IgG-k/IgG-λ Para-Osseous Plasmacytoma Relapsed as Soft-Tissue Plasmacytoma with IgA-k Immunophenotype: A Case Report and Review of the Literature on Related Biochemical Aspects
Abstract
1. Introduction
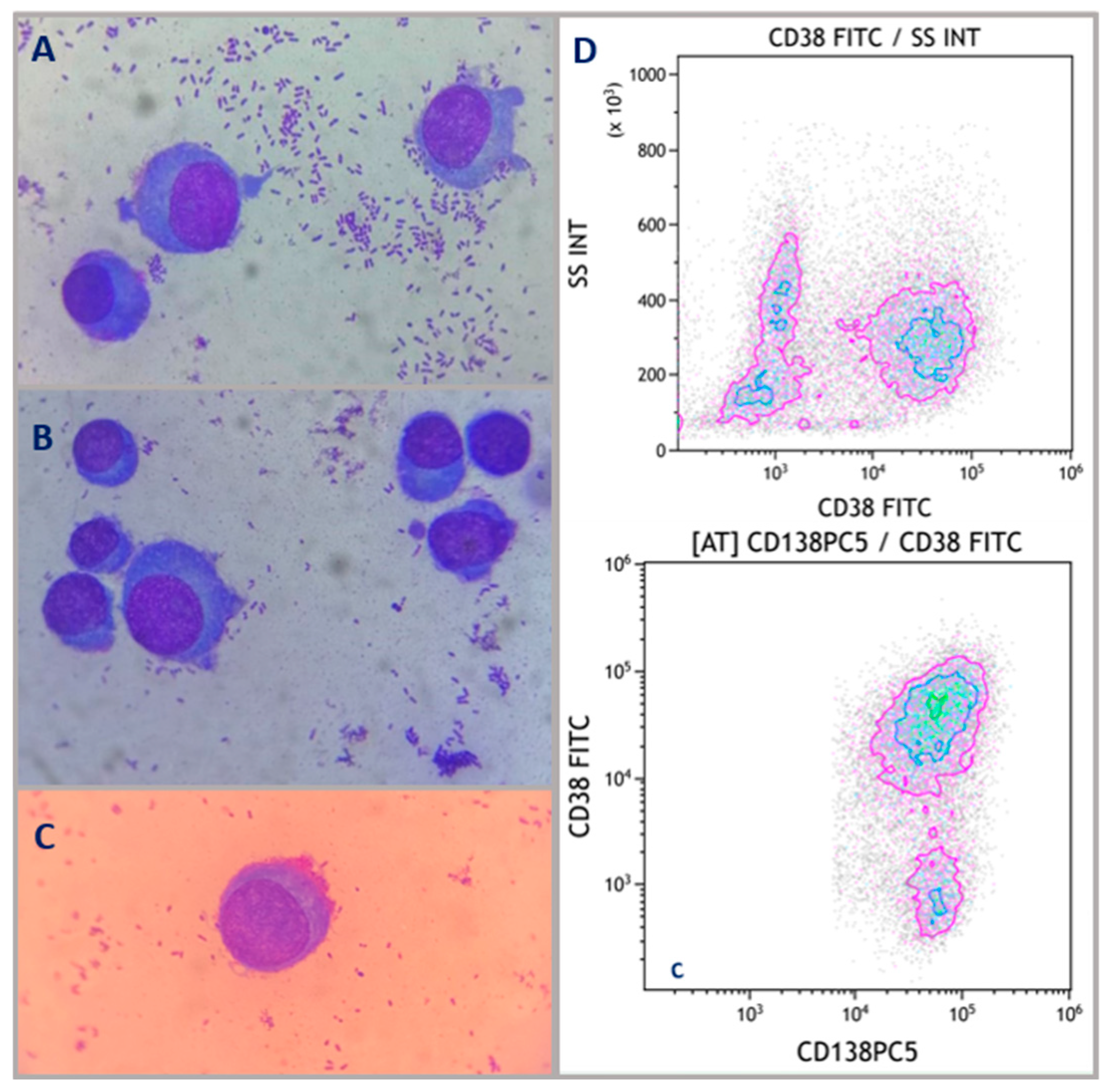
