Psychiatric Comorbidities and Quality of Life in Patients with Vestibular Migraine and Migraine without Vertigo: A Cross-Sectional Study from a Tertiary Clinic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
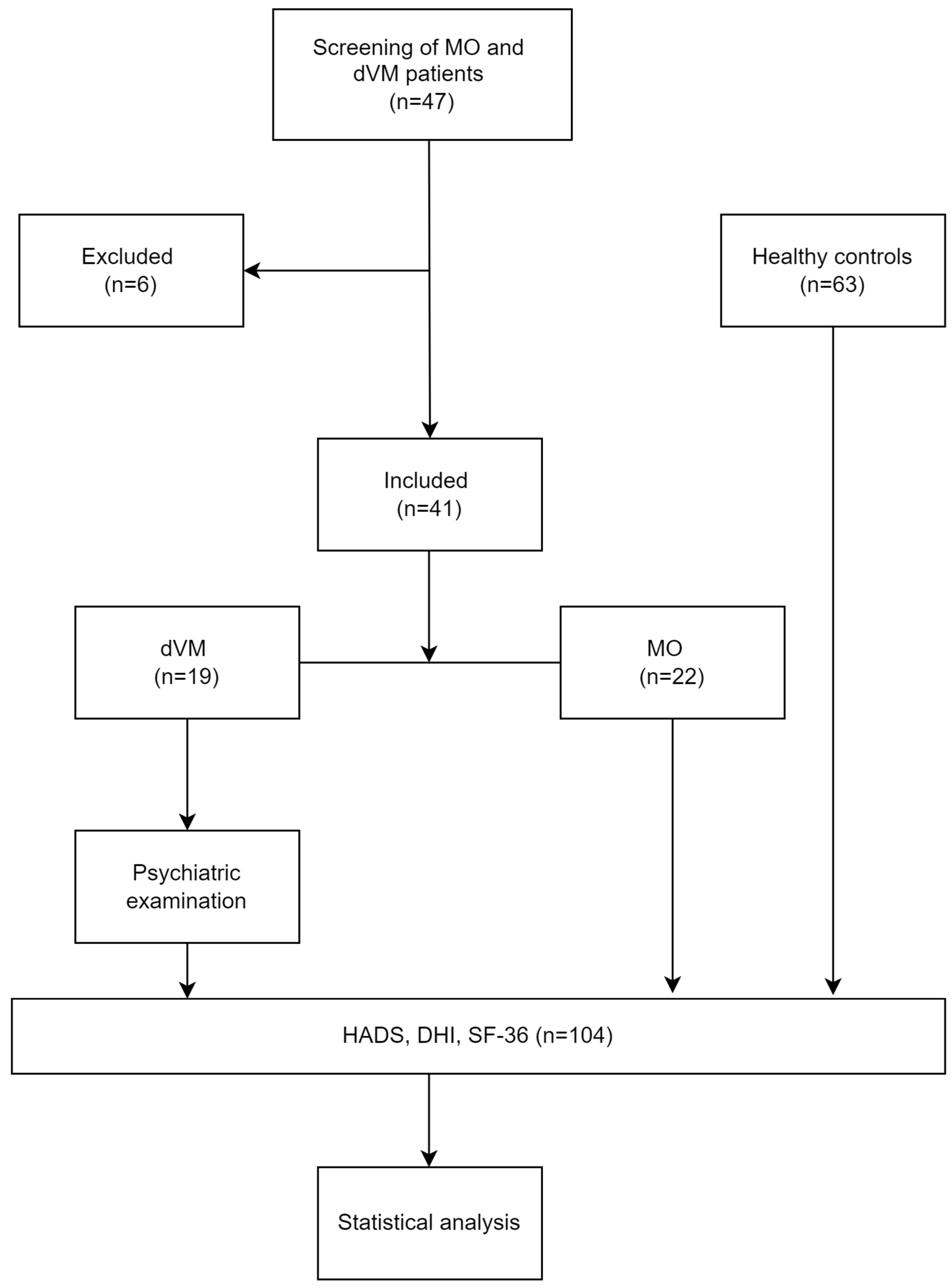
2.2. Participants
2.3. Questionnaires
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Comparison of HADS Scores between the Groups
3.3. Comparison of DHI Scores between the Groups
3.4. Comparison of SF-36 Scores between the Groups
3.5. Correlation of Different Questionnaire Scores in Each Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furman, J.M.; Marcus, D.A.; Balaban, C.D. Vestibular migraine: Clinical aspects and pathophysiology. Lancet Neurol. 2014, 12, 706–715. [Google Scholar] [CrossRef]
- Neuhauser, H.K.; Radtke, A.; von Brevern, M.; Feldmann, M.; Lezius, F.; Ziese, T.; Lempert, T. Migrainous vertigo: Prevalence and impact on quality of life. Neurology 2006, 67, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Formeister, E.J.; Rizk, H.G.; Kohn, M.A.; Sharon, J.D. The epidemiology of vestibular migraine: A population-based survey study. Otol. Neurotol. 2018, 39, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Teggi, R.; Colombo, B.; Albera, R.; Libonati, G.A.; Balzanelli, C.; Caletrio, A.B.; Casani, A.; Espinoza-Sanchez, J.M.; Gamba, P.; Lopez-Escamez, J.A.; et al. Clinical Features, Familial History, and Migraine Precursors in Patients with Definite Vestibular Migraine: The VM-Phenotypes Projects. Headache 2018, 58, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Lempert, T.; Olesen, J.; Furman, J.; Waterston, J.; Seemungal, B.; Carey, J.; Bisdorff, A.; Versino, M.; Evers, S.; Newman-Toker, D. Vestibular migraine: Diagnostic criteria. J. Vestib. Res. Equilib. Orientat. 2012, 22, 167–172. [Google Scholar] [CrossRef]
- Olesen, J. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Kim, T.S.; Lee, W.H.; Heo, Y. Prevalence and Contributing Factors of Anxiety and Depression in Patients with Vestibular Migraine. Ear Nose Throat J. 2023, 5, 1–8. [Google Scholar] [CrossRef]
- Hilber, P.; Cendelin, J.; Le Gall, A.; Machado, M.-L.; Tuma, J.; Besnard, S. Cooperation of the vestibular and cerebellar networks in anxiety disorders and depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 310–321. [Google Scholar] [CrossRef]
