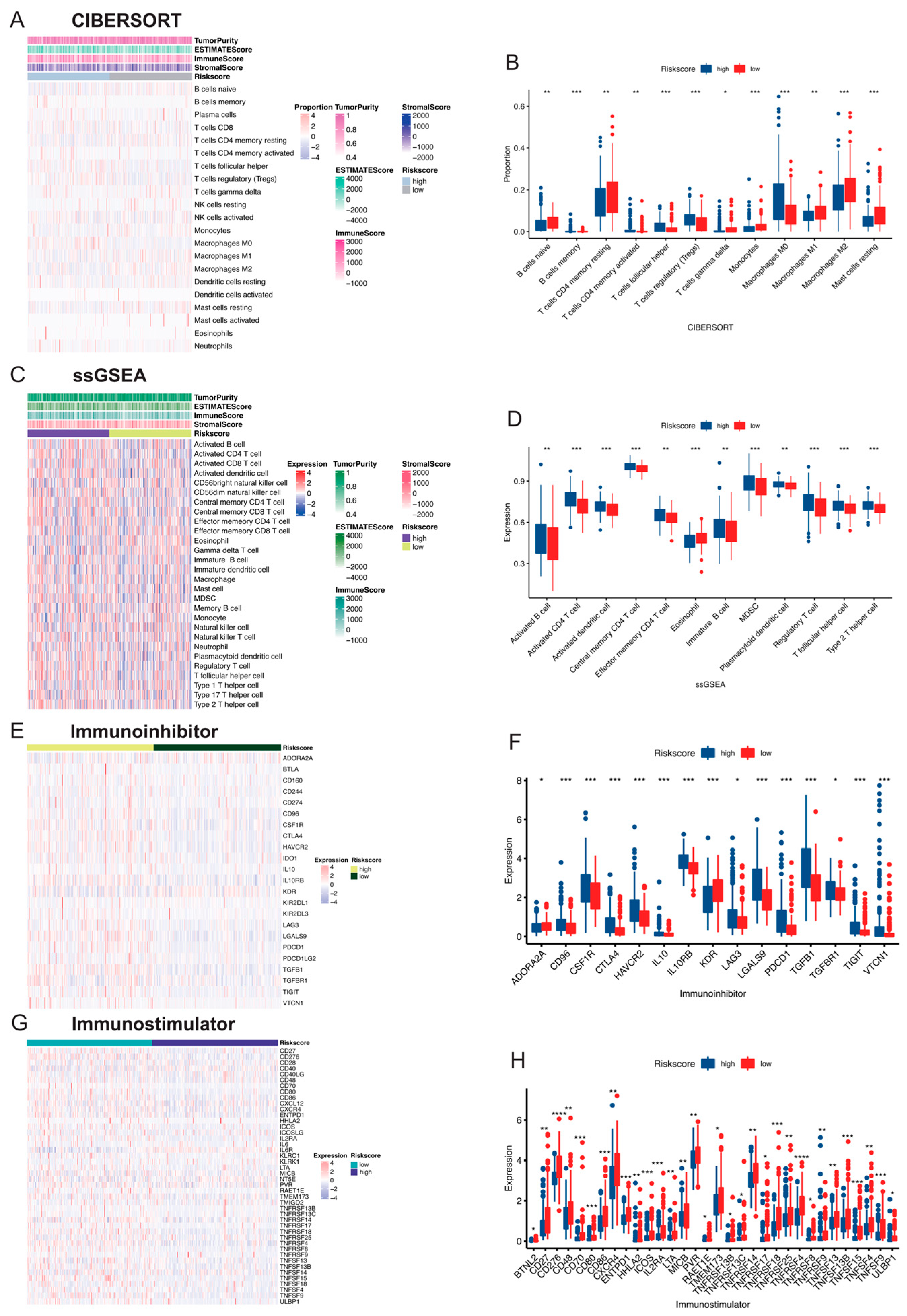
2.4. Risk Score, Clinical Characteristics, and Immune Infiltration
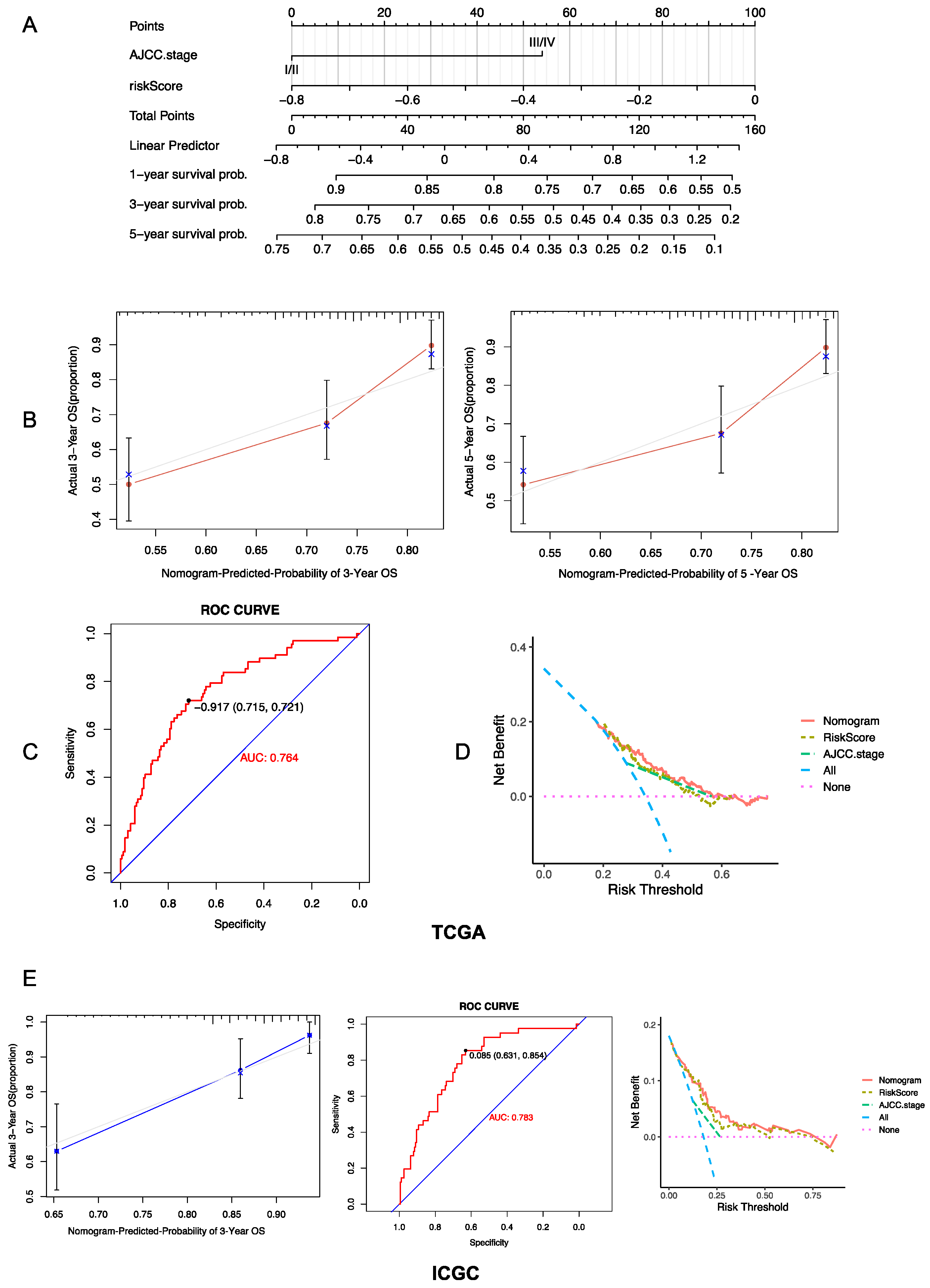
2.5. Establishment of a Survival Prediction Nomogram
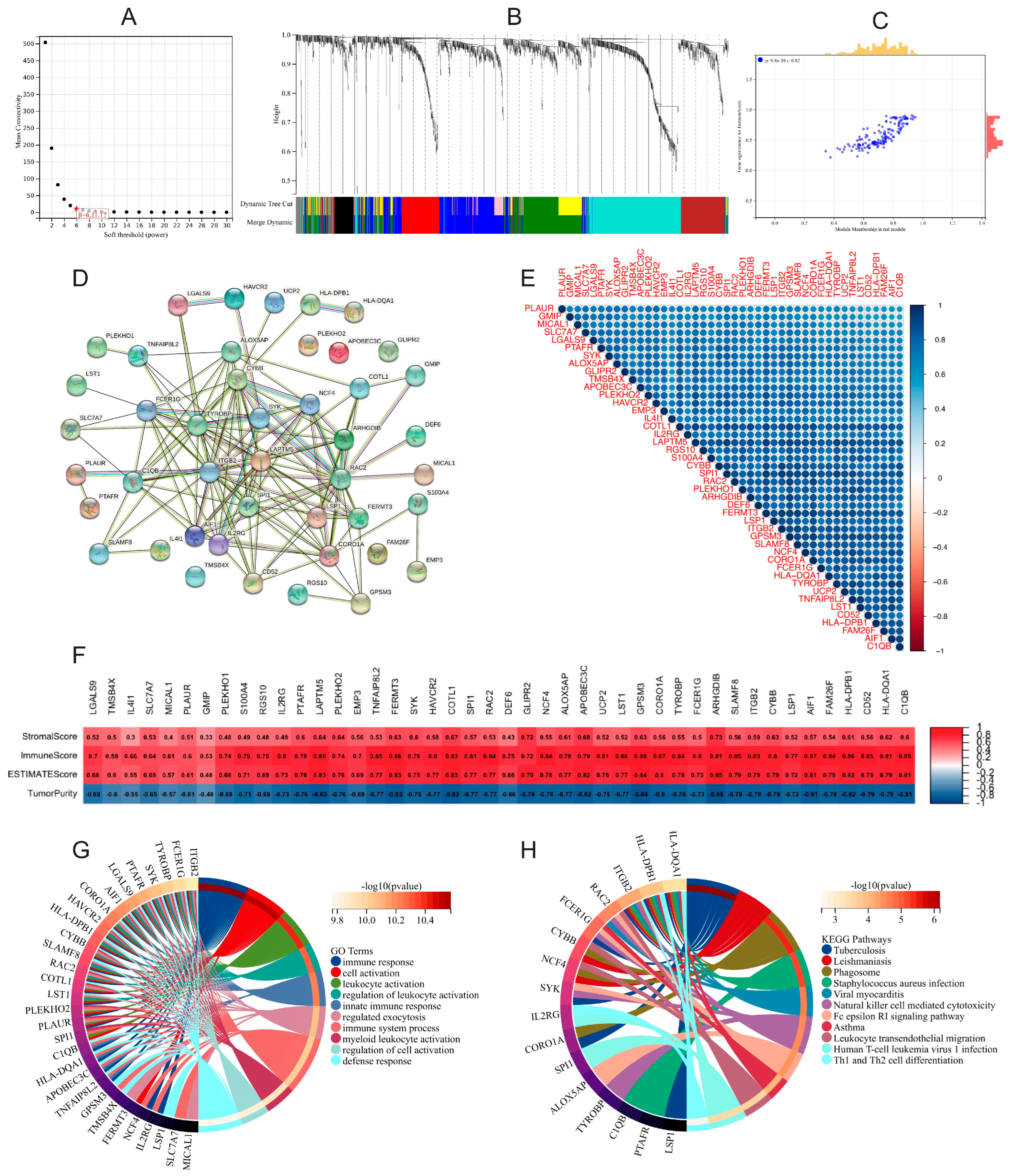
2.6. Weighted Gene Co-Expression Network Analysis
2.7. The Human Protein Atlas
2.8. Western Blotting and Antibodies
2.9. Reverse Transcription Quantitative-Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results
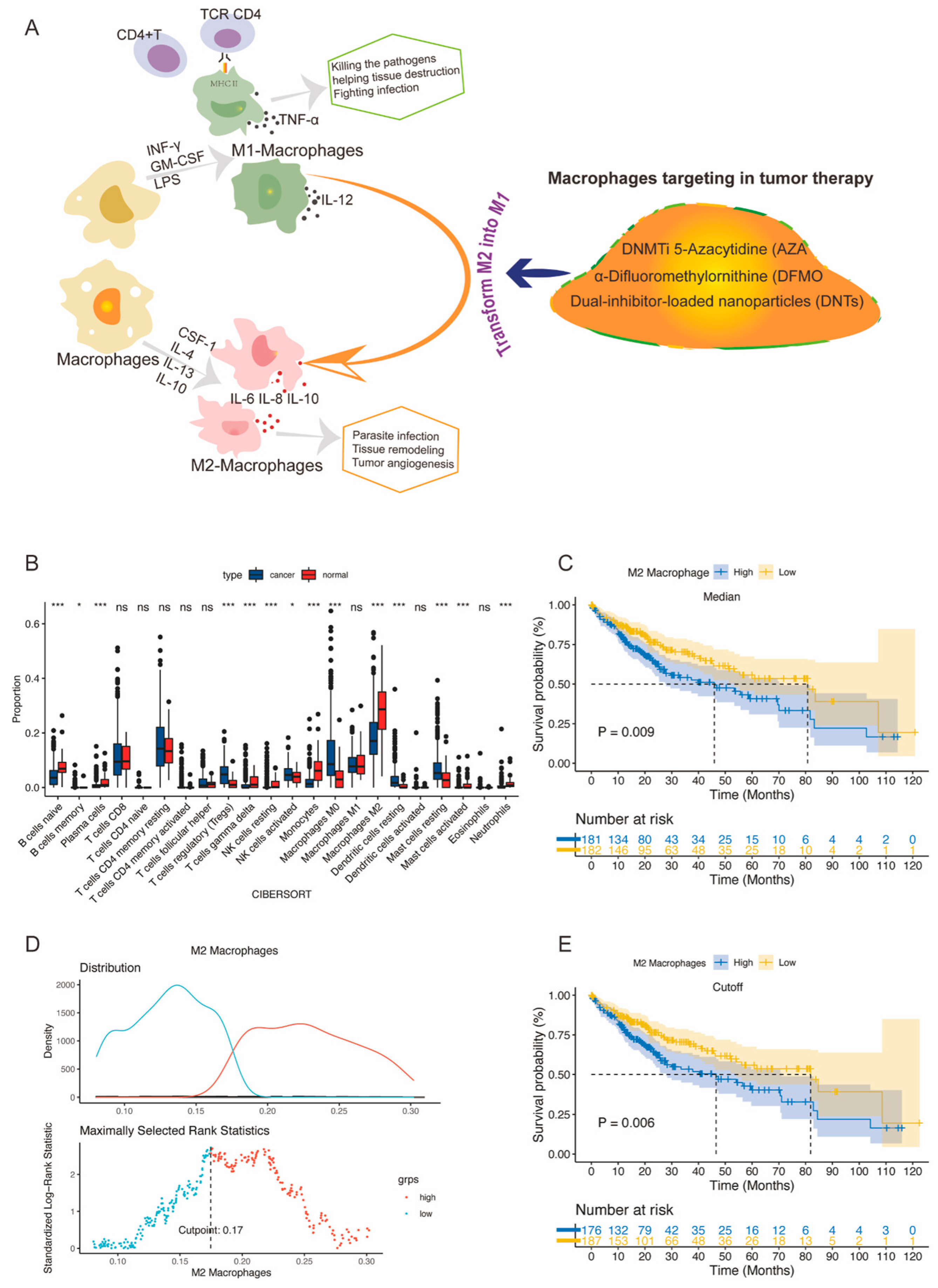
3.1. Analyses of the Immune Cell Infiltration and Prognostic Significance of M2 Macrophages
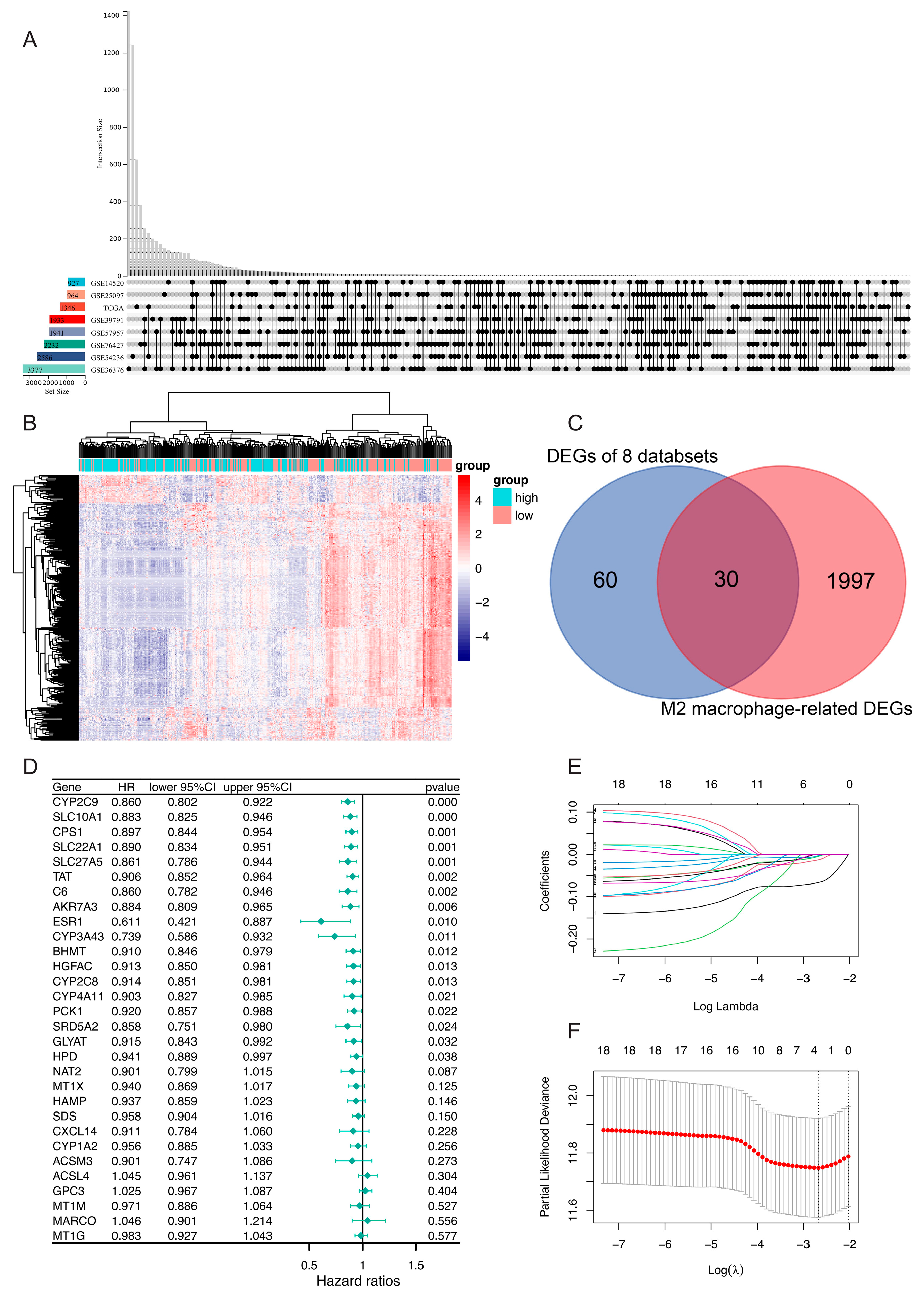
3.2. Identification of DEGs and Establishment of a Prognostic Risk Model
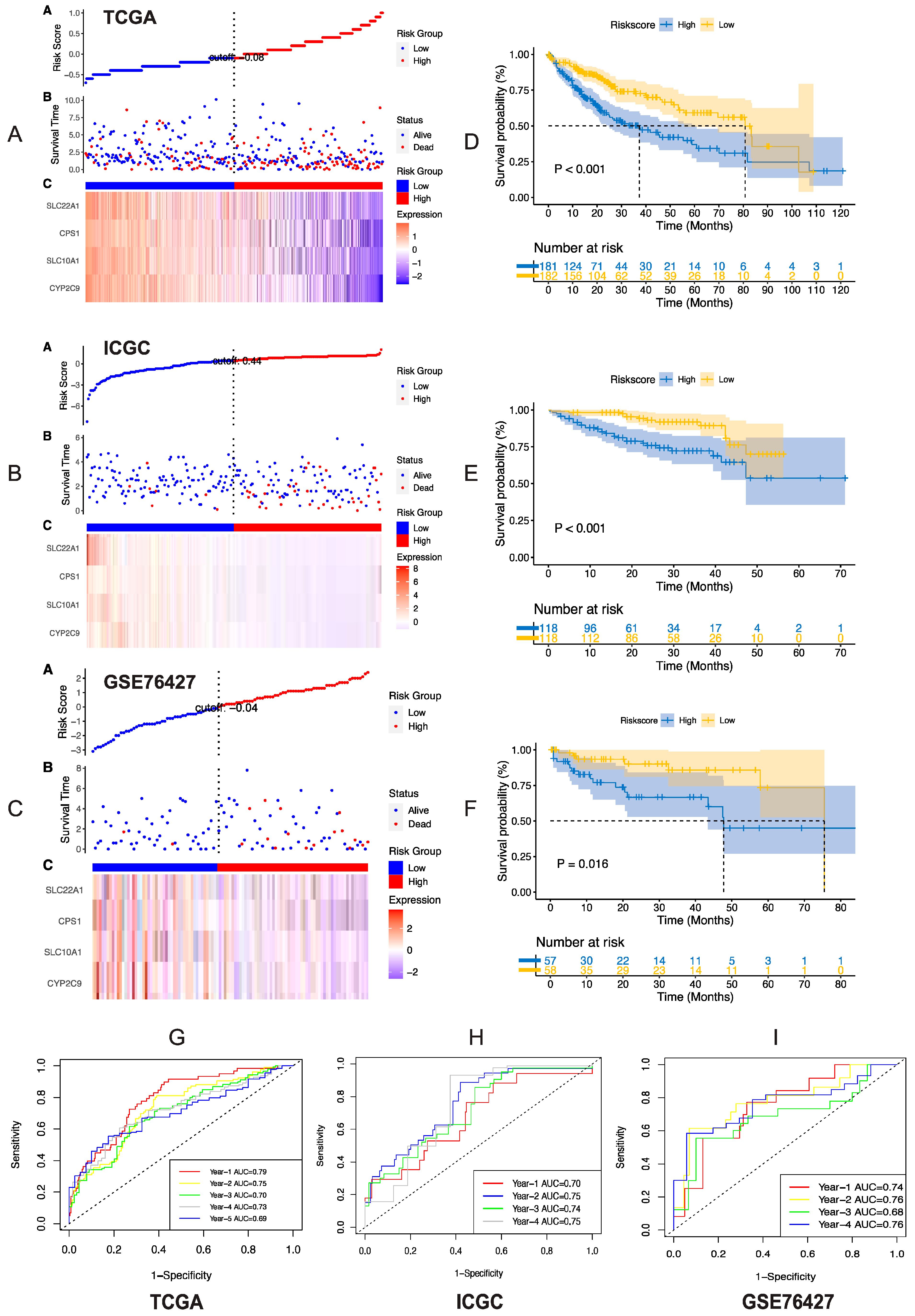
3.3. Validation of Risk Model
3.4. Establishment and Evaluation of a Prognostic Risk Score-Based Nomogram
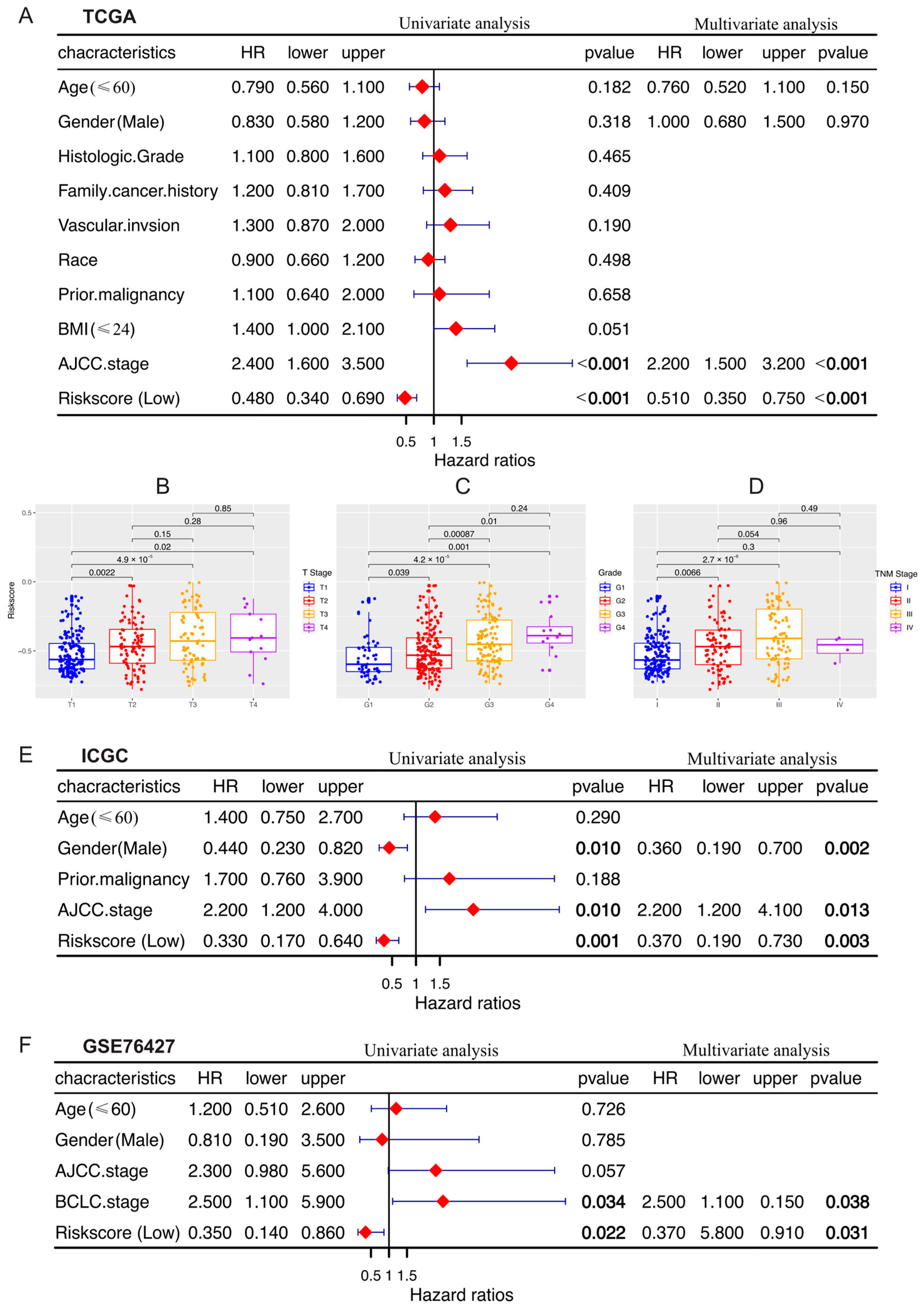
3.5. Comparison of Immune Activity between Risk Score Subgroups
3.6. Functional Enrichment and WGCNA of DEGs in High- and Low-Risk-Score Groups
3.7. Expression and Prognostic Role of 4 M2 Macrophage-Related Genes
3.8. The mRNA and Protein Expression Patterns of the Four M2 Macrophage-Related Genes in HCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | The American Joint Committee on Cancer |
| BMI | Body mass index |
| BrMC | 8-Bromo-7-methoxychrysin |
| CPS1 | Carbamoyl-Phosphate Synthase 1 |
| CYP2C9 | Cytochrome P450 Family 2 Subfamily C Member 9 |
| CSF-1 | Colony-stimulating factor-1 |
| DEGs | Differentially expressed genes |
| HCC | Hepatocellular carcinoma |
| ICGC | International Cancer Genome Consortium |
| IFN-γ | Interferon-gamma |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| GS | Gene significance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LASSO | Least absolute shrinkage and selection operator |
