Management of Cartilage Conduction Hearing Aids in Pediatric Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Hearing Assessment
2.3. Adjustment and Fitting of the Devices and Ethical Standards
2.4. Purchase Rate and the Evaluation of Cases That Did and Did Not Purchase CC-HA(s)
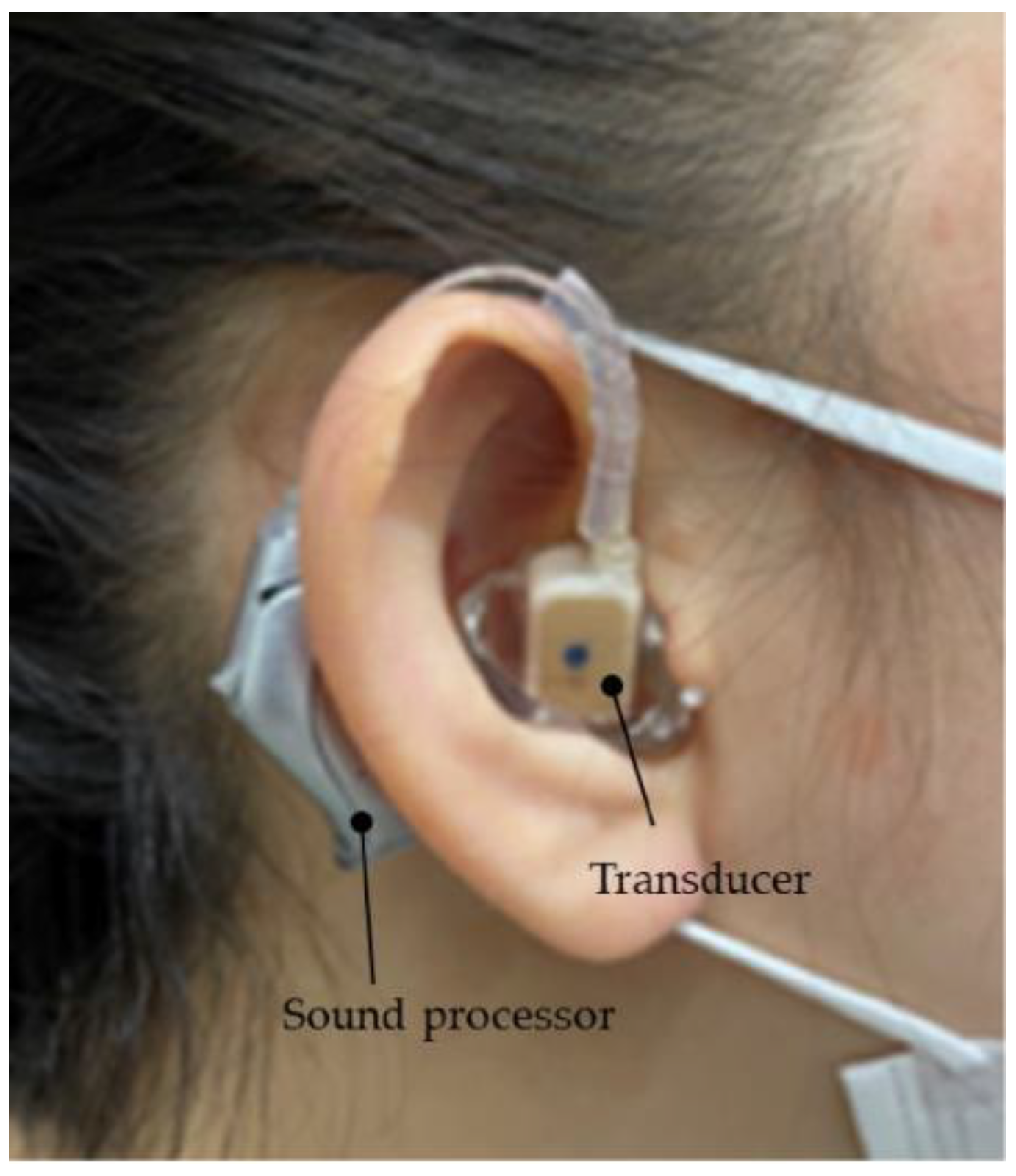
2.5. A Simple Way to Improve Hearing Aid Fixation
2.6. Evaluation after Purchase
3. Results
3.1. Purchase Rates and Differences between the Participants Who Did and Did Not Purchase CC-HAs
3.2. Participants Who Did Not Purchase CC-HAs
3.3. Aided and Unaided Hearing Thresholds of the Purchase and Non-Purchase Groups
3.4. Post-Purchase Evaluation-Wearing Status According to the Questionnaire Survey
3.5. Case Reports
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shimokura, R.; Hosoi, H.; Nishimura, T.; Yamanaka, T.; Levitt, H. Cartilage conduction hearing. J. Acoust. Soc. Am. 2014, 135, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, S. Model predictions for bone conduction perception in the human. Hear. Res. 2016, 340, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, H. Approach in the Use of Cartilage Conduction Speaker. Japanese patent number 4541111, 2004. [Google Scholar]
- Hosoi, H.; Nishimura, T.; Shimokura, R.; Kitahara, T. Cartilage conduction as the third pathway for sound transmission. Auris Nasus Larynx 2019, 46, 151–159. [Google Scholar] [CrossRef]
- Nishimura, T.; Hosoi, H.; Saito, O.; Miyamae, R.; Shimokura, R.; Matsui, T.; Yamanaka, T.; Levitt, H. Is cartilage conduction classified into air or bone conduction? Laryngoscope 2014, 124, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hosoi, H.; Saito, O.; Miyamae, R.; Shimokura, R.; Yamanaka, T.; Kitahara, T.; Levitt, H. Cartilage conduction is characterized by vibrations of the cartilaginous portion of the ear canal. PLoS ONE 2015, 10, e0120135. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, S.; Nishimura, T.; Hosoi, H.; Saito, O.; Shimokura, R.; Morimoto, C.; Kitahara, T. Benefits of cartilage conduction hearing aids for speech perception in unilateral aural atresia. Audiol. Res. 2021, 11, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hosoi, H.; Saito, O.; Shimokura, R.; Yamanaka, T.; Kitahara, T. Cartilage conduction hearing aids for severe conduction hearing loss. Otol. Neurotol. 2018, 39, 65–72. [Google Scholar] [CrossRef]
- Stenfelt, S.; Goode, R.L. Bone-conducted sound: Physiological and clinical aspects. Otol. Neurotol. 2005, 26, 1245–1261. [Google Scholar] [CrossRef]
- Dillon, H. CROS, bone-conduction, and implanted hearing aids. In Hearing Aids; Dillon, H., Ed.; Thieme: Stuttgart, Germany, 2001; pp. 434–450. [Google Scholar]
- Lo, J.F.; Tsang, W.S.; Yu, J.Y.; Ho, O.Y.; Ku, P.K.; Tong, M.C. Contemporary hearing rehabilitation options with aural atresia. Biomed Res. Int. 2014, 2014, 761579. [Google Scholar] [CrossRef]
- Nishimura, T.; Hosoi, H.; Shimokura, R.; Kitahara, T. Cartilage conduction hearing aids in clinical practice. Audiol. Res. 2023, 13, 506–515. [Google Scholar] [CrossRef]
- Hosoi, H. Cartilage Conduction Hearing Aids: The Third Pathway for Sound Transmission and Its Application, ENT & Audiology News. 2020. Available online: https://www.entandaudiologynews.com/features/audiology-features/post/cartilage-conduction-hearing-aids-the-third-pathway-for-sound-transmission-and-itsapplication (accessed on 22 September 2020).
