A Review of Neural Data and Modelling to Explain How a Semicircular Canal Dehiscence (SCD) Causes Enhanced VEMPs, Skull Vibration Induced Nystagmus (SVIN), and the Tullio Phenomenon
Abstract
1. Introduction
- Skull vibration-induced nystagmus (SVIN) reviewed in [11];
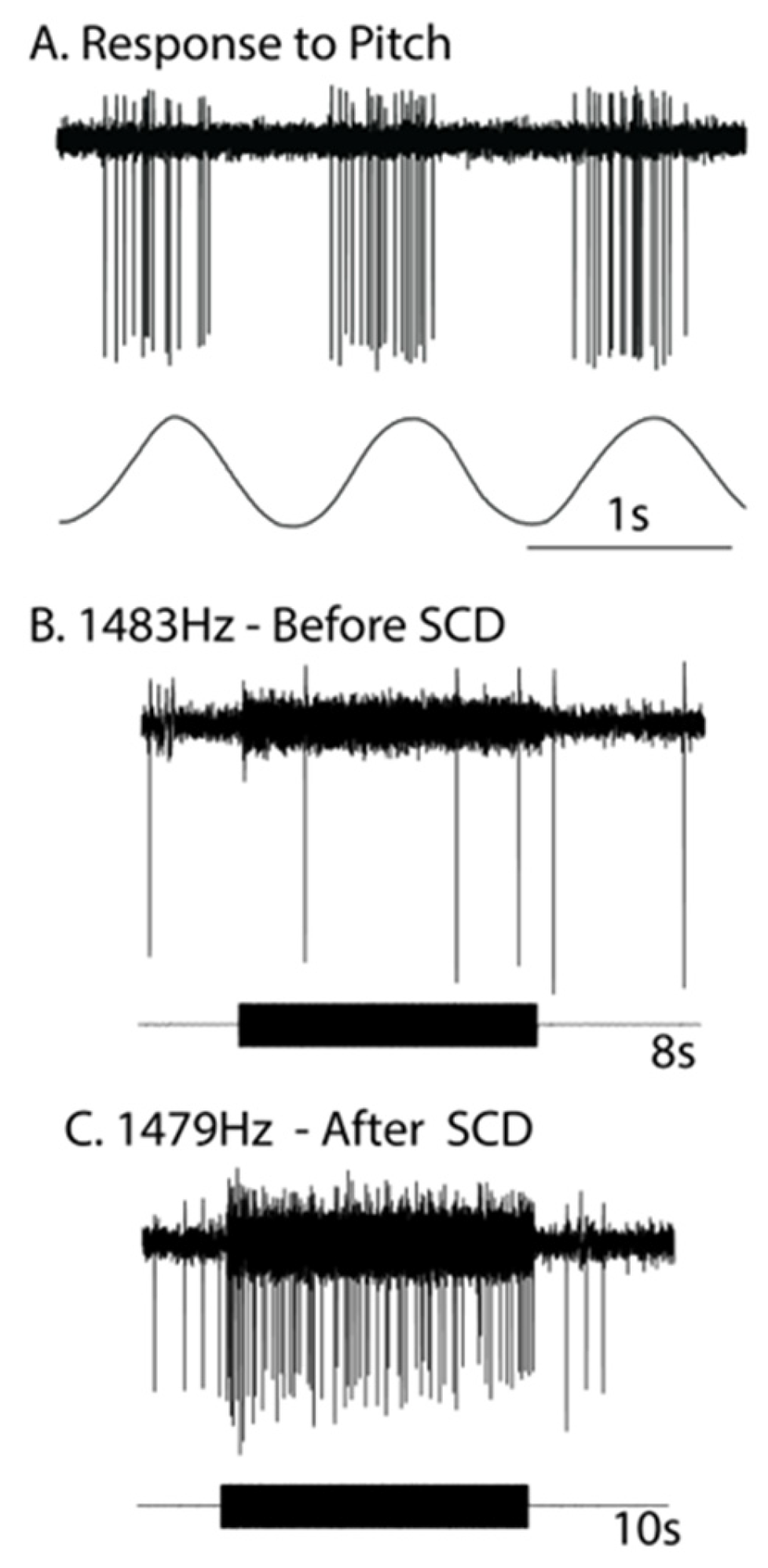
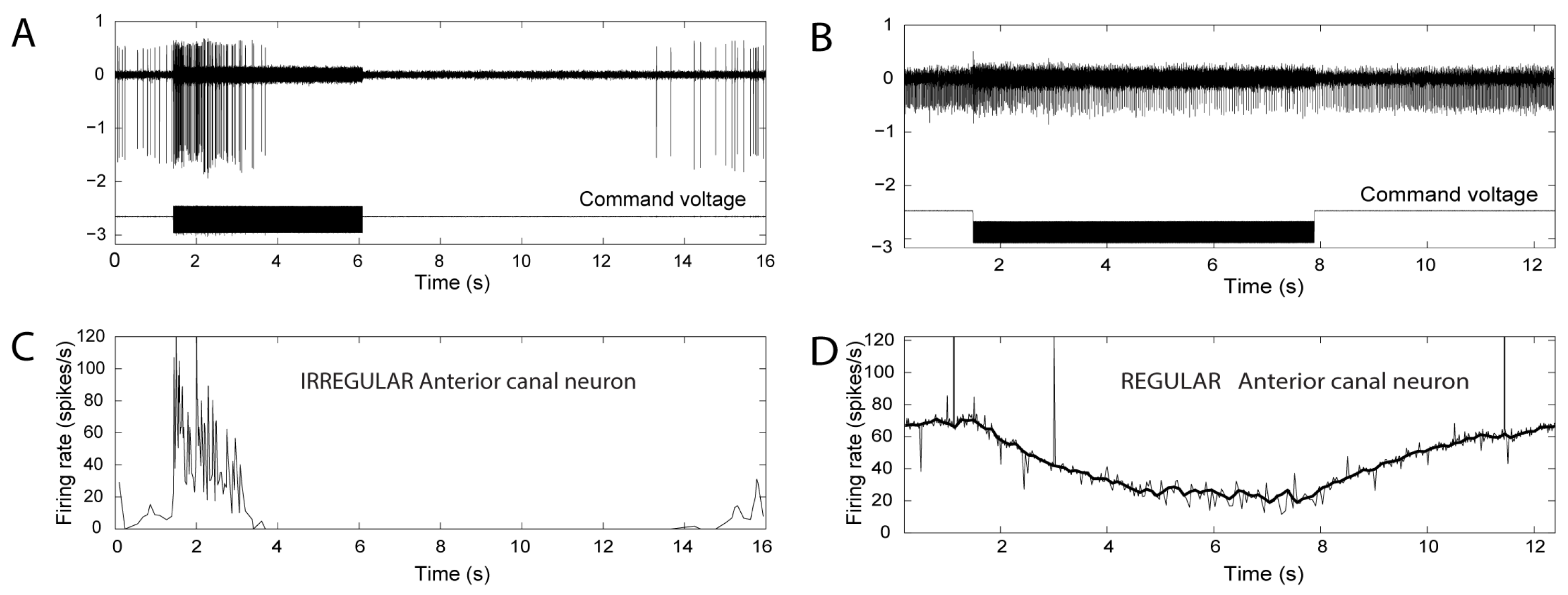
2. Neural Results after SCD—History
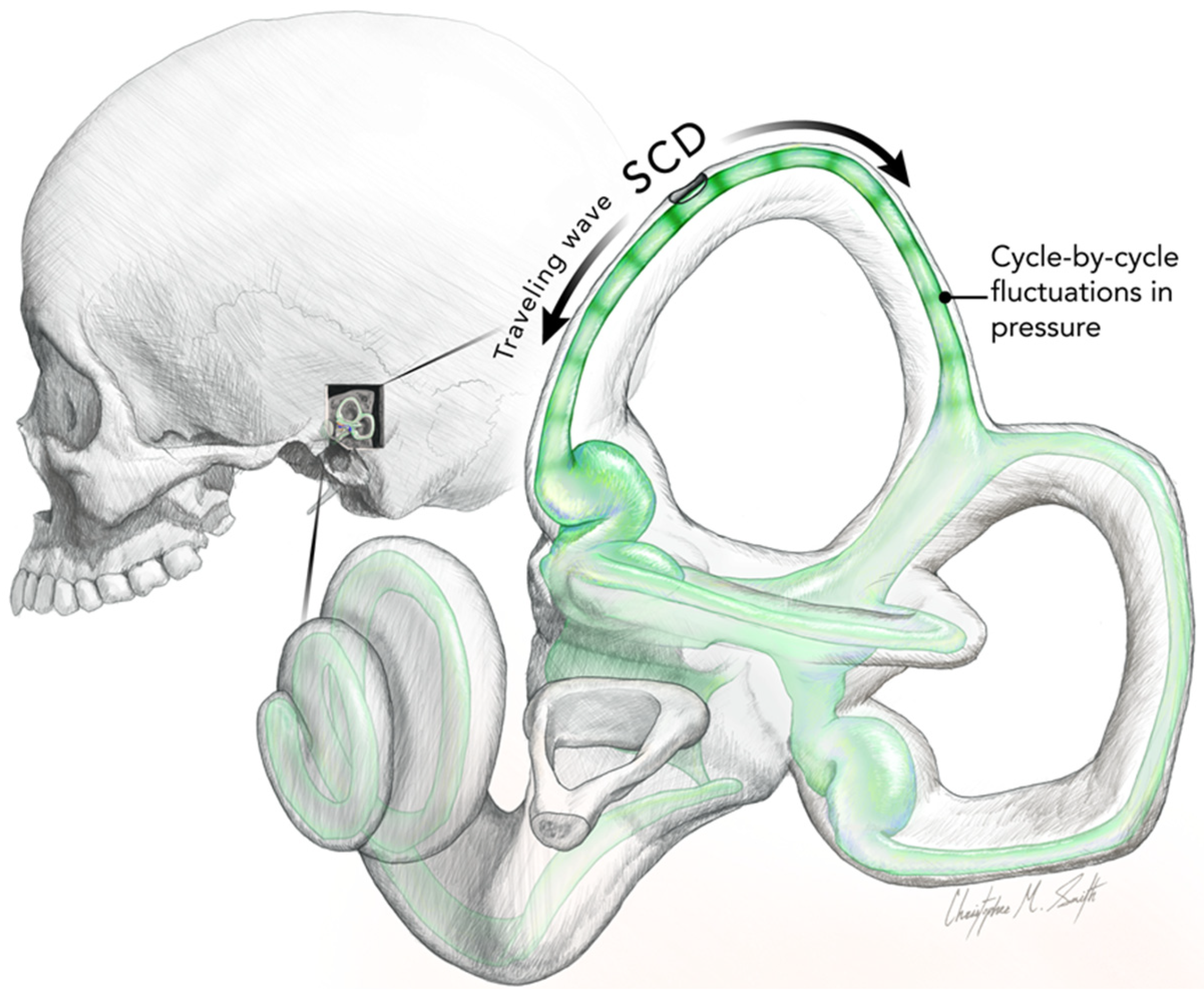
3. The Iversen/Rabbitt Model
- Activation may reverse to inhibition at particular frequencies, as Carey showed (see Figure 5 of Carey et al., 2004 [15]), depending on SCD location and stimulus frequency;
- There can be a spread of activation of canals other than the canal with the SCD-Figures 4 and 5 of [15] show the excitation of a horizontal canal neuron after dehiscence of the anterior canal.
4. VEMPs
5. Skull Vibration-Induced Nystagmus (SVIN)
6. Discussion
Implications for Diagnosis
- (1)
- Test with ACS or BCV at high frequencies (500 or 750 Hz). The standard SVIN stimulus frequency of 100 Hz is almost ideal for activating irregular semicircular canal neurons if the duct is encased in bone [11]. However, the neural data from recordings of primary afferents after SCD showed that irregular canal neurons could be activated up to very high frequencies of ACS or BCV [21]. So Curthoys urged his colleague Georges Dumas to try high frequencies in SCD patients, and he did so and found clear SVIN response at high frequencies (e.g., 500 and 750 Hz) [40], far above the effective frequencies for total or partial unilateral vestibular loss. In human patients, the presence of SVIN at high frequencies (such as 500 or 750 Hz) is an indicator of SCD [43,44]);
- (2)
- It is valuable to test different BCV stimulus locations: BCV stimulation at the vertex of the skull in healthy people causes small or absent oVEMPs [30] and little or no nystagmus response [43]. However, in SCD patients, there is a very clear SVIN nystagmus generated by such vertex stimulation [43]. This is in agreement with the fact that vertex (Cz) BCV stimulation causes small or absent oVEMPs in healthy people, but in SCD patients there is a clear oVEMP n10 [30].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Air-conducted sound |
| BCV | bone-conducted vibration |
| IO | inferior oblique eye muscle |
| QP: | quick phases |
| SCD | semicircular canal dehiscence |
| SCM | sternocleidomastoid neck muscle |
| SPV | slow phase eye velocity |
| SVIN | skull vibration-induced nystagmus |
| TUVL | total unilateral vestibular loss |
| VEMP | vestibular evoked myogenic potential |