| LPS | Lipopolysaccharide |
| MM | Module membership |
| OS | Overall survival |
| PPI | Protein–protein interaction |
| ROC | Receiver operating characteristic |
| RT | Room temperature |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| SLC10A1 | Solute Carrier Family 11 Member 1 |
| SLC22A1 | Solute Carrier Family 22 Member 1 |
| TAMs | Tumor-associated macrophages |
| TCGA | The Cancer Genome Atlas |
| TILs | Tumor-infiltrating lymphocytes |
| Treg | Regulatory T cells |
| WGCNA | Weighted gene co-expression network analysis |
References
- Duran, S.R.; Jaquiss, R.D.B. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 381, e2. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e820. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Sprinzl, M.F.; Galle, P.R. Current progress in immunotherapy of hepatocellular carcinoma. J. Hepatol. 2017, 66, 482–484. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nature reviews. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Degroote, H.; Van Dierendonck, A.; Geerts, A.; Van Vlierberghe, H.; Devisscher, L. Preclinical and Clinical Therapeutic Strategies Affecting Tumor-Associated Macrophages in Hepatocellular Carcinoma. J. Immunol. Res. 2018, 2018, 7819520. [Google Scholar] [CrossRef]
- Fu, X.T.; Dai, Z.; Song, K.; Zhang, Z.J.; Zhou, Z.J.; Zhou, S.L.; Zhao, Y.M.; Xiao, Y.S.; Sun, Q.M.; Ding, Z.B.; et al. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int. J. Oncol. 2015, 46, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [CrossRef]
- Heusinkveld, M.; van der Burg, S.H. Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med. 2011, 9, 216. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Pomaznoy, M.; Ha, B.; Peters, B. GOnet: A tool for interactive Gene Ontology analysis. BMC Bioinform. 2018, 19, 470. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. The lasso method for variable selection in the Cox model. Stat. Med. 1997, 16, 385–395. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Q.; Huang, Y.; Fan, Z.; Qin, W.; Liu, B.; Yuan, X. Integrative Analysis Constructs an Extracellular Matrix-Associated Gene Signature for the Prediction of Survival and Tumor Immunity in Lung Adenocarcinoma. Front. Cell Dev. Biol. 2022, 10, 835043. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, Y.; Xu, J.; Li, J.; Liu, H.; Liu, N. Bioinformatics screening the novel and promising targets of curcumin in hepatocellular carcinoma chemotherapy and prognosis. BMC Complement. Med. Ther. 2022, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Fang, S.; Weng, Q.; Lv, X.; Meng, M.; Zhu, J.; Zheng, L.; Hu, Y.; Gao, Y.; Wu, X.; et al. Integrated analysis reveals critical glycolytic regulators in hepatocellular carcinoma. Cell Commun. Signal 2020, 18, 97. [Google Scholar] [CrossRef]
- Lautem, A.; Heise, M.; Grasel, A.; Hoppe-Lotichius, M.; Weiler, N.; Foltys, D.; Knapstein, J.; Schattenberg, J.M.; Schad, A.; Zimmermann, A.; et al. Downregulation of organic cation transporter 1 (SLC22A1) is associated with tumor progression and reduced patient survival in human cholangiocellular carcinoma. Int. J. Oncol. 2013, 42, 1297–1304. [Google Scholar] [CrossRef]
- Chapman, J.R.; Waldenstrom, J. With Reference to Reference Genes: A Systematic Review of Endogenous Controls in Gene Expression Studies. PLoS ONE 2015, 10, e0141853. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Binatti, E.; Gerussi, A.; Barisani, D.; Invernizzi, P. The Role of Macrophages in Liver Fibrosis: New Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 6649. [Google Scholar] [CrossRef]
- Yu, L.X.; Ling, Y.; Wang, H.Y. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis. Oncol. 2018, 2, 6. [Google Scholar] [CrossRef]
