Sound Localization and Lateralization by Bilateral Bone Conduction Devices, Middle Ear Implants, and Cartilage Conduction Hearing Aids
Abstract
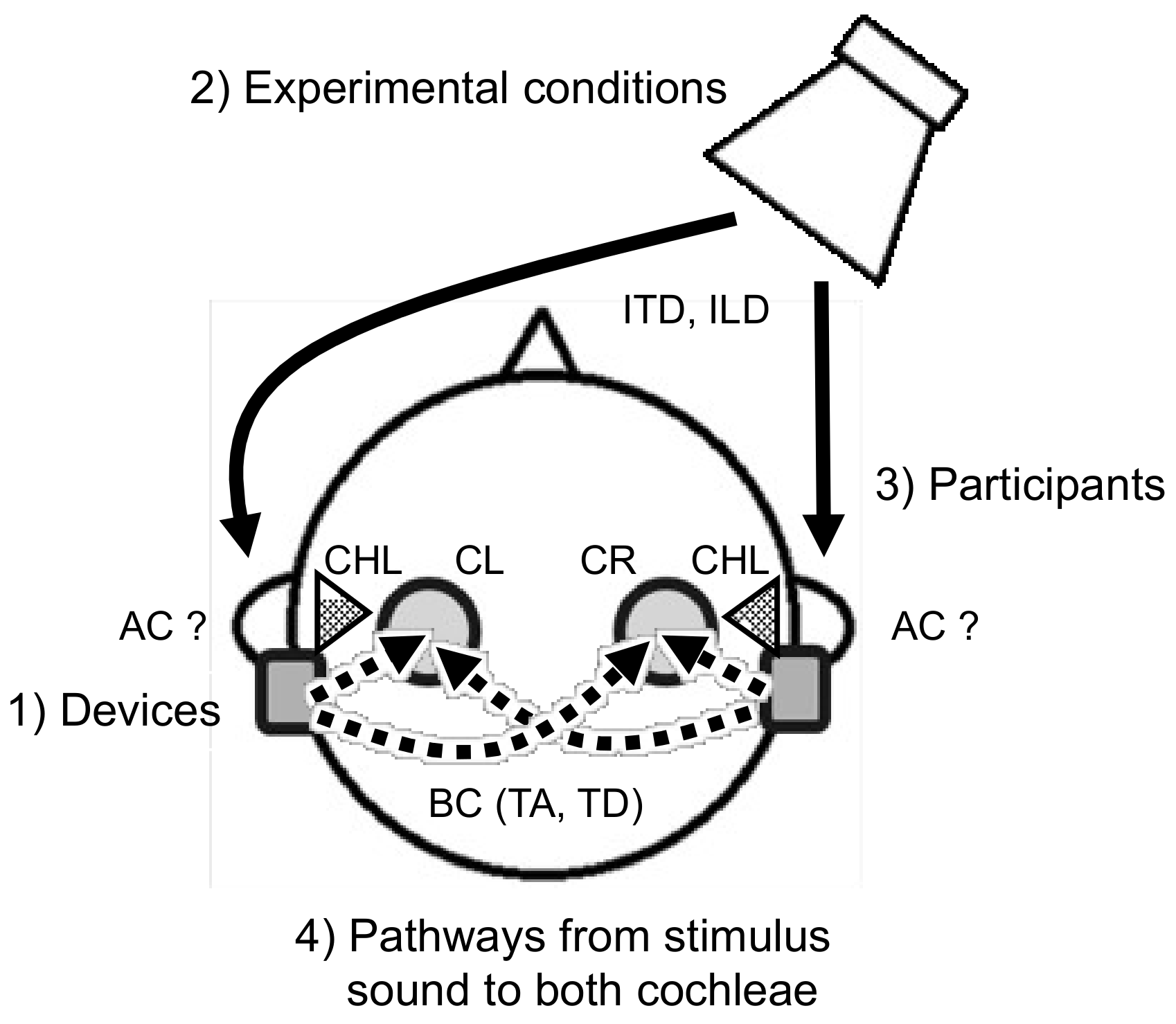
:1. Introduction
2. Factors Affecting the Accuracy of Sound Localization or Lateralization
2.1. Devices
2.2. Experimental Conditions
2.2.1. Measurement Methods
2.2.2. Stimulus Conditions
2.3. Participants
2.3.1. Normal-Hearing Participants with Simulated CHL
2.3.2. Patients with Bilateral CHL
2.4. Pathways from the Sound Source to the Cochleae
2.4.1. Pathways from the Sound Source to the Microphones of the Bilateral Devices
2.4.2. Pathways from Bone-Conducted Sound induced by Devices to the Cochleae
- Transcranial attenuation (TA):
- Transcranial delay (TD):
3. Accuracy of Sound Localization and Lateralization Using Device(s)
3.1. Normal-Hearing Participants with Simulated CHL
3.2. Patients with Bilateral CHL
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aBCD | adhesive bone conduction device |
| BAHA | bone-anchored hearing aid |
| BB | Bonebridge |
| BC | bone conduction |
| BCDs | bone conduction devices |
| BCHAs | bone conduction hearing aids |
| CCHAs | cartilage conduction hearing aids |
| CHL | conductive hearing loss |
| DM | directional microphone |
| HL | hearing level |
| HRTF | head-related transfer function |
| ILD | interaural level difference |
| ITD | interaural time difference |
| MAA | minimum audible angle |
| MAE | mean absolute localization error |
| μs | microsecond |
| RMSE | root mean square error |
| TA | transcranial attenuation |
| TD | transcranial delay |
References
- Moore, B.C.J. 7. Space perception. In An Introduction to the Psychology of Hearing, 5th ed.; Academic Press: San Diego, CA, USA, 2003; pp. 233–298. [Google Scholar]
- Plack, C.J. 9. Spatial hearing. In The Sense of Hearing; Psychology Press: New York, NY, USA, 2005; pp. 173–192. [Google Scholar]
- Bosman, A.J.; Snik, A.F.; van der Pouw, C.T.; Mylanus, E.A.; Cremers, C.W. Audiometric evaluation of bilaterally fitted bone-anchored hearing aids. Audiology 2001, 40, 158–167. [Google Scholar] [CrossRef]
- Häusler, R.; Colburn, S.; Marr, E. Sound localization in subjects with impaired hearing. Spatial-discrimination and interaural-discrimination tests. Acta Otolaryngol. Suppl. 1983, 400, 1–62. [Google Scholar] [CrossRef]
- Kramer, S.E.; Kapteyn, T.S.; Festen, J.M.; Tobi, H. Factors in subjective hearing disability. Audiology 1995, 34, 311–320. [Google Scholar] [CrossRef]
- Kramer, S.E.; Kapteyn, T.S.; Festen, J.M. The self-reported handicapping effect of hearing disabilities. Audiology 1998, 37, 302–312. [Google Scholar] [CrossRef]