| VN | vestibular nuclei |
References
- Curthoys, I.S. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin. Neurophysiol. 2010, 121, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S. The new vestibular stimuli: Sound and vibration-anatomical, physiological and clinical evidence. Exp. Brain Res. 2017, 235, 957–972. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Vulovic, V.; Burgess, A.M.; Manzari, L.; Sokolic, L.; Pogson, J.; Robins, M.; Mezey, L.E.; Goonetilleke, S.; Cornell, E.D.; et al. Neural basis of new clinical vestibular tests: Otolithic neural responses to sound and vibration. Clin. Exp. Pharmacol. Physiol. 2014, 41, 371–380. [Google Scholar] [CrossRef] [PubMed]
- McCaslin, D.L.; Jacobson, G.P. Current role of the videonystagmography examination in the context of the multidimensional balance function test battery. Semin. Hear. 2009, 30, 242–252. [Google Scholar] [CrossRef]
- Rosengren, S.M.; Colebatch, J.G. The contributions of vestibular evoked myogenic potentials and acoustic vestibular stimulation to our understanding of the vestibular system. Front. Neurol. 2018, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Dlugaiczyk, J.; Burgess, A.M.; Curthoys, I.S. Activation of Guinea Pig Irregular Semicircular Canal Afferents by 100 Hz Vibration: Clinical Implications for Vibration-induced Nystagmus and Vestibular-evoked Myogenic Potentials. Otol. Neurotol. 2020, 41, E961–E970. [Google Scholar] [CrossRef]
- Wackym, P.A.; Agrawal, Y.; Ikezono, T.; Balaban, C.D. Editorial: Third Window Syndrome. Front. Neurol. 2021, 12, 704095. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Dlugaiczyk, J. Physiology, clinical evidence and diagnostic relevance of sound-induced and vibration-induced vestibular stimulation. Curr. Opin. Neurol. 2020, 33, 126–135. [Google Scholar] [CrossRef]
- Watson, S.R.; Halmagyi, G.M.; Colebatch, J.G. Vestibular hypersensitivity to sound (Tullio phenomenon): Structural and functional assessment. Neurology 2000, 54, 722–728. [Google Scholar] [CrossRef]
- Bronstein, A.M.; Faldon, M.; Rothwell, J.; Gresty, M.A.; Colebatch, J.; Ludman, H. Clinical and electrophysiological findings in the Tullio phenomenon. Acta Otolaryngol. Suppl. 1995, 520 Pt 1, 209–211. [Google Scholar] [CrossRef]
- Dumas, G.; Curthoys, I.S.; Lion, A.; Perrin, P.; Schmerber, S. The skull vibration-induced nystagmus test of vestibular function-a review. Front. Neurol. 2017, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Tullio, P. L’orecchio; Cappelli: Bologna, Italy, 1928. [Google Scholar]