- Furman, J.M.; Balaban, C.D.; Jacob, R.G.; A Marcus, D. Migraine-anxiety related dizziness (MARD): A new disorder? J. Neurol. Neurosurg. Psychiatry 2005, 76, 1–8. [Google Scholar] [CrossRef]
- Chen, J.J.; Zeng, B.S.; Su, K.P.; Wu, Y.-C.; Tu, Y.-K.; Stubbs, B.; Chen, T.-Y.; Chen, Y.-W.; Hsu, C.-W.; Tseng, P.-T. Network Meta-analysis of Different Treatments for Vestibular Migraine. CNS Drugs 2023, 37, 837–847. [Google Scholar] [CrossRef]
- Tschan, R.; Best, C.; Beutel, M.E.; Knebel, A.; Wiltink, J.; Dieterich, M.; Eckhardt-Henn, A. Patients’ psychological well-being and resilient coping protect from secondary somatoform vertigo and dizziness (SVD) 1 year after vestibular disease. J. Neurol. 2011, 258, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lahmann, C.; Henningsen, P.; Brandt, T.; Strupp, M.; Jahn, K.; Dieterich, M.; Eckhardt-Henn, A.; Feuerecker, R.; Dinkel, A.; Schmid, G. Psychiatric comorbidity and psychosocial impairment among patients with vertigo and dizziness. J. Neurol. Neurosurg. Psychiatry 2015, 86, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Maslovara, S.; Soldo, S.B.; Puksec, M.; Balaban, B.; Penavic, I.P. Benign Paroxysmal Positional Vertigo (BPPV): Influence of pharmacotherapy and rehabilitation therapy on patients’ recovery rate and life quality. NeuroRehabilitation 2012, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Chirilă, M.; Bolboacă, S.D.; Cosgarea, M. Health-related quality of life and disability in patients with acute unilateral peripheral vestibular disorders. Braz. J. Otorhinolaryngol. 2017, 83, 611–618. [Google Scholar] [CrossRef]
- Ak, A.K.; Çelebisoy, N.; Özdemir, H.N.; Gökçay, F. Vestibular migraine and persistent postural perceptual dizziness: Handicap, emotional comorbidities, quality of life and personality traits. Clin. Neurol. Neurosurg. 2022, 1, 107409. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, S.H.; Kim, J.S. Central vertigo. Curr. Opin. Neurol. 2018, 31, 81–89. [Google Scholar] [CrossRef]