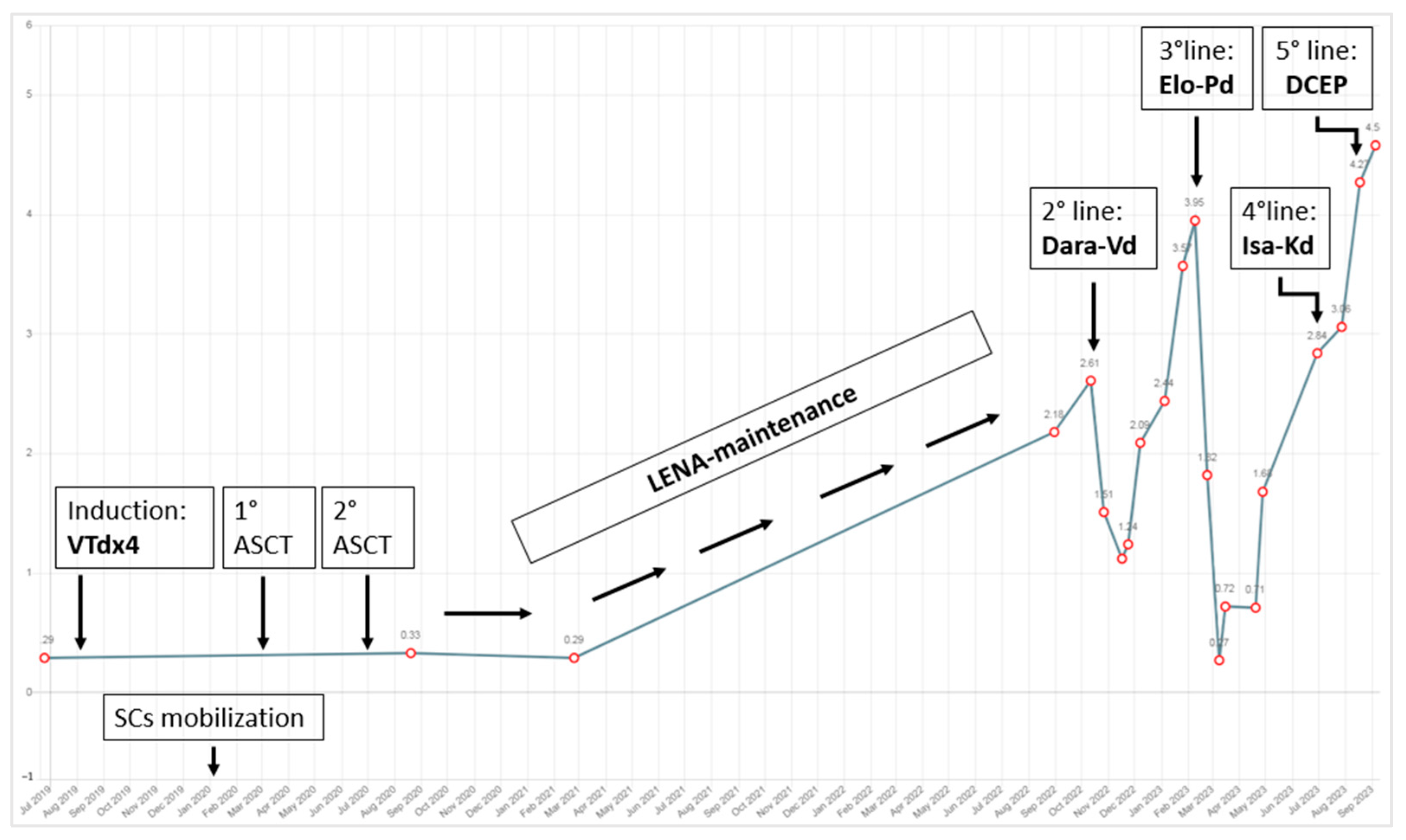
2. Case Description
3. Discussion
3.1. Simultaneous Presence or Consecutive Development of a Second Monoclonal Component: Peculiar Immunoglobulin Patterns and Their Clinical and Prognostic Significance