- Halmagyi, G.M.; Curthoys, I.S.; Colebatch, J.G.; Aw, S.T. Vestibular responses to sound. Ann. N. Y. Acad. Sci. 2005, 1039, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Iversen, M.M.; Zhu, H.; Zhou, W.; Della Santina, C.C.; Carey, J.P.; Rabbitt, R.D. Sound abnormally stimulates the vestibular system in canal dehiscence syndrome by generating pathological fluid-mechanical waves. Sci. Rep. 2018, 8, 10257. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.P.; Hirvonen, T.P.; Hullar, T.E.; Minor, L.B. Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol. Neurotol. 2004, 25, 345–352. [Google Scholar] [CrossRef]
- Rosowski, J.J.; Songer, J.E.; Nakajima, H.H.; Brinsko, K.M.; Merchant, S.N. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol. Neurotol. 2004, 25, 323–332. [Google Scholar] [CrossRef]
- Songer, J.E.; Rosowski, J.J. The effect of superior canal dehiscence on cochlear potential in response to air-conducted stimuli in chinchilla. Hear. Res. 2005, 210, 53–62. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Fernandez, C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J. Neurophysiol. 1971, 34, 635–660. [Google Scholar] [CrossRef]
- Grieser, B.J.; Kleiser, L.; Obrist, D. Identifying Mechanisms Behind the Tullio Phenomenon: A Computational Study Based on First Principles. Jaro J. Assoc. Res. Otolaryngol. 2016, 17, 103–118. [Google Scholar] [CrossRef]
- Liebau, G. Uber ein ventilloses pumpprinzip. Naturwissenschaften 1954, 41, 327. [Google Scholar] [CrossRef]
- Dlugaiczyk, J.; Burgess, A.M.; Goonetilleke, S.C.; Sokolic, L.; Curthoys, I.S. Superior Canal Dehiscence syndrome: Relating clinical findings with vestibular neural responses from a guinea pig model. Otol. Neurotol. 2019, 40, e406–e414. [Google Scholar] [CrossRef]
- Curthoys, I.S. The Neural Basis of Skull Vibration Induced Nystagmus (SVIN). Audiol. Res. 2021, 11, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Kim, J.; McPhedran, S.K.; Camp, A.J. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp. Brain Res. 2006, 175, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Vulovic, V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp. Brain Res. 2011, 210, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Burgess, A.M.; Goonetilleke, S.C. Phase-locking of irregular guinea pig primary vestibular afferents to high frequency (>250 Hz) sound and vibration. Hear. Res. 2019, 373, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Grant, J.W. In what way is an air conducted sound an otolithic stimulus? Assoc. Res. Otolaryngol. Abstr. 2016, 39, 568. [Google Scholar]