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Sprinzl, M.F.; Reisinger, F.; Puschnik, A.; Ringelhan, M.; Ackermann, K.; Hartmann, D.; Schiemann, M.; Weinmann, A.; Galle, P.R.; Schuchmann, M.; et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology 2013, 57, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef]
- Chew, V.; Lai, L.; Pan, L.; Lim, C.J.; Li, J.; Ong, R.; Chua, C.; Leong, J.Y.; Lim, K.H.; Toh, H.C.; et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc. Natl. Acad. Sci. USA 2017, 114, E5900–E5909. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Man, K.; Tsao, S.W.; Che, C.M.; Feng, Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015, 6, e1942. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Ren, K.; Quan, M.; Song, Z.; Zou, H.; Li, D.; Zheng, Y.; Cao, J. 8-bromo-7-methoxychrysin Reversed M2 Polarization of Tumor-associated Macrophages Induced by Liver Cancer Stem-like Cells. Anticancer. Agents Med. Chem. 2017, 17, 286–293. [Google Scholar] [CrossRef]
- Ao, J.Y.; Zhu, X.D.; Chai, Z.T.; Cai, H.; Zhang, Y.Y.; Zhang, K.Z.; Kong, L.Q.; Zhang, N.; Ye, B.G.; Ma, D.N.; et al. Colony-Stimulating Factor 1 Receptor Blockade Inhibits Tumor Growth by Altering the Polarization of Tumor-Associated Macrophages in Hepatocellular Carcinoma. Mol. Cancer Ther. 2017, 16, 1544–1554. [Google Scholar] [CrossRef]
- Gao, X.; Huang, H.; Wang, Y.; Pan, C.; Yin, S.; Zhou, L.; Zheng, S. Tumor Immune Microenvironment Characterization in Hepatocellular Carcinoma Identifies Four Prognostic and Immunotherapeutically Relevant Subclasses. Front. Oncol. 2020, 10, 610513. [Google Scholar] [CrossRef] [PubMed]
- Onuma, A.E.; Zhang, H.; Huang, H.; Williams, T.M.; Noonan, A.; Tsung, A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020, 20, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 127. [Google Scholar] [CrossRef]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef]