- Machner, B.; Erber, K.; Choi, J.H.; Sprenger, A.; Helmchen, C.; Trillenberg, P. A Simple Gain-Based Evaluation of the Video Head Impulse Test Reliably Detects Normal Vestibulo-Ocular Reflex Indicative of Stroke in Patients with Acute Vestibular Syndrome. Front. Neurol. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Chang, T.P.; Winnick, A.A.; Hsu, Y.C.; Sung, P.-Y.; Schubert, M.C. The bucket test differentiates patients with MRI confirmed brainstem/cerebellar lesions from patients having migraine and dizziness alone. BMC Neurol. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Huang, T.C.; Wang, S.J.; Kheradmand, A. Vestibular migraine: An update on current understanding and future directions. Cephalalgia 2020, 40, 107–121. [Google Scholar] [CrossRef]
- Wakefield, J.C. Diagnostic Issues and Controversies in DSM-5: Return of the False Positives Problem. Annu. Rev. Clin. Psychol. 2016, 12, 105–132. [Google Scholar] [CrossRef]
- Pallant, J.F.; Tennant, A. An introduction to the Rasch measurement model: An example using the Hospital Anxiety and Depression Scale (HADS). Br. J. Clin. Psychol. 2007, 46, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cassiani-Miranda, C.A.; Scoppetta, O.; Cabanzo-Arenas, D.F. Validity of the Hospital Anxiety and Depression Scale (HADS) in primary care patients in Colombia. Gen. Hosp. Psychiatry 2022, 74, 102–109. [Google Scholar] [CrossRef]
- Jerković, A.; Proroković, A.; Matijaca, M.; Vuko, J.; Poljičanin, A.; Mastelić, A.; Katić, A.; Košta, V.; Kustura, L.; Dolić, K.; et al. Psychometric Properties of the HADS Measure of Anxiety and Depression Among Multiple Sclerosis Patients in Croatia. Front. Psychol. 2021, 12, 794353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, Y.; Ju, Y.; Zhao, X. Dizziness handicap and anxiety depression among patients with benign paroxysmal positional vertigo and vestibular migraine. Medicine 2020, 99, e23752. [Google Scholar] [CrossRef]
- Jureša, V.; Ivanković, D.; Vuletić, G.; Babić-Banaszak, A.; Srček, I.; Mastilica, M.; Budak, A. The Croatian health survey—SF-36: I. General quality of life assessment. Coll. Antropol. 2000, 24, 69–78. [Google Scholar] [PubMed]
- Lupi-Ferandin, S.; Glumac, S.; Poljak, N.; Galic, T.; Ivkovic, N.; Brborovic, O.; Pecotic, R.; Dogas, Z. Health-related quality of life in patients after surgically treated midface fracture: A comparison with the Croatian population norm. Ther. Clin. Risk Manag. 2020, 16, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Team, C.R.; R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: Foundation for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 12 October 2023).