- Rabbitt, R.D.; Iversen, M.M. Elasto-hydrodynamic Model of the Deformable Semicircular Canals. Assoc. Res. Otolaryngol. Abstr. 2017, 40, 412. [Google Scholar]
- Smith, C.M.; Curthoys, I.S.; Mukherjee, P.; Wong, C.; Laitman, J.T. Three-dimensional visualization of the human membranous labyrinth: The membrana limitans and its role in vestibular form. Anat. Rec. 2022, 305, 1037–1050. [Google Scholar] [CrossRef]
- Rabbitt, R.D.; Breneman, K.D.; King, C.; Yamauchi, A.M.; Boyle, R.; Highstein, S.M. Dynamic Displacement of Normal and Detached Semicircular Canal Cupula. Jaro J. Assoc. Res. Otolaryngol. 2009, 10, 497–509. [Google Scholar] [CrossRef]
- Manzari, L.; Burgess, A.M.; McGarvie, L.A.; Curthoys, I.S. Ocular and cervical vestibular evoked myogenic potentials to 500 Hz fz bone-conducted vibration in superior semicircular canal dehiscence. Ear Hear. 2012, 33, 508–520. [Google Scholar] [CrossRef]
- Manzari, L.; Burgess, A.M.; MacDougall, H.G.; Curthoys, I.S. Enhanced otolithic function in semicircular canal dehiscence. Acta Otolaryngol. 2011, 131, 107–112. [Google Scholar] [CrossRef]
- Fernandez, C.; Lysakowski, A.; Goldberg, J.M. Hair-cell counts and afferent innervation patterns in the cristae ampullares of the squirrel monkey with a comparison to the chinchilla. J. Neurophysiol. 1995, 73, 1253–1269. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Vulovic, V.; Burgess, A.M.; Sokolic, L.; Goonetilleke, S.C. The response of guinea pig primary utricular and saccular irregular neurons to bone-conducted vibration (BCV) and air-conducted, sound (ACS). Hear. Res. 2016, 331, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.I.; Tokumasu, K.; Goto, K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969, 68, 350–362. [Google Scholar] [CrossRef]
- Uchino, Y.; Kushiro, K. Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci. Res. 2011, 71, 315–327. [Google Scholar] [CrossRef]
- Streubel, S.O.; Cremer, P.D.; Carey, J.P.; Weg, N.; Minor, L.B. Vestibular-evoked myogenic potentials in the diagnosis of superior canal dehiscence syndrome. Acta Otolaryngol. Suppl. 2001, 545, 41–49. [Google Scholar] [CrossRef]
- Welgampola, M.S.; Myrie, O.A.; Minor, L.B.; Carey, J.P. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology 2008, 70, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Grant, J.W.; Burgess, A.M.; Pastras, C.J.; Brown, D.J.; Manzari, L. Otolithic Receptor Mechanisms for Vestibular-Evoked Myogenic Potentials: A Review. Front. Neurol. 2018, 9, 366. [Google Scholar] [CrossRef]