- Littler, T.S.; Knight, J.J.; Strange, P.H. Hearing by bone conduction and the use of bone-conduction hearing aids. Proc. R. Soc. Med. 1952, 45, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Snik, A.F.M.; Beynon, A.J.; van der Pouw, C.T.M.; Mylanus, E.A.M.; Cremers, C.W.R.J. Binaural application of the bone-anchored hearing aid. Ann Otol. Rhinol. Laryngol. 1998, 107, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tjellström, A.; Håkansson, B.; Lindström, J.; Brånemark, P.I.; Hallén, O.; Rosenhall, U.; Leijon, A. Analysis of the mechanical impedance of bone-anchored hearing aids. Acta Otolaryngol. 1980, 89, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tjellström, A.; Granström, G. Long-term follow-up with the bone-anchored hearing aid: A review of the first 100 patients between 1977 and 1985. Ear Nose Throat J. 1994, 73, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Priwin, C.; Stenfelt, S.; Granström, G.; Tjellström, A.; Håkansson, B. Bilateral bone-anchored hearing aids (BAHAs): An audiometric evaluation. Laryngoscope 2004, 114, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, R.M.; Hong, P.; Chadha, N.K. Bilateral bone-anchored hearing aids for bilateral permanent conductive hearing loss: A systematic review. Otolaryngol. Head Neck Surg. 2012, 147, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priwin, C.; Jönsson, R.; Hultcrantz, M.; Granström, G. BAHA in children and adolescents with unilateral or bilateral conductive hearing loss: A study of outcome. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 135–145. [Google Scholar] [CrossRef]
- Kaga, K.; Setou, M.; Nakamura, M. Bone-conducted sound lateralization of interaural time difference and interaural intensity difference in children and a young adult with bilateral microtia and atresia of the ears. Acta Otolaryngol. 2001, 121, 274–277. [Google Scholar]
- Schmerber, S.; Sheykholeslami, K.; Kermany, M.H.; Hotta, S.; Kaga, K. Time-intensity trading in bilateral congenital aural atresia patients. Hear. Res. 2005, 202, 248–257. [Google Scholar] [CrossRef]
- Reinfeldt, S.; Håkansson, B.; Taghavi, H.; Olofsson, M.E. New developments in bone-conduction hearing implants: A review. Med. Devices 2015, 8, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ADHEAR. Available online: https://blog.medel.pro/adhear-hearing-system/ (accessed on 31 March 2021).
- Hosoi, H.; Yanai, S.; Nishimura, T.; Sakaguchi, T.; Iwakura, T.; Yoshino, K. Development of cartilage conduction hearing aid. Arch. Mater. Sci. Eng. 2010, 42, 104–110. [Google Scholar]