- Malla, R.R.; Vasudevaraju, P.; Vempati, R.K.; Rakshmitha, M.; Merchant, N.; Nagaraju, G.P. Regulatory T cells: Their role in triple-negative breast cancer progression and metastasis. Cancer 2022, 128, 1171–1183. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Rizzo, A.; Cusmai, A.; Gadaleta-Caldarola, G.; Palmiotti, G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev. Gastroenterol. Hepatol. 2022, 16, 333–339. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Di Federico, A.; Frega, G.; Palloni, A.; Tavolari, S.; Brandi, G. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: Where Do We Stand? Front. Oncol. 2021, 11, 803133. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Gebauer, L.; Brockmoller, J.; Ducker, C. Relationships between Inhibition, Transport and Enhanced Transport via the Organic Cation Transporter 1. Int. J. Mol. Sci. 2022, 23, 2007. [Google Scholar] [CrossRef] [PubMed]
- Heise, M.; Lautem, A.; Knapstein, J.; Schattenberg, J.M.; Hoppe-Lotichius, M.; Foltys, D.; Weiler, N.; Zimmermann, A.; Schad, A.; Gründemann, D.; et al. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer 2012, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Herraez, E.; Lozano, E.; Macias, R.I.; Vaquero, J.; Bujanda, L.; Banales, J.M.; Marin, J.J.; Briz, O. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology 2013, 58, 1065–1073. [Google Scholar] [CrossRef]
- Spector, A.A.; Kim, H.Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 2015, 1851, 356–365. [Google Scholar] [CrossRef]
- Yasar, U.; Eliasson, E.; Dahl, M.L. Association of CYP2C9 genotypes leading to high enzyme activity and colorectal cancer risk. Carcinogenesis 2002, 23, 665; author reply 667–668. [Google Scholar] [CrossRef]