- Brandt, T.; Dieterich, M. ‘Excess anxiety’ and ‘less anxiety’: Both depend on vestibular function. Curr. Opin. Neurol. 2020, 33, 136–141. [Google Scholar] [CrossRef]
- Eckhardt-Henn, A.; Best, C.; Bense, S.; Breuer, P.; Diener, G.; Tschan, R.; Dieterich, M. Psychiatric comorbidity in different organic vertigo syndromes. J. Neurol. 2008, 255, 420–428. [Google Scholar] [CrossRef]
- Best, C.; Eckhardt-Henn, A.; Tschan, R.; Dieterich, M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes: Results of a prospective longitudinal study over one year. J. Neurol. 2008, 256, 58–65. [Google Scholar] [CrossRef]
- Ketola, S.; Havia, M.; Appelberg, B.; Kentala, E. Psychiatric symptoms in vertiginous patients. Nord. J. Psychiatry 2015, 69, 287–291. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Semenov, Y.R.; Du Lac, S.; Hoffman, H.J.; Agrawal, Y. Vestibular vertigo and comorbid cognitive and psychiatric impairment: The 2008 National Health Interview Survey. J. Neurol. Neurosurg. Psychiatry 2016, 87, 367–372. [Google Scholar] [CrossRef]
- Yuan, Q.; Yu, L.; Shi, D.; Ke, X.; Zhang, H. Anxiety and depression among patients with different types of vestibular peripheral vertigo. Medicine 2015, 94, 453. [Google Scholar] [CrossRef]
- Minen, M.T.; De Dhaem, O.B.; Van Diest, A.K.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Kutay, Ö.; Akdal, G.; Keskinoğlu, P.; Balcı, B.D.; Alkın, T. Vestibular migraine patients are more anxious than migraine patients without vestibular symptoms. J. Neurol. 2017, 264, 37–41. [Google Scholar] [CrossRef]
- Neuhauser, H.; Lempert, T. Vertigo and dizziness related to migraine: A diagnostic challenge. Cephalalgia 2004, 24, 83–91. [Google Scholar] [CrossRef]
- Beh, S.C.; Masrour, S.; Smith, S.V.; Friedman, D.I. The Spectrum of Vestibular Migraine: Clinical Features, Triggers, and Examination Findings. Headache 2019, 59, 727–740. [Google Scholar] [CrossRef]
- Balcı, B.; Akdal, G. Imbalance, motion sensitivity, anxiety and handicap in vestibular migraine and migraine only patients. Auris Nasus Larynx 2020, 47, 747–751. [Google Scholar] [CrossRef]
- Wang, N.; Huang, H.L.; Zhou, H.; Yu, C.-Y. Cognitive impairment and quality of life in patients with migraine-associated vertigo. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4913–4917. [Google Scholar] [PubMed]
- Kim, E.K.; Hum, M.; Sharon, J.D. Correlating Vestibular Migraine Patient Assessment Tool and Handicap Inventory to Daily Dizziness Symptoms. Otol. Neurotol. 2023, 10, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Gentile, E.; Delussi, M.; Abagnale, C.; Caponnetto, V.; De Cesaris, F.; Frattale, I.; Guaschino, E.; Marcinnò, A.; Ornello, R.; Pistoia, F.; et al. Migraine during COVID-19: Data from second wave pandemic in an Italian cohort. Brain Sci. 2021, 11, 482. [Google Scholar] [CrossRef]
| Definite Vestibular Migraine (dVM) n = 19 | Migraine (MO) n = 22 | Healthy Controls (HC) n = 63 | p-Value | Pairwise Comparisons | ||
|---|---|---|---|---|---|---|
| Comparison | p-Value | |||||
| Age (years) | 50 (25–70) | 41 (22–75) | 28 (20–63) | <0.0001 a | VM-MO | 0.276 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.011 | |||||