- Zeitooni, M.; Mäki-Torkko, E.; Stenfelt, S. Binaural hearing ability with bilateral bone conduction stimulation in subjects with normal hearing: Implications for bone conduction hearing aids. Ear Hear. 2016, 37, 690–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatehouse, S.; Noble, W. The speech, spatial and qualities of hearing scale (SSQ). Int J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Gawliczek, T.; Munzinger, F.; Anschuetz, L.; Caversaccio, M.; Kompis, M.; Wimmer, W. Unilateral and bilateral audiological benefit with an adhesively attached, noninvasive bone conduction hearing system. Otol. Neurotol. 2018, 39, 1025–1030. [Google Scholar] [CrossRef]
- Gawliczek, T.; Wimmer, W.; Munzinger, F.; Caversaccio, M.; Kompis, M. Speech understanding and sound localization with a new nonimplantable wearing option for Baha. BioMed. Res. Int. 2018, 2018, 5264124. [Google Scholar] [CrossRef] [Green Version]
- Snapp, H.; Vogt, K.; Agterberg, M.J.H. Bilateral bone conduction stimulation provides reliable binaural cues for localization. Hear. Res. 2020, 388, 107881. [Google Scholar] [CrossRef]
- Dun, C.A.J.; Agterberg, M.J.; Cremers, C.W.; Hol, M.K.; Snik, A.F. Bilateral bone conduction devices: Improved hearing ability in children with bilateral conductive hearing loss. Ear Hear. 2013, 34, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ping, L.; Yang, T.; Niu, X.; Chen, Y.; Xia, X.; Gao, R.; Fan, Y.; Chen, X. Comparative effects of unilateral and bilateral bone conduction hearing devices on functional hearing and sound localization abilities in patients with bilateral microtia-atresia. Acta Otolaryngol. 2020, 140, 575–582. [Google Scholar] [CrossRef]
- Besten, C.A.D.; Vogt, K.; Bosman, A.J.; Snik, A.F.M.; Hol, M.K.S.; Agterberg, M.J.H. The merits of bilateral application of bone-conduction devices in children with bilateral conductive hearing loss. Ear Hear. 2020, 41, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hosoi, H.; Saito, O.; Shimokura, R.; Yamanaka, T.; Kitahara, T. Sound localisation ability using cartilage conduction hearing aids in bilateral aural atresia. Int. J. Audiol. 2020, 59, 891–896. [Google Scholar] [CrossRef]
- Ren, L.; Duan, Y.; Yu, J.; Xie, Y.; Zhang, T. Instant auditory benefit of an adhesive BCHD on children with bilateral congenital microtia. Clin. Otolaryngol. 2021, 46, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Caspers, C.J.I.; Janssen, A.M.; Agterberg, M.J.H.; Cremers, C.W.R.J.; Hol, M.K.S.; Bosman, A.J. Sound localization with bilateral bone conduction devices. Eur. Arch. Otorhinolaryngol. 2021. [Google Scholar] [CrossRef]
- Sakai, Y.; Karino, S.; Kaga, K. Bone-conducted auditory brainstem-evoked responses and skull vibratory velocity measurement in rats at frequencies of 0.5–30 kHz with a new giant magnetostrictive bone conduction transducer. Acta Otolaryngol. 2016, 126, 926–933. [Google Scholar] [CrossRef]
- Zahorik, P.; Bangayan, P.; Sundareswaran, V.; Wang, K.; Tam, C. Perceptual recalibration in human sound localization: Learning to remediate front-back reversals. J. Acoust Soc. Am. 2006, 120, 343–359. [Google Scholar] [CrossRef]