- Chan, A.T.; Tranah, G.J.; Giovannucci, E.L.; Hunter, D.J.; Fuchs, C.S. A prospective study of genetic polymorphisms in the cytochrome P-450 2C9 enzyme and the risk for distal colorectal adenoma. Clin. Gastroenterol. Hepatol. 2004, 2, 704–712. [Google Scholar] [CrossRef]
- London, S.J.; Sullivan-Klose, T.; Daly, A.K.; Idle, J.R. Lung cancer risk in relation to the CYP2C9 genetic polymorphism among Caucasians in Los Angeles County. Pharmacogenetics 1997, 7, 401–404. [Google Scholar] [CrossRef]
- Chen, X.; Cheung, S.T.; So, S.; Fan, S.T.; Barry, C.; Higgins, J.; Lai, K.M.; Ji, J.; Dudoit, S.; Ng, I.O.; et al. Gene expression patterns in human liver cancers. Mol. Biol. Cell 2002, 13, 1929–1939. [Google Scholar] [CrossRef]
- Vaz, F.M.; Paulusma, C.C.; Huidekoper, H.; de Ru, M.; Lim, C.; Koster, J.; Ho-Mok, K.; Bootsma, A.H.; Groen, A.K.; Schaap, F.G.; et al. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: Conjugated hypercholanemia without a clear clinical phenotype. Hepatology 2015, 61, 260–267. [Google Scholar] [CrossRef]
- Hu, H.H.; Liu, J.; Lin, Y.L.; Luo, W.S.; Chu, Y.J.; Chang, C.L.; Jen, C.L.; Lee, M.H.; Lu, S.N.; Wang, L.Y.; et al. The rs2296651 (S267F) variant on NTCP (SLC10A1) is inversely associated with chronic hepatitis B and progression to cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis B. Gut 2016, 65, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Zollner, G.; Wagner, M.; Fickert, P.; Silbert, D.; Fuchsbichler, A.; Zatloukal, K.; Denk, H.; Trauner, M. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 2005, 25, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Nitzahn, M.; Lipshutz, G.S. CPS1: Looking at an ancient enzyme in a modern light. Mol. Genet. Metab. 2020, 131, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ridder, D.A.; Schindeldecker, M.; Weinmann, A.; Berndt, K.; Urbansky, L.; Witzel, H.R.; Heinrich, S.; Roth, W.; Straub, B.K. Key Enzymes in Pyrimidine Synthesis, CAD and CPS1, Predict Prognosis in Hepatocellular Carcinoma. Cancers 2021, 13, 744. [Google Scholar] [CrossRef]