- Dumas, G.; Perrin, P.; Schmerber, S. Nystagmus induced by high frequency vibrations of the skull in total unilateral peripheral vestibular lesions. Acta Otolaryngol. 2008, 128, 255–262. [Google Scholar] [CrossRef]
- Dumas, G.; Tan, H.; Dumas, L.; Perrin, P.; Lion, A.; Schmerber, S. Skull vibration induced nystagmus in patients with superior semicircular canal dehiscence. Eur. Ann. Otorhinolaryngol.-Head Neck Dis. 2019, 136, 263–272. [Google Scholar] [CrossRef]
- Dumas, G.; Lion, A.; Karkas, A.; Perrin, P.; Perottino, F.; Schmerber, S. Skull vibration-induced nystagmus test in unilateral superior canal dehiscence and otosclerosis: A vestibular Weber test. Acta Otolaryngol. 2014, 134, 588–600. [Google Scholar] [CrossRef]
- Schmerber, S.; Dumas, G.; Perrin, P. Anterior semicircular canal dehiscence and cranial vibration-induced nystagmus test. Otol. Neurotol. 2008, 29, 573–574. [Google Scholar] [CrossRef]
- Dumas, G.; Curthoys, I.S.; Catellucci, A.; Perrin, P.; Dumas, L.; Schmerber, S. A bone conducted Tullio phenomenon—A bridge to understand Skull Vibration Induced Nystagmus in Superior Canal Dehiscence. Front. Neurol. 2023, 14, accepted. [Google Scholar]
- Dumas, G.; Quatre, R.; Schmerber, S. How to do and why perform the skull vibration-induced nystagmus test. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Vulovic, V.; Sokolic, L.; Pogson, J.; Burgess, A.; Grant, W. The frequency responses of irregular primary utricular afferent neurons to bone conducted vibration (BCV) and air-conducted sound (ACS). J. Vestib. Res. 2014, 24, 70–71. [Google Scholar]
- Curthoys, I.S.; Vulovic, V.; Sokolic, L.; Pogson, J.; Robins, M.; Burgess, A. The neural basis of clinical vestibular responses to bone conducted vibration (BCV) and air-conducted sound (ACS). ARO Abstr. 2013, 36, 262. [Google Scholar]
- Curthoys, I.S.; MacDougall, H.G.; Vidal, P.P.; de Waele, C. Sustained and transient vestibular systems: A physiological basis for interpreting vestibular function. Front. Neurol. 2017, 8, 117. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Manzari, L. A simple specific functional test for SCD: VEMPs to high frequency (4000Hz) stimuli-their origin and explanation. Front. Neurol. 2020, 11, 612075. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Vulovic, V.; Sokolic, L.; Pogson, J.; Burgess, A.M. Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound. Brain Res. Bull. 2012, 89, 16–21. [Google Scholar] [CrossRef]
- Manzari, L.; Burgess, A.M.; McGarvie, L.A.; Curthoys, I.S. An Indicator of Probable Semicircular Canal Dehiscence: Ocular Vestibular Evoked Myogenic Potentials to High Frequencies. Otolaryngol. Head Neck Surg. 2013, 149, 142–145. [Google Scholar] [CrossRef]