- ADHEAR SYSTEM Including the ADHEAR Audio Processor and the ADHEAR Adhesive Adapter Technical Data. Available online: https://s3.medel.com/pdf/28867_40_ADHEAR_Factsheet_English.pdf (accessed on 31 March 2021).
- Cochlear™ Baha®5 Sound Processor Data Sheet. Available online: https://www.cochlear.com/3116aa05-0d7d-4806-9ed4-7ddb058d8998/BUN335-ISS2-OCT17-Baha5-SP-Datasheet.pdf?MOD=AJPERES&CVID=nlcuPdQ (accessed on 31 March 2021).
- Cire, G. The Baha 5 Sound Processor Overview. Audio Online, 2015. Available online: https://www.audiologyonline.com/articles/baha-5-sound-processor-overview-14427(accessed on 23 July 2021).
- The BonebridgeTM Bone Conduction Implant System Data Sheet. Available online: https://s3.medel.com/pdf/INT/professionals_brochure2014.pdf (accessed on 31 March 2021).
- Shimokura, R.; Hosoi, H.; Iwakura, T.; Nishimura, T.; Matsui, T. Development of monaural and binaural behind-the-ear cartilage conduction hearing aids. Appl. Acoust 2013, 74, 1234–1240. [Google Scholar] [CrossRef]
- Denk, F.; Ewert, S.D.; Kollmeier, B. On the limitations of sound localization with hearing devices. J. Acoust Soc. Am. 2019, 146, 1732–1744. [Google Scholar] [CrossRef]
- Keidser, G.; Rohrseitz, K.; Dillon, H.; Hamacher, V.; Carter, L.; Rass, U.; Convery, E. The effect of multi-channel wide dynamic range compression, noise reduction, and the dsirectional microphone on horizontal localization performance in hearing aid wearers. Int. J. Audiol. 2006, 45, 563–579. [Google Scholar] [CrossRef]
- Van den Bogaert, T.; Klasen, T.J.; Moonen, M.; Van Deun, L.; Wouters, J. Horizontal localization with bilateral hearing aids: Without is better than with. J. Acoust Soc. Am. 2006, 119, 515–526. [Google Scholar] [CrossRef]
- Ibrahim, I.; Parsa, V.; Macpherson, E.; Cheesman, M. Evaluation of speech intelligibility and sound localization abilities with hearing aids using binaural wireless technology. Audiol. Res. 2013, 3, e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.A.; Xu, J.; Cox, R.M. Impact of Hearing Aid Technology on Outcomes in Daily Life III: Localization. Ear Hear. 2017, 38, 746–759. [Google Scholar] [CrossRef]
- Stenfelt, S. Transcranial attenuation of bone-conducted sound when stimulation is at the mastoid and at the bone conduction hearing aid position. Otol. Neurotol. 2012, 33, 105–114. [Google Scholar] [CrossRef]
- Dobrev, I.; Stenfelt, S.; Röösli, C.; Bolt, L.; Pfiffner, F.; Gerig, R.; Huber, A.; Sim, J.H. Influence of stimulation position on the sensitivity for bone conduction hearing aids without skin penetration. Int. J. Audiol. 2016, 55, 439–446. [Google Scholar] [CrossRef]