- Hernandez-Alias, X.; Benisty, H.; Schaefer, M.H.; Serrano, L. Translational efficiency across healthy and tumor tissues is proliferation-related. Mol. Syst. Biol. 2020, 16, e9275. [Google Scholar] [CrossRef]
- Gingold, H.; Pilpel, Y. Determinants of translation efficiency and accuracy. Mol. Syst. Biol. 2011, 7, 481. [Google Scholar] [CrossRef]
| Characteristics | Training Set | Validation Set | |
|---|---|---|---|
| TCGA-LICH | ICGC-LIRI-JR | GSE76427 | |
| Sample size * | 363 | 240 | 115 |
| Median OS (year) | 1.63 (0.08–10.7) | 2.14 (0.08–5.92) | 1.16 (0.08–7.76) |
| Number of deaths | 130 (35.8%) | 42 (18.5%) | 23 (20.0%) |
| Age (year) | |||
| ≤65 | 227 (62.5%) | 88 (38.8%) | 65 (56.5%) |
| >65 | 136 (37.5%) | 139 (61.2%) | 50 (43.5%) |
| Gender | |||
| Male | 245 (67.5%) | 170 (70.8%) | 93 (80.9%) |
| Female | 118 (32.5%) | 70 (29.2%) | 22 (19.1%) |
| AJCC stage | |||
| Stage I–II | 263 (77.6%) | 138 (60.8%) | 90 (78.9%) |
| Stage III–IV | 76 (22.4%) | 89 (39.2%) | 24 (21.1%) |
| Histologic grade | |||
| G1–2 | 230 (64.2%) | - | - |
| G3–4 | 128 (35.8%) | - | - |
| Vascular invasion | |||
| Positive | 205 (66.3%) | - | - |
| Negative | 104 (33.7%) | - | - |
| Prior malignancy | |||
| Yes | 34 (9.4%) | 43 (17.9%) | - |
| No | 329 (90.6%) | 197 (82.1%) | - |
| Family cancer history | |||
| Yes | 110 (35.1%) | - | |
| No | 204 (64.9%) | - | |
| BCLC stage | |||
| 0–A | - | - | 78 (67.8%) |
| B–C | - | - | 37 (32.2%) |
| Characteristics | TCGA Cohort * | p Value ** | |
|---|---|---|---|
| High Risk Score (n = 181) | Low Risk Score (n = 182) | ||
| Age (year) | 0.432 | ||
| ≤60 | 91 (50.2%) | 82 (54.9%) | |
