Skull Vibration-Induced Nystagmus and High Frequency Ocular Vestibular-Evoked Myogenic Potentials in Superior Canal Dehiscence
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. SVIN
2.2.1. Stimulation
- VIN starts and stops with stimulation, does not present any secondary reversal, is constant on both mastoid processes, and beats in the same direction.
- The slow-phase velocity (SPV) of the VIN as measured in the horizontal canal must be >2.5°/s.
- It is reproducible and must be identical or similar on two successive tests.
2.2.2. VEMP Testing
2.2.3. CT Scan
2.2.4. Statistics
3. Results
3.1. SCD Finding in CT Scan
3.2. SVIN
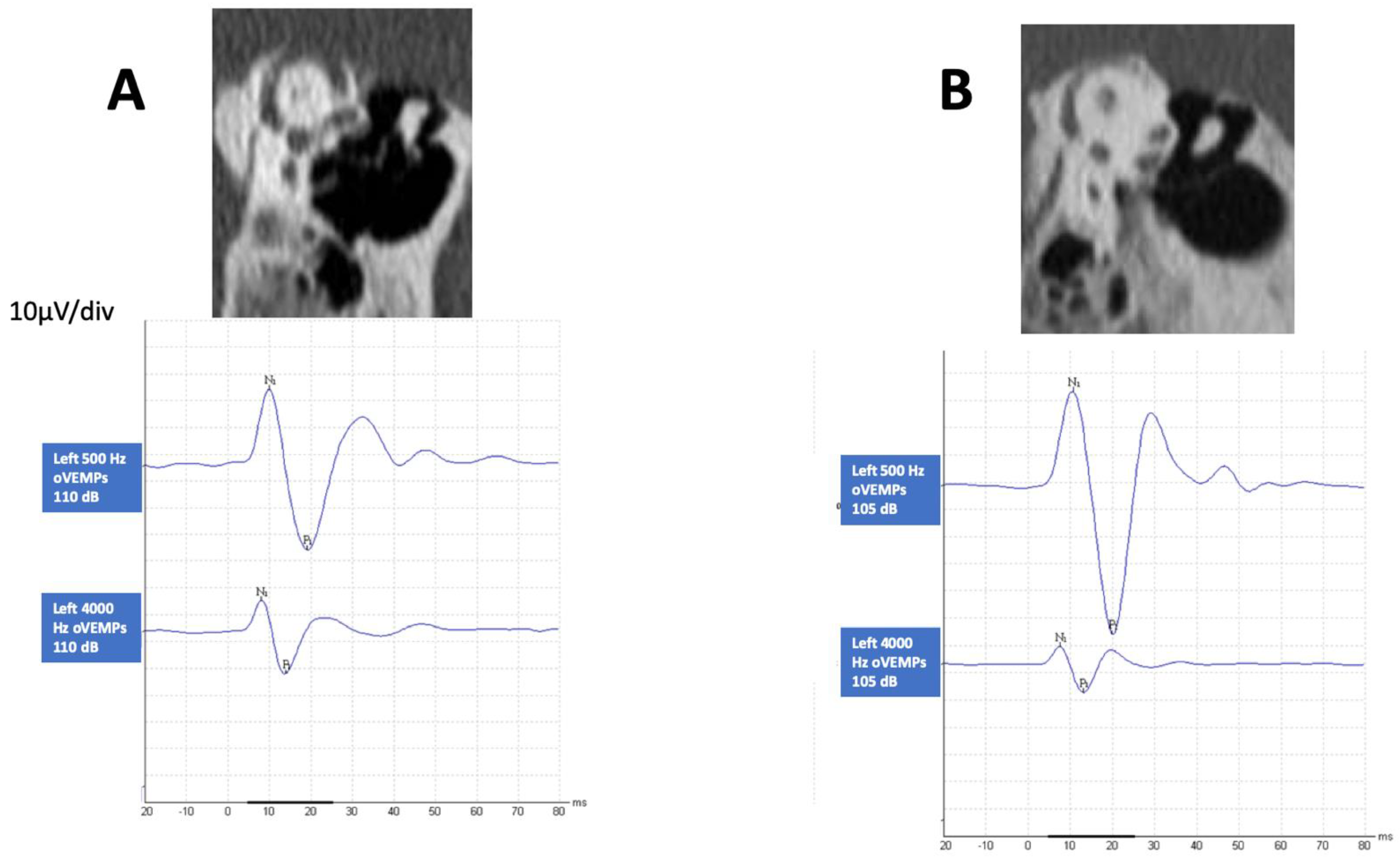
3.3. HFoVEMP
3.4. SVIN and HFoVEMP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minor, L.B.; Solomon, D.; Zinreich, J.S.; Zee, D.S. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, J.P.; Minor, L.B.; Nager, G.T. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, T.; Nadol, J.B. Evidence of Osteoclastic Activity in the Human Temporal Bone. Audiol. Neurootol. 2017, 22, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Iversen, M.M.; Rabbitt, R.D. Biomechanics of Third Window Syndrome. Front Neurol. 2020, 11, 891. [Google Scholar] [CrossRef]
- Ward, B.K.; van de Berg, R.; van Rompaey, V.; Bisdorff, A.; Hullar, T.E.; Welgampola, M.S.; Carey, J.P. Superior Semicircular Canal Dehiscence Syndrome: Diagnostic Criteria Consensus Document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. J. Ves. Res. 2021, 31, 131–141. [Google Scholar] [CrossRef]
- Ward, B.K.; Carey, J.P.; Minor, L.B. Superior Canal Dehiscence Syndrome: Lessons from the First 20 Years. Front. Neurol. 2017, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Dumas, G.; Lion, A.; Karkas, A.; Perrin, P.; Perottino, F.; Schmerber, S. Skull vibration-induced nystagmus test in unilateral superior canal dehiscence and otosclerosis: A vestibular Weber test. Acta Otolaryngol. 2014, 134, 588–600. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Dlugaiczyk, J. Physiology, clinical evidence and diagnostic relevance of sound-induced and vibration-induced vestibular stimulation. Curr. Opin. Neurol. 2020, 33, 126–135. [Google Scholar] [CrossRef]
- Dumas, G.; Curthoys, I.S.; Lion, A.; Perrin, P.; Schmerber, S. The Skull Vibration-Induced Nystagmus Test of Vestibular Function—A Review. Front. Neurol. 2017, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Perez, N. Vibration induced nystagmus in normal subjects and in patients with dizziness. A videonystagmography study. Rev. Laryngol. Otol. Rhinol. 2003, 124, 85–90. [Google Scholar]