- Asakura, T.; Takai, K. Effect of the contact condition of the actuator on the sound localization of bone-conducted reproduction. Acoust Sci. Technol 2019, 40, 259–264. [Google Scholar] [CrossRef]
- Middlebrooks, J.C.; Green, D.M. Sound localization by human listeners. Annu Rev. Psychol. 1991, 42, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.A. The bandwidth effect on monaural and binaural localization. Hear. Res. 1986, 21, 67–73. [Google Scholar] [CrossRef]
- Mills, A.W. On the minimum audible angle. J. Acoust Soc. Am. 1958, 30, 237–246. [Google Scholar] [CrossRef]
- Lovett, R.E.S.; Kitterick, P.T.; Huang, S.; Summerfield, A.Q. The developmental trajectory of spatial listening skills in normal-hearing children. J. Speech Lang. Hear. Res. 2012, 55, 865–878. [Google Scholar] [CrossRef]
- Asp, F.; Olofsson, Å.; Berninger, E. Corneal-reflection eye-tracking technique for the assessment of horizontal sound localization accuracy from 6 months of age. Ear Hear. 2016, 37, e104–e118. [Google Scholar] [CrossRef] [Green Version]
- Yost, W.A.; Zhong, X. Sound source localization identification accuracy: Bandwidth dependencies. J. Acoust Soc. Am. 2014, 136, 2737–2746. [Google Scholar] [CrossRef]
- Rakerd, B.; Hartmann, W.M. Localization of sound in rooms, III: Onset and duration effects. J. Acoust Soc. Am. 1986, 80, 1695–1706. [Google Scholar] [CrossRef]
- Asp, F.; Jakobsson, A.; Berminger, E. The effect of simulated unilateral hearing loss on horizontal sound localization accuracy and recognition of speech in spatially separate competing speech. Hear. Res. 2018, 357, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kaga, K.; Asato, H. Sound lateralization test in patients with unilateral microtia and atresia after reconstruction of the auricle and external canal and fitting of canal-type hearing aids. Acta Otolaryngol. 2016, 136, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Studebaker, G.A. Clinical masking of the nontest ear. J. Speech Hear. Disord 1967, 32, 360–371. [Google Scholar] [CrossRef]
- Kaga, M. Development of sound localization. Acta Paediatr. Jpn. 1992, 34, 134–138. [Google Scholar] [CrossRef]
- Firszt, J.B.; Reeder, R.M.; Dwyer, N.Y.; Burton, H.; Holden, L.K. Localization training results in individuals with unilateral severe to profound hearing loss. Hear. Res. 2015, 319, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Klumpp, R.G.; Eady, H.R. Some measurements of interaural time difference thresholds. J. Acoust Soc. Am. 1956, 28, 859–860. [Google Scholar] [CrossRef]
- Eeg-Olofsson, M. Transmission of Bone-Conducted Sound in the Human Skull Based on Vibration and Perceptual Measures. 2012. Available online: https://gupea.ub.gu.se/bitstream/2077/28248/1/gupea_2077_28248_1.pdf (accessed on 31 March 2021).