3.1.1. Bi-Phenotypical/Bi-Clonal Paraproteinemia: A Possible Risk Sub-Category?
3.1.2. Patient’s Relapse with IgA-k Switch: Role of sFLC Assay in R/R MM and Prognostic Significance of IgA MC
3.1.3. Immunoglobulin Isotype Switch: Association with EMD and Correlation with IMiDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bladé, J.; Beksac, M.; Caers, J.; Jurczyszyn, A.; von Lilienfeld-Toal, M.; Moreau, P.; Rasche, L.; Rosiñol, L.; Usmani, S.Z.; Zamagni, E.; et al. Extramedullary disease in multiple myeloma: A systematic literature review. Blood Cancer J. 2022, 12, 45. [Google Scholar] [CrossRef]
- Ullah, T.R. The role of CXCR4 in multiple myeloma: Cells’ journey from bone marrow to beyond. J. Bone Oncol. 2019, 17, 100253. [Google Scholar] [CrossRef]
- McAvera, R.; Quinn, J.; Murphy, P.; Glavey, S. Genetic Abnormalities in Extramedullary Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 11259. [Google Scholar] [CrossRef]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2020, 34, 1–20. [Google Scholar] [CrossRef]
- Shin, H.; Kim, K.; Lee, J.W.; Song, M.; Lee, J.; Lee, H.; Lee, W.S.; Kim, S.J.; Chung, J.S. Comparison of outcomes after autologous stem cell transplantation between myeloma patients with skeletal and soft tissue plasmacytoma. Eur. J. Haematol. 2014, 93, 414–421. [Google Scholar] [CrossRef]
- Bansal, R.; Rakshit, S.; Kumar, S. Extramedullary disease in multiple myeloma. Blood Cancer J. 2021, 11, 161. [Google Scholar] [CrossRef]
- Di, K.; Lloyd, G.K.; Abraham, V.; MacLaren, A.; Burrows, F.J.; Desjardins, A.; Trikha, M.; Bota, D.A. Marizomib activity as a single agent in malignant gliomas: Ability to cross the blood-brain barrier. Neuro Oncol. 2016, 18, 840–848. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Sakellis, C.; Jacene, H.; Shinagare, A.B.; Munshi, N.C.; Ramaiya, N.H.; Abbeele, A.D.V.D. Role of FDG-PET/CT in Extramedullary Multiple Myeloma. Clin. Nucl. Med. 2016, 41, e7–e13. [Google Scholar] [CrossRef]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood J. Am. Soc. Hematol. 2011, 118, 5989–5995. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Suresh, J.; Wu, Y.; Sabaratnam, R.; Brijlall, S.; Kyle, B.; Torlakovic, E.E. Dual Expression of Immunoglobulin Light Chains in Plasma Cell Myeloma: A Case Report and Literature Review. Appl. Immunohistochem. Mol. Morphol. 2023, 31, 447–451. [Google Scholar] [CrossRef]
- Jiwani, S.; Bornhost, J.; Alapat, D. Biphenotypic plasma cell myeloma: Two cases of plasma cell neoplasm with a coexpression of kappa and lambda light chains. Int. J Clin. Exp. Pathol. 2015, 8, 8536–8544. [Google Scholar]
- Gentry, M.; Pettenati, M.; Pang, C.S. Biclonal light chain gammopathy with aberrant CD33 expression in secondary plasma cell leukemia. Int. J. Clin. Exp. Pathol. 2013, 6, 2224–2229. [Google Scholar]
- Lu, M.; Chu, B.; Wang, Y.; Shi, L.; Gao, S.; Fang, L.; Xiang, Q.; Liu, X.; Ding, Y.; Chen, Y.; et al. Clinical characteristics and prognostic significance of immunoglobulin isotype switch in patients with multiple myeloma. Cancer Pathog. Ther. 2023, 1, 149–153. [Google Scholar] [CrossRef]
- Dawson, M.A.; Patil, S.; Spencer, A. Extramedullary relapse of multiple myeloma associated with a shift in secretion from intact immunoglobulin to light chains. Haematologica 2007, 92, 143–144. [Google Scholar] [CrossRef]
- Shapiro-Shelef, M.; Calame, K. Regulation of plasma-cell development. Nat. Rev. Immunol. 2005, 5, 230–242. [Google Scholar] [CrossRef]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Kim, N.Y.; Gong, S.J.; Kim, J.; Youn, S.M.; Lee, J.-A. Multiple Myeloma with Biclonal Gammopathy Accompanied by Prostate Cancer. Ann. Lab. Med. 2011, 31, 285–289. [Google Scholar] [CrossRef]
- Kyle, R.A.; Robinson, R.A.; Katzmann, J.A. The clinical aspects of biclonal gammopathies. Am. J. Med. 1981, 71, 999–1008. [Google Scholar] [CrossRef]
- Jiang, A.S.; Wu, Z.; Wei, E.X.; Ni, H.; You, B.; Yang, T.; Jiang, J.-G. Plasma cell myeloma with dual expression of kappa and lambda light chains. Int. J. Clin. Exp. Pathol. 2018, 11, 4718–4723. [Google Scholar]