| Female | 16 (84.21%) | 20 (90.91%) | 44 (69.84%) | 0.092 b | - | - |
| HADS | 17.6 (4.99) | 10.8 (4.66) | 8.16 (4.25) | <0.0001 c | VM-MO | <0.0001 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.065 | |||||
| HADS-A | 9.37 (2.27) | 6.5 (2.50) | 5.08 (2.55) | <0.0001 c | VM-MO | 0.0007 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.047 | |||||
| HADS-D | 8.26 (3.05) | 4.32 (2.71) | 3.08 (2.43) | <0.0001 c | VM-MO | <0.0001 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.207 | |||||
| DHI | 37.6 (16.1) | 18 (17.2) | 2.32 (5.99) | <0.0001 c | VM-MO | <0.0001 |
| VM-HC | <0.0001 | |||||
| MO-HC | <0.0001 | |||||
| Definite Vestibular Migraine (dVM) n = 19 | Migraine (MO) n = 22 | Healthy Controls (HC) n = 63 | p-Value | Post-hoc Test (Tukey) | ||
|---|---|---|---|---|---|---|
| Comparison | p-Value | |||||
| Physical functioning (PF, min = 1, max = 3) | 2.34 (0.279) | 2.61 (0.432) | 2.86 (0.328) | <0.0001 | VM-MO | 0.026 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.017 | |||||
| Role-Physical (RF, min = 1, max = 2) | 1.18 (0.201) | 1.43 (0.431) | 1.83 (0.308) | <0.0001 | VM-MO | 0.039 |
| VM-HC | <0.0001 | |||||
| MO-HC | <0.0001 | |||||
| MO-HC | 0.048 | |||||
| Bodily Pain (BP, min = 1, max = 5.5) | 2.61 (0.542) | 3.52 (0.970) | 4.65 (0.883) | <0.0001 | VM-MO | 0.002 |
| VM-HC | <0.0001 | |||||
| MO-HC | <0.0001 | |||||
| General Health (GH, min = 1, max = 5) | 2.31 (0.555) | 3.35 (0.851) | 3.92 (0.655) | <0.0001 | VM-MO | <0.0001 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.004 | |||||
| Vitality (VT, min = 1, max = 6) | 2.91 (0.630) | 3.69 (0.626) | 4.03 (0.779) | <0.0001 | VM-MO | 0.001 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.082 | |||||
| Social Functioning (SF, min = 1, max = 5) | 2.76 (0.562) | 3.66 (0.714) | 4.29 (0.749) | <0.0001 | VM-MO | 0.0002 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.0021 | |||||
| Role-Emotional (RE, min = 1, max = 2) | 1.14 (0.231) | 1.62 (0.415) | 1.78 (0.369) | <0.0001 | VM-MO | 0.0001 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.155 | |||||
| Mental Health (MH, min = 1, max = 6) | 3.76 (0.536) | 4.15 (0.401) | 4.28 (0.454) | <0.0001 | VM-MO | 0.011 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.325 | |||||
| Health Transition (HT, min = 1, max = 5) | 2.05 (0.405) | 3.32 (0.995) | 3.35 (0.889) | <0.0001 | VM-MO | <0.0001 |
| VM-HC | <0.0001 | |||||
| MO-HC | 0.773 | |||||
| Scale | PF | RP | BP | GH | VT | SF | RE | MH | HT | DHI |
|---|---|---|---|---|---|---|---|---|---|---|
| HC | ||||||||||
| HADS | 0.02 (0.920) | −0.376 (0.005) | −0.396 (0.003) | −0.473 (<0.001) | −0.512 (<0.001) | −0.515 (<0.001) | −0.275 (0.043) | −0.313 (0.020) | −0.248 (0.072) | 0.437 (<0.001) |
| HADS-A | 0.004 (0.987) | −0.22 (0.126) | −0.401 (0.003) | −0.433 (0.001) | −0.466 (<0.001) | −0.464 (<0.001) | −0.211 (0.129) | −0.356 (0.007) | −0.222 (0.112) | 0.391 (0.003) |
| HADS-D | 0.032 (0.882) | −0.426 (0.001) | −0.27 (0.051) | −0.372 (0.004) | −0.406 (0.002) | −0.414 (0.001) | −0.258 (0.055) | −0.173 (0.218) | −0.216 (0.12) | 0.354 (0.006) |