- Batuecas-Caletrío, A.; Martínez-Carranza, R.; García-Nuñez, G.M.; Fernández-Nava, M.J.; Sánchez-Gómez, H.; Santacruz-Ruiz, S.; Pérez-Guillén, V.; Pérez-Fernández, N. Skull vibration-induced nystagmus in vestibular neuritis. Acta Otolaryngol. 2020, 140, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Junet, P.; Karkas, A.; Dumas, G.; Quesada, J.L.; Schmerber, S. Vestibular results after intratympanic gentamicin therapy in disabling Menière’s disease. Eur. Arch. Otorhinolaryngol. 2016, 273, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanz, E.; Esteban-Sánchez, J.; González-Márquez, R.; Larrán-Jiménez, A.; Cuesta, Á.; Batuecas-Caletrio, A. Vibration-induced nystagmus and head impulse test screening for vestibular schwannoma. Acta Otolaryngol. 2021, 141, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Dumas, G.; Tan, H.; Dumas, L.; Perrin, P.; Lion, A.; Schmerber, S. Skull vibration induced nystagmus in patients with superior semicircular canal dehiscence. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 263–272. [Google Scholar] [CrossRef]
- Dumas, G.; Quatre, R.; Schmerber, S. How to do and why perform the skull vibration-induced nystagmus test. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 287–290. [Google Scholar] [CrossRef]
- Belden, C.J.; Weg, N.; Minor, L.B.; Zinreich, S.J. CT Evaluation of Bone Dehiscence of the Superior Semicircular Canal as a Cause of Sound- and/or Pressure-induced Vertigo. Radiology 2003, 226, 337–343. [Google Scholar] [CrossRef]
- Branstetter IV, B.F.; Harrigal, C.; Escott, E.J.; Hirsch, B.E. Superior Semicircular Canal Dehiscence: Oblique Reformatted CT Images for Diagnosis. Radiology 2006, 238, 938–942. [Google Scholar] [CrossRef]
- Janky, K.L.; Nguyen, K.D.; Welgampola, M.; Zuniga, M.G.; Carey, J.P. Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol. Neurotol. 2013, 34, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Verrecchia, L.; Brantberg, K.; Tawfique, Z.; Maoli, D. Diagnostic Accuracy of Ocular Vestibular Evoked Myogenic Potentials for Superior Canal Dehiscence Syndrome in a Large Cohort of Dizzy Patients. Ear Hear. 2019, 40, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Zuniga, M.G.; Janky, K.L.; Nguyen, K.D.; Welgampola, M.S.; Carey, J.P. Ocular versus cervical VEMPs in the diagnosis of superior semicircular canal dehiscence syndrome. Otol. Neurotol. 2013, 34, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Manzari, L.; Burgess, A.M.; McGarvie, L.A.; Curthoys, I.S. An indicator of probable semicircular canal dehiscence: Ocular vestibular evoked myogenic potentials to high frequencies. Otolaryngol. Head Neck Surg. 2013, 149, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S. The new vestibular stimuli: Sound and vibration-anatomical, physiological and clinical evidence. Exp. Brain Res. 2017, 235, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S. The Neural Basis of Skull Vibration Induced Nystagmus (SVIN). Audiol. Res. 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Hughes, G.B.; Ruggieri, P.N. Vibration-Induced Nystagmus as an Office Procedure for the Diagnosis of Superior Semicircular Canal Dehiscence. Otol Neurotol. 2007, 7, 911–916. [Google Scholar] [CrossRef]