- Markovic, U.; Romano, A.; Bellofiore, C.; Condorelli, A.; Garibaldi, B.; Bulla, A.; Duminuco, A.; Del Fabro, V.; Di Raimondo, F.; Conticello, C. Role of Serum Free Light Chain Assay in Relapsed/Refractory Multiple Myeloma. A Real-Life Unicentric Retrospective Study. Cancers 2021, 13, 6017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, F.-Y.; Li, Y.; Sun, J.-N.; Zhang, J.-J.; Tang, R.; Zhong, Y.-P. IgA Type Multiple Myeloma, Clinical Features, and Prognosis. Chin. Med. J. 2018, 131, 1249–1250. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Kimura, K.; Yasukawa, K.; Aikawa, K. Metastatic Cutaneous Plasmacytoma: A Case Report Associated with IgA λ Multiple Myeloma and a Review of the Literature of Metastatic Cutaneous Plasmacytomas Associated with Multiple Myeloma and Primary Cutaneous Plasmacytomas. J. Dermatol. 1999, 26, 587–594. [Google Scholar] [CrossRef]
- Behera, B.; Pattnaik, M.; Sahu, B.; Mohanty, P.; Jena, S.; Mohapatra, L. Cutaneous manifestations of multiple myeloma. Indian J. Dermatol. 2016, 61, 668. [Google Scholar] [PubMed]
- Balleari, E.; Ghio, R.; Falcone, A.; Musto, P. Possible Multiple Myeloma Dedifferentiation Following Thalidomide Therapy: A Report of Four Cases. Leuk Lymphoma 2004, 45, 735–738. [Google Scholar] [CrossRef]
- Avigdor, A.; Raanani, P.; Levi, I.; Hardan, I.; Ben-Bassat, I. Extramedullary Progression Despite a Good Response in the Bone Marrow in Patients Treated with Thalidomide for Multiple Myeloma. Leuk Lymphoma 2001, 42, 683–687. [Google Scholar] [CrossRef]
- Alejandre, M.E.; Madalena, L.B.; Pavlovsky, M.A.; Facio, M.L.; Corrado, C.; Milone, G.; Bresciani, P.D.; Fraind, S.A.; Pavlovsky, S.; Pizzolato, M.A. Oligoclonal bands and immunoglobulin isotype switch during monitoring of patients with multiple myeloma and autologous hematopoietic cell transplantation: A 16-year experience. Clin. Chem. Lab. Med. 2010, 48, 727–731. [Google Scholar] [CrossRef]
- Ye, R.; Kundrapu, S.; Gerson, S.L.; Driscoll, J.J.; Beck, R.; Ali, N.; Landgren, O.; VanHeeckeren, W.; Luo, G.; Kroger, N.; et al. Immune Signatures Associated With Clonal Isotype Switch After Autologous Stem Cell Transplantation for Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e213–e220. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, M.; Sorbello, C.M.C.; Del Fabro, V.; Romano, A.; Cannizzaro, M.T.; Parrinello, N.L.; Esposito, B.; Frazzetto, S.; Elia, F.; Di Raimondo, F.; et al. IgG-k/IgG-λ Para-Osseous Plasmacytoma Relapsed as Soft-Tissue Plasmacytoma with IgA-k Immunophenotype: A Case Report and Review of the Literature on Related Biochemical Aspects. Hematol. Rep. 2024, 16, 541-551. https://doi.org/10.3390/hematolrep16030052
Fazio M, Sorbello CMC, Del Fabro V, Romano A, Cannizzaro MT, Parrinello NL, Esposito B, Frazzetto S, Elia F, Di Raimondo F, et al. IgG-k/IgG-λ Para-Osseous Plasmacytoma Relapsed as Soft-Tissue Plasmacytoma with IgA-k Immunophenotype: A Case Report and Review of the Literature on Related Biochemical Aspects. Hematology Reports. 2024; 16(3):541-551. https://doi.org/10.3390/hematolrep16030052
Chicago/Turabian StyleFazio, Manlio, Chiara Maria Catena Sorbello, Vittorio Del Fabro, Alessandra Romano, Maria Teresa Cannizzaro, Nunziatina Laura Parrinello, Benedetta Esposito, Sara Frazzetto, Federica Elia, Francesco Di Raimondo, and et al. 2024. "IgG-k/IgG-λ Para-Osseous Plasmacytoma Relapsed as Soft-Tissue Plasmacytoma with IgA-k Immunophenotype: A Case Report and Review of the Literature on Related Biochemical Aspects" Hematology Reports 16, no. 3: 541-551. https://doi.org/10.3390/hematolrep16030052
APA StyleFazio, M., Sorbello, C. M. C., Del Fabro, V., Romano, A., Cannizzaro, M. T., Parrinello, N. L., Esposito, B., Frazzetto, S., Elia, F., Di Raimondo, F., & Conticello, C. (2024). IgG-k/IgG-λ Para-Osseous Plasmacytoma Relapsed as Soft-Tissue Plasmacytoma with IgA-k Immunophenotype: A Case Report and Review of the Literature on Related Biochemical Aspects. Hematology Reports, 16(3), 541-551. https://doi.org/10.3390/hematolrep16030052