- Nishimura, T.; Hosoi, H.; Saito, O.; Miyamae, R.; Shimokura, R.; Matsui, T.; Iwakura, T. Benefit of a new hearing device utilizing cartilage conduction. Auris Nasus Larynx 2013, 40, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hosoi, H.; Saito, O.; Miyamae, R.; Shimokura, R.; Matsui, T.; Yamanaka, T.; Kitahara, T.; Levitt, H. Cartilage conduction efficiently generates airborne sound in the ear canal. Auris Nasus Larynx 2015, 42, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, C.; Nishimura, T.; Hosoi, H.; Saito, O.; Fukuda, F.; Shimokura, R.; Yamanaka, T. Sound transmission by cartilage conduction in ear with fibrotic aural atresia. J. Rehabil. Res. Dev. 2014, 51, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Oishi, N.; Ogawa, K. Who are good adult candidates for cartilage conduction hearing aids? Eur. Arch. Otorhinolaryngol. 2020, 278, 1789–1798. [Google Scholar] [CrossRef]
- Nishiyama, T.; Oishi, N.; Ogawa, K. Efficacy of cartilage conduction hearing aids in children. Int. J. Pediatr. Otorhinolaryngol. 2021, 142, 110628. [Google Scholar] [CrossRef] [PubMed]
- Suwento, R.; Widodo, D.W.; Airlangga, T.J.; Alviandi, W.; Watanuki, K.; Nakanowatari, N.; Hosoi, H.; Nishimura, T. Clinical trial for cartilage conduction hearing aid in Indonesia. Audiol. Res. 2021, 11, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Mitsuzawa, H.; Shintani, T.; Go, M.; Himi, T. Audiological chronological findings in children with congenital anomalies of the central nervous system. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1105–1110. [Google Scholar] [CrossRef]
- Scollie, S.; Seewald, R.; Cornelisse, L.; Moodie, S.; Bagatto, M.; Laurnagaray, D.; Beaulac, S.; Pumford, J. The desired sensation level multistage input/output algorithm. Trends Amplif. 2005, 9, 159–197. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997, 277, 925–926. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare, Ethical Guidelines for Medical and Health Research Involving Human Subjects Provisional Translation. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf. (accessed on 20 September 2020).
- Yamamoto-Shimojima, K.; Imaizumi, T.; Akagawa, H.; Kanno, H.; Yamamoto, T. Primrose syndrome associated with unclassified immunodeficiency and a novel ZBZB20 mutation. Am. J. Med. Genet. A 2020, 182, 521–526. [Google Scholar] [CrossRef]
- Lieu, J.E.C.; Kenna, M.; Anne, S.; Davidson, L. Hearing loss in children: A review. JAMA 2020, 324, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Yoshida, T.; Fukunaga, Y.; Motegi, A.; Saito, K.; Kobayashi, M.; Sone, M. Comparative analysis of cartilage conduction hearing aid users and non-users: An investigative study. Audiol. Res. 2023, 13, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Calzolari, F.; Sensi, A. Genetic syndromes involving hearing. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Plomp, R.G.; Bredero-Boelhouwer, H.H.; Joosten, K.F.; Wolvius, E.B.; Hoeve, H.L.; Poublon, R.M.; Mathiijssen, I.M. Obstructive sleep apnoea in Treacher Collins syndrome: Prevalence, severity and cause. Int. J. Oral. Maxillofac. Surg. 2012, 41, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Katsanis, S.H.; Jabs, E.W. Treacher Collins syndrome. In Gene Reviews; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; Thieme Medical: Seattle, WA, USA, 1993. [Google Scholar]
- Hylton, J.B.; Leon-Salazar, V.; Anderson, G.C.; De Felippe, N.L. Multidisciplinary treatment approach in Treacher Collins syndrome. J. Dent. Child. 2012, 79, 15–21. [Google Scholar]
- Lesinskas, E.; Stankeviciute, V.; Petrulionis, M. Application of the Vibrant Sound bridge middle-ear implant for aural atresia in patients with Treacher Collins syndrome. J. Laryngol. Otol. 2012, 126, 1216–1223. [Google Scholar] [CrossRef]