- Noij, K.S.; Rauch, S.D. Vestibular Evoked Myogenic Potential (VEMP) Testing for Diagnosis of Superior Semicircular Canal Dehiscence. Front Neurol. 2020, 11, 695. [Google Scholar] [CrossRef]
- Maheu, M.; Elblidi, A.; Saliba, I. Investigating Performance of cVEMP and oVEMP in the Identification of Superior Canal Dehiscence in Relation to Dehiscence Location and Size. Audiol. Res. 2021, 11, 42. [Google Scholar] [CrossRef]
- Dumas, G.; Perrin, P.; Morel, N.; N’Guyen, D.Q.; Schmerber, S. Le test vibratoire osseux crânien dans les lésions vestibu-laires périphériques partielles-Influence de la fréquence du stimulus sur le sens du nystagmus. Rev. Laryngol. Otol. Rhinol. 2005, 126, 235–242. [Google Scholar]




| Left Ear | Total | ||||
|---|---|---|---|---|---|
| NO | NEAR D | YES | |||
| NO | - | 4 | 2 | 6 | |
| Right Ear | NEAR D | 9 | 1 | - | 10 |
| YES | 7 | 2 | 5 | 14 | |
| Total | 16 | 7 | 7 | 30 |
| Horizontal SVIN | Vertical SVIN | |||
|---|---|---|---|---|
| Findings in TB | N | SPV (Mean ± SD) | N | SPV |
| NO&NearSSCD | 1/13 | 2.9°s−1 | 5/13 | 1.7 ± 0.3 |
| NO&SSCD | 4/9 | 4.1 ± 2.3 | 4/9 | 1.9 ± 0.7 |
| SSCD&SSCD | 1/5 | 4.5 | 4/5 | 2.3 ± 0.8 |
| SSCD&NearSSCD | 0/2 | 0/2 | ||
| NearSSCD&NearSSCD | 0/1 | 0/1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batuecas-Caletrío, Á.; Jara, A.; Suarez-Vega, V.M.; Marcos-Alonso, S.; Sánchez-Gómez, H.; Pérez-Fernández, N. Skull Vibration-Induced Nystagmus and High Frequency Ocular Vestibular-Evoked Myogenic Potentials in Superior Canal Dehiscence. Audiol. Res. 2022, 12, 202-211. https://doi.org/10.3390/audiolres12020023
Batuecas-Caletrío Á, Jara A, Suarez-Vega VM, Marcos-Alonso S, Sánchez-Gómez H, Pérez-Fernández N. Skull Vibration-Induced Nystagmus and High Frequency Ocular Vestibular-Evoked Myogenic Potentials in Superior Canal Dehiscence. Audiology Research. 2022; 12(2):202-211. https://doi.org/10.3390/audiolres12020023
Chicago/Turabian StyleBatuecas-Caletrío, Ángel, Alejandra Jara, Victor Manuel Suarez-Vega, Susana Marcos-Alonso, Hortensia Sánchez-Gómez, and Nicolas Pérez-Fernández. 2022. "Skull Vibration-Induced Nystagmus and High Frequency Ocular Vestibular-Evoked Myogenic Potentials in Superior Canal Dehiscence" Audiology Research 12, no. 2: 202-211. https://doi.org/10.3390/audiolres12020023
APA StyleBatuecas-Caletrío, Á., Jara, A., Suarez-Vega, V. M., Marcos-Alonso, S., Sánchez-Gómez, H., & Pérez-Fernández, N. (2022). Skull Vibration-Induced Nystagmus and High Frequency Ocular Vestibular-Evoked Myogenic Potentials in Superior Canal Dehiscence. Audiology Research, 12(2), 202-211. https://doi.org/10.3390/audiolres12020023