| >60 | 90 (49.8%) | 100 (45.1%) | |
| Gender | <0.001 | ||
| Female | 75 (41.4%) | 43 (23.6%) | |
| Male | 106 (58.6%) | 139 (76.4%) | |
| AJCC stage | 0.025 | ||
| Stage I–II | 122 (72.2%) | 141 (82.9%) | |
| Stage III–Iv | 47 (27.8%) | 29 (17.1%) | |
| Histologic grade | 0.001 | ||
| G1-2 | 97 (54.5%) | 129 (71.7%) | |
| G3-4 | 81 (45.5%) | 51 (28.2%) | |
| T Stage | 0.009 | ||
| T1/T2 | 125 (56.7%) | 146 (72.8%) | |
| T3/T4 | 56 (43.3%) | 33 (27.2%) | |
| Vascular invasion | 0.016 | ||
| Positive | 60 (40.8%) | 44 (27.2%) | |
| Negative | 87 (59.2%) | 118 (72.8%) | |
| BMI (kg/m2) | 0.011 | ||
| ≤24 | 90 (54.2%) | 66 (36.2%) | |
| >24 | 76 (45.8%) | 100 (63.8%) | |
| Family cancer history | 0.731 | ||
| Yes | 52 (33.8%) | 58 (26.3%) | |
| No | 102 (66.2%) | 102 (63.7%) | |
| Prior malignancy | 0.377 | ||
| Yes | 14 (2.3%) | 20 (11.0%) | |
| No | 167 (97.7%) | 162 (89.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, Y.; Wang, G.; Dahl, E.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The Role of Macrophage Polarization-Associated Gene Expression in the Oncological Prognosis of Hepatocellular Carcinoma. Gastroenterol. Insights 2024, 15, 764-785. https://doi.org/10.3390/gastroent15030055
Liu D, Li Y, Wang G, Dahl E, Luedde T, Neumann UP, Bednarsch J. The Role of Macrophage Polarization-Associated Gene Expression in the Oncological Prognosis of Hepatocellular Carcinoma. Gastroenterology Insights. 2024; 15(3):764-785. https://doi.org/10.3390/gastroent15030055
Chicago/Turabian StyleLiu, Dong, Yankun Li, Guanwu Wang, Edgar Dahl, Tom Luedde, Ulf Peter Neumann, and Jan Bednarsch. 2024. "The Role of Macrophage Polarization-Associated Gene Expression in the Oncological Prognosis of Hepatocellular Carcinoma" Gastroenterology Insights 15, no. 3: 764-785. https://doi.org/10.3390/gastroent15030055