- Marsella, P.; Scorpecci, A.; Pacifico, C.; Tieri, L. Bone-anchored hearing aid (Baha) in patients with Treacher Collins syndrome: Tips and pitfalls. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1308–1312. [Google Scholar] [CrossRef]
- Marres, H.A. Hearing loss in the Treacher-Collins syndrome. Adv. Otorhinolaryngol. 2002, 61, 209–215. [Google Scholar]
- Thompson, J.T.; Anderson, P.J.; David, D.J. Treacher Collins syndrome: Protocol management from birth to maturity. J. Cranofac. Surg. 2009, 20, 2028–2035. [Google Scholar] [CrossRef]
- Verhagen, C.V.; Hol, M.K.; Coppens-Schellekens, W.; Snik, A.F.; Cremers, C.W. The Baha Softband. A new treatment for young children with bilateral congenital aural atresia. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 1455–1459. [Google Scholar] [CrossRef]
- House, J.W.; Kutz, J.W., Jr. Bone-anchored hearing aids: Incidence and management of postoperative complications. Otol. Neurotol. 2007, 28, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hobson, J.C.; Roper, A.J.; Andrew, R.; Rothera, M.P.; Hill, P.; Green, K.M. Complications of bone-anchored hearing aid implantation. J. Laryngol. Otol. 2010, 124, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Gbekie, C.; Siper, P.M.; Srivastava, S.; Saland, J.M.; Sethuram, S.; Tang, L.; Drapeau, E.; Frank, Y.; Buxbaum, J.D.; et al. FOXP1 syndrome: A review of the literature and practice parameters for medical assessment and monitoring. J. Neurodev. Disord. 2021, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L. Trisomy 18 Syndrome. Smith’s Recognizable Patterns of Human Malformation, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 13–17. [Google Scholar]
- Carey, J.C. Trisomy 18 and Trisomy 13 Syndromes. In Management of Genetic Syndromes, 2nd ed.; Cassidy, S.B., Allanson, J.E., Eds.; Wiley-Liss: Hoboken, NY, USA, 2005; pp. 555–568. [Google Scholar]
- Kaga, K.; Setou, M.; Nakamura, M. Bone-conducted sound lateralization of inte-raural time difference and interaural intensity difference in children and a young adult with bilateral microtia and atresia of the ears. Acta. Otolaryngol. 2001, 121, 274–277. [Google Scholar] [CrossRef]

















| Characteristics | Purchase Group | Non-Purchase Group | p Value |
|---|---|---|---|
| Age at fitting (year, Mean ± SD) | 5.3 ± 2.6 (n = 36) | 4.4 ± 3.0 (n = 13) | 0.376 b |
| Bilateral hearing loss, Average hearing threshold a of the better ear (dB HL, Mean ± SD) | 46.5 ± 17.3 (n = 8) | 56.7 ± 16.7 (n = 3) | 0.427 b |
| Threshold a of the worse ear (dB HL, Mean ± SD) | 65.8 ± 20.3 (n = 8) | 61.7 ± 22.5 (n = 3) | 0.796 b |
| Unilateral hearing loss, Average hearing threshold a of the better ear (dB HL, Mean ± SD) | 9.7 ± 5.2 (n = 24) | 9.6 ± 3.4 (n = 4) | 0.948 b |
| Threshold a of the worse ear (dB HL, Mean ± SD) | 70.4 ± 12.2 (n = 24) | 59.6 ± 16.3 (n = 4) | 0.281 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakawa, S.; Sugiuchi, T.; Myojin, R.; Sato, K.; Murakami, T.; Miyoshi, Y.; Sugio, Y. Management of Cartilage Conduction Hearing Aids in Pediatric Patients. Audiol. Res. 2023, 13, 871-888. https://doi.org/10.3390/audiolres13060076
Yakawa S, Sugiuchi T, Myojin R, Sato K, Murakami T, Miyoshi Y, Sugio Y. Management of Cartilage Conduction Hearing Aids in Pediatric Patients. Audiology Research. 2023; 13(6):871-888. https://doi.org/10.3390/audiolres13060076
Chicago/Turabian StyleYakawa, Satomi, Tomoko Sugiuchi, Rika Myojin, Kiyoko Sato, Takako Murakami, Yuki Miyoshi, and Yuichiro Sugio. 2023. "Management of Cartilage Conduction Hearing Aids in Pediatric Patients" Audiology Research 13, no. 6: 871-888. https://doi.org/10.3390/audiolres13060076
APA StyleYakawa, S., Sugiuchi, T., Myojin, R., Sato, K., Murakami, T., Miyoshi, Y., & Sugio, Y. (2023). Management of Cartilage Conduction Hearing Aids in Pediatric Patients. Audiology Research, 13(6), 871-888. https://doi.org/10.3390/audiolres13060076