- Colebatch, J.G.; Day, B.L.; Bronstein, A.M.; Davies, R.A.; Gresty, M.A.; Luxon, L.M.; Rothwell, J.C. Vestibular hypersensitivity to clicks is characteristic of the Tullio phenomenon. J. Neurol. Neurosurg. Psychiatry 1998, 65, 670–678. [Google Scholar] [CrossRef]
- Colebatch, J.G.; Rothwell, J.C.; Bronstein, A.; Ludman, H. Click-evoked vestibular activation in the Tullio phenomenon. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1538–1540. [Google Scholar] [CrossRef]
- Kaski, D.; Bronstein, A.M. Patients with vestibular loss, tullio phenomenon, and pressure-induced nystagmus: Vestibular atelectasis? Otol. Neurotol. 2016, 37, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Kaski, D.; Davies, R.; Luxon, L.; Bronstein, A.M.; Rudge, P. The Tullio phenomenon: A neurologically neglected presentation. J. Neurol. 2012, 259, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Fabre, C.; Tan, H.Y.; Dumas, G.; Giraud, L.; Perrin, P.; Schmerber, S. Skull Vibration Induced Nystagmus Test: Correlations with Semicircular Canal and Otolith Asymmetries. Audiol. Res. 2021, 11, 618–628. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Hughes, G.B.; Ruggieri, P.N. Vibration-induced nystagmus as an office procedure for the diagnosis of superior semicircular canal dehiscence. Otol. Neurotol. 2007, 28, 911–916. [Google Scholar] [CrossRef]


| Clinical Observation after Unilateral SCD | References for Clinical Observations | Probable Neural Cause | References for Probable Neural Cause |
|---|---|---|---|
| Enhanced oVEMP to 500 Hz or clicks (ACS or BCV) | [30] | Cycle-by-cycle phase-locked activation of irregular canal afferent neurons to ACS and BCV after SCD to 500 Hz | [2,3,8,15,23,24,25,33,45,46,47,48,49] |
| Enhanced oVEMP to very high-frequency ACS or BCV (4000 Hz) | [48,50] | Cycle-by-cycle phase-locked activation of irregular canal afferent neurons to ACS and BCV to very high frequencies after SCD | [21,25,45] |
| Nystagmus and vertigo in response to maintained ACS or BCV (Tullio phenomenon). The direction of quick phases usually towards the ear with the SCD but may be opposite (determined by many factors, such as skull location of BCV stimulation (see text)) | [9,51,52,53,54] | Acoustic streaming of endolymph generated by repeated compression of the semicircular duct. The “traveling wave hypothesis” of Iversen and Rabbitt. Factors such as location of the SCD (proximity to ampulla), stimulus frequency, BCV stimulator location, and membranous labyrinth impedance determine acoustic streaming. (Non-linear process). Complemented by a cycle-by-cycle phase-locked afferent activation | [14,15,19,26] |