- Stenfelt, S.; Zeitooni, M. Binaural hearing ability with mastoid applied bilateral bone conduction stimulation in normal hearing subjects. J. Acoust Soc. Am. 2013, 134, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röösli, C.; Dobrev, I.; Pfiffner, F. Transcranial attenuation in bone conduction stimulation. Ear Hear. 2021, in press. [Google Scholar]
- Franke, E.K. Response of the human skull to mechanical vibrations. J. Acoust Soc. Am. 1956, 28, 1277–1284. [Google Scholar] [CrossRef]
- Wigand, M.E.; Borucki, H.J.; Schmitt, H.G. Bone Conduction of sound pulse waves. Physical measurement of velocity. Acta Otolaryngol. 1964, 58, 95–104. (In German) [Google Scholar] [CrossRef] [PubMed]
- Tonndorf, J.; Jahn, A.F. Velocity of propagation of bone-conducted sound in a human head. J. Acoust Soc. Am. 1981, 70, 1294–1297. [Google Scholar] [CrossRef]
- Stenfelt, S.; Goode, R.L. Transmission properties of bone conducted sound: Measurements in cadaver heads. J. Acoust Soc. Am. 2005, 118, 2373–2391. [Google Scholar] [CrossRef]
- Farrell, N.F.; Hartl, R.M.B.; Benichoux, V.; Brown, A.D.; Cass, S.P.; Tollin, D.J. Intracochlear measurements of interaural time and level differences conveyed by bilateral bone conduction systems. Otol. Neurotol. 2017, 38, 1476–1483. [Google Scholar] [CrossRef] [PubMed]

| No. | Authors (Year) | Participants | Devices | Stimuli | Presentation Levels | Setup | Test Conditions |
|---|---|---|---|---|---|---|---|
| 1 | Gawliczek et al. (2018a) [21] | 15 participants with induced bilateral CHL | aBCD(s) (ADHEAR) and BCD(s) attached to a softband (Baha5) | White noise (200 ms duration) | Between 60 and 65 dB SPL | Twelve loudspeakers (aligned in a horizontal circular set up: an angular interval of 30°) | Evaluation of BCD1 (ADHEAR), comparison between unilateral and bilateral fitting, and comparison between BCD1 and BCD2 (Baha 5) |
| 2 | Gawliczek et al. (2018b) [22] | 15 participants with simulated bilateral CHL with a combination of ear plugs and silicon mould material | BCD(s) (Baha5) (SoundArc and a softband) | White noise (200 ms duration) | 60, 65, and 70 dB SPL | Twelve loudspeakers (aligned in a horizontal circular set up: an angular interval of 30°) | (i) Unaided, (ii) aided with one BAHS sound processor mounted on a SoundArc, (iii) aided with 2 BAHS sound processors on a single SoundArc, (iv) aided with one BAHS sound processor on a softband, and (v) aided with 2 BAHS sound processors on a single soft band |
| 3 | Snapp et al. (2020) [23] | 11 listeners with simulated CHL with plug(s) | aBCD(s) (ADHER) | Broadband (BB; 0.5–20 kHz), high-pass (HP; 3–20 kHz), and low-pass (LP; 0.5–1.5 kHz) noise bursts (150 ms duration) | 45, 55, and 65 dB A | Twenty-four loudspeakers (the horizontal (±70°) and vertical (+40°/−30°) planes (The speakers were covered by a black, sound emitting curtain) | Normal hearing, unilateral plug, unilateral plug + ipsilateral aBCD, unilateral plug +ipsilateral aBCD+ contralateral pinna mold, bilateral plugs, bilateral plugs + bilateral aBCDs, and bilateral plugs + unilateral aBCD |
| No. | Authors (Year) | Participants | Devices | Stimuli | Presentation Levels | Setup | Test Conditions |
|---|---|---|---|---|---|---|---|