| DHI | −0.124 (0.395) | −0.253 (0.078) | −0.471 (<0.001) | −0.395 (0.003) | −0.372 (0.006) | −0.508 (<0.001) | −0.415 (0.002) | −0.388 (0.004) | −0.384 (0.004) | - |
| dVM | ||||||||||
| HADS | −0.384 (0.185) | −0.292 (0.332) | −0.085 (0.764) | −0.401 (0.145) | −0.267 (0.332) | −0.143 (0.575) | −0.295 (0.281) | −0.281 (0.291) | 0.198 (0.471) | 0.467 (0.065) |
| HADS-A | −0.359 (0.242) | −0.15 (0.616) | −0.263 (0.412) | −0.215 (0.456) | −0.227 (0.442) | −0.197 (0.483) | −0.337 (0.262) | −0.21 (0.456) | 0.181 (0.543) | 0.493 (0.074) |
| HADS-D | −0.361 (0.213) | −0.366 (0.241) | 0.056 (0.856) | −0.496 (0.053) | −0.268 (0.332) | −0.087 (0.740) | −0.232 (0.387) | −0.303 (0.263) | 0.188 (0.485) | 0.397 (0.145) |
| DHI | −0.862 (<0.001) | −0.529 (0.185) | −0.62 (0.088) | −0.366 (0.290) | −0.463 (0.200) | −0.649 (0.050) | −0.389 (0.262) | −0.391 (0.262) | 0.027 (0.924) | - |
| MO | ||||||||||
| HADS | −0.218 (0.475) | −0.523 (0.040) | −0.465 (0.081) | −0.773 (0.019) | −0.581 (0.003) | −0.528 (0.030) | −0.184 (0.531) | −0.471 (0.081) | −0.12 (0.664) | 0.337 (0.253) |
| HADS-A | −0.165 (0.572) | −0.422 (0.122) | −0.47 (0.081) | −0.679 (0.040) | −0.375 (0.086) | −0.461 (0.082) | −0.292 (0.365) | −0.555 (0.039) | −0.008 (0.971) | 0.334 (0.268) |
| HADS-D | −0.222 (0.45) | −0.509 (0.040) | −0.364 (0.173) | −0.7 (0.030) | −0.651 (<0.001) | −0.481 (0.057) | −0.046 (0.881) | −0.295 (0.300) | −0.198 (0.499) | 0.27 (0.368) |
| DHI | −0.015 (0.961) | −0.463 (0.09) | −0.212 (0.499) | −0.152 (0.671) | −0.193 (0.401) | −0.247 (0.433) | −0.498 (0.081) | −0.205 (0.506) | 0.146 (0.623) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batinović, F.; Sunara, D.; Košta, V.; Pernat, M.; Mastelić, T.; Paladin, I.; Pleić, N.; Krstulović, J.; Đogaš, Z. Psychiatric Comorbidities and Quality of Life in Patients with Vestibular Migraine and Migraine without Vertigo: A Cross-Sectional Study from a Tertiary Clinic. Audiol. Res. 2024, 14, 778-789. https://doi.org/10.3390/audiolres14050065
Batinović F, Sunara D, Košta V, Pernat M, Mastelić T, Paladin I, Pleić N, Krstulović J, Đogaš Z. Psychiatric Comorbidities and Quality of Life in Patients with Vestibular Migraine and Migraine without Vertigo: A Cross-Sectional Study from a Tertiary Clinic. Audiology Research. 2024; 14(5):778-789. https://doi.org/10.3390/audiolres14050065
Chicago/Turabian StyleBatinović, Franko, Davor Sunara, Vana Košta, Milena Pernat, Tonći Mastelić, Ivan Paladin, Nikolina Pleić, Jure Krstulović, and Zoran Đogaš. 2024. "Psychiatric Comorbidities and Quality of Life in Patients with Vestibular Migraine and Migraine without Vertigo: A Cross-Sectional Study from a Tertiary Clinic" Audiology Research 14, no. 5: 778-789. https://doi.org/10.3390/audiolres14050065
APA StyleBatinović, F., Sunara, D., Košta, V., Pernat, M., Mastelić, T., Paladin, I., Pleić, N., Krstulović, J., & Đogaš, Z. (2024). Psychiatric Comorbidities and Quality of Life in Patients with Vestibular Migraine and Migraine without Vertigo: A Cross-Sectional Study from a Tertiary Clinic. Audiology Research, 14(5), 778-789. https://doi.org/10.3390/audiolres14050065