| Skull Vibration Induced Nystagmus (SVIN). The direction of quick phases is usually towards the ear with the SCD but may be opposite (determined by many factors, such as skull location of BCV stimulation (see text)). Anterior canal SCD generates vertical nystagmus | [11,40,41,42,55,56] | Probably mainly due to cycle-by-cycle phase-locked activation. The direction of the nystagmus is determined by factors such as the location of the SCD (proximity to ampulla), stimulus frequency, BCV stimulator location, and membranous labyrinth impedance, which determine acoustic streaming. (Non-linear process). | [14,15] |
| SVIN components in canal planes different from the canal with the SCD (e.g., horizontal components after anterior canal dehiscence). | [15] | The cycle-by-cycle compression is not restricted to only the canal with the SCD, so afferents from other canals show phase-locked neural responses. (Figures 4 and 5 of [15]) Iversen et al.). Also, otolithic afferents show enhanced responses (Curthoys 2017 [2]) | [14,15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curthoys, I.S.; Smith, C.M.; Burgess, A.M.; Dlugaiczyk, J. A Review of Neural Data and Modelling to Explain How a Semicircular Canal Dehiscence (SCD) Causes Enhanced VEMPs, Skull Vibration Induced Nystagmus (SVIN), and the Tullio Phenomenon. Audiol. Res. 2023, 13, 418-430. https://doi.org/10.3390/audiolres13030037
Curthoys IS, Smith CM, Burgess AM, Dlugaiczyk J. A Review of Neural Data and Modelling to Explain How a Semicircular Canal Dehiscence (SCD) Causes Enhanced VEMPs, Skull Vibration Induced Nystagmus (SVIN), and the Tullio Phenomenon. Audiology Research. 2023; 13(3):418-430. https://doi.org/10.3390/audiolres13030037
Chicago/Turabian StyleCurthoys, Ian S., Christopher M. Smith, Ann M. Burgess, and Julia Dlugaiczyk. 2023. "A Review of Neural Data and Modelling to Explain How a Semicircular Canal Dehiscence (SCD) Causes Enhanced VEMPs, Skull Vibration Induced Nystagmus (SVIN), and the Tullio Phenomenon" Audiology Research 13, no. 3: 418-430. https://doi.org/10.3390/audiolres13030037
APA StyleCurthoys, I. S., Smith, C. M., Burgess, A. M., & Dlugaiczyk, J. (2023). A Review of Neural Data and Modelling to Explain How a Semicircular Canal Dehiscence (SCD) Causes Enhanced VEMPs, Skull Vibration Induced Nystagmus (SVIN), and the Tullio Phenomenon. Audiology Research, 13(3), 418-430. https://doi.org/10.3390/audiolres13030037