| 1 | Dun et al. (2013) [24] | 10 children with severe bilateral CHL due to congenital major or minor ear anomalies with microtia or anotia or due to resistant chronic inflammation | Unilateral (right or left) BCD and bilateral BCDs (Baha) | Broadband noise; (0.5 to 20 kHz, 500 ms duration) | Randomly in 10 dB steps within the 40 to 70 dB SPL range | MAA: two loudspeakers (±90°, ±60°, ±30°, ±15°, ±10°, and ±5°) | Unilateral condition (right or left) and bilateral condition |
| 2 | Fan et al. (2020) [25] | 32 patients with congenital bilateral microtia-atresia | Bilateral BCDs (BB and l ADHEAR) | White noise | 65, 70, and 75 dB SPL | Seven loudspeakers (30° interval from −90° to +90°) | Three conditions: unaided, unilateral BCD (BB) and bilateral BCDs (BB plus contralateral ADHEAR) |
| 3 | Besten et al. (2020) [26] | 10 children with congenital bilateral CHL and 1 child with acquired bilateral CHL | One or two percutaneous BCDs (Baha Divino, Baha BP100, or Baha4) | MAA: a broadband noise burst (bandwidth 0.5–20 kHz, 500 ms duration) Sound localization: broadband noise bursts (bandwidth 0.5–8 kHz, 150 ms duration) | MAA: 55, 60 or 65 dB SPL Sound localization: 50, 60, or 70 dB SPL | MAA: two loudspeakers (±90°, ±60°, ±30°, ±15°, ±10°, and ±5°) Sound localization: −75° (left) to +75° (right). | Unilateral BCD on the left side, unilateral BCD on the right side, and bilateral BCDs |
| 4 | Nishimura et al. (2020) [27] | 13 patients with bilateral aural atresia | CC hearing aids | Pink noise (500 ms duration including a rise/fall time of 50 ms) | 65 dB SPL | Eight loudspeakers (full circle at 45° interval) | Unaided, aided with previously used hearing aids (air conduction or BC hearing aids), and aided with CC hearing aids |
| 5 | Ren et al. (2021) [28] | 12 children with mild-to-severe bilateral conductive hearing loss due to congenital microtia | Unilateral or Bilateral BCD(s) (ADHEAR) | A recorded gunshot sound | 65 dB SPL | Twelve loudspeakers (a semi-circle, ranging from −82.5° to 82.5° with 15° intervals | Unaided, unilateral aided and bilateral aided |
| 6 | Caspers et al. (2021) [29] | 15 adults with bilateral CHL and mixed HL (congenital and acquired HL) | Percutaneous BCDs (Baha4 or Baha5) | Broadband noise (BB; 0.5 to 20 kHz), high-pass (HP; 3–20 kHz) and low-pass (LP; 0.5–1.5 kHz) | 45, 55, and 65 dB SPL for BB stimuli, and 55 dB SPL for HL and LP stimuli | Twenty-four loudspeakers positioned on an arc between +70° (right) and −70° (left) azimuth and between +40° (up) and −30° (down) | Unilateral aided conditions (right and left) and bilateral aided condition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiraishi, K. Sound Localization and Lateralization by Bilateral Bone Conduction Devices, Middle Ear Implants, and Cartilage Conduction Hearing Aids. Audiol. Res. 2021, 11, 508-523. https://doi.org/10.3390/audiolres11040046
Shiraishi K. Sound Localization and Lateralization by Bilateral Bone Conduction Devices, Middle Ear Implants, and Cartilage Conduction Hearing Aids. Audiology Research. 2021; 11(4):508-523. https://doi.org/10.3390/audiolres11040046
Chicago/Turabian StyleShiraishi, Kimio. 2021. "Sound Localization and Lateralization by Bilateral Bone Conduction Devices, Middle Ear Implants, and Cartilage Conduction Hearing Aids" Audiology Research 11, no. 4: 508-523. https://doi.org/10.3390/audiolres11040046





