Effects of Digital Noise Reduction Processing on Subjective and Objective (Pupillometry) Assays of Listening Effort
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
2.3. Hearing Aids with Digital Noise Reduction
2.4. Speech Processing
2.5. Task
2.6. Self-Report Measures
2.7. Pupillometry
2.8. Statistical Analyses
3. Results
3.1. Behavioral and Self-Report Data
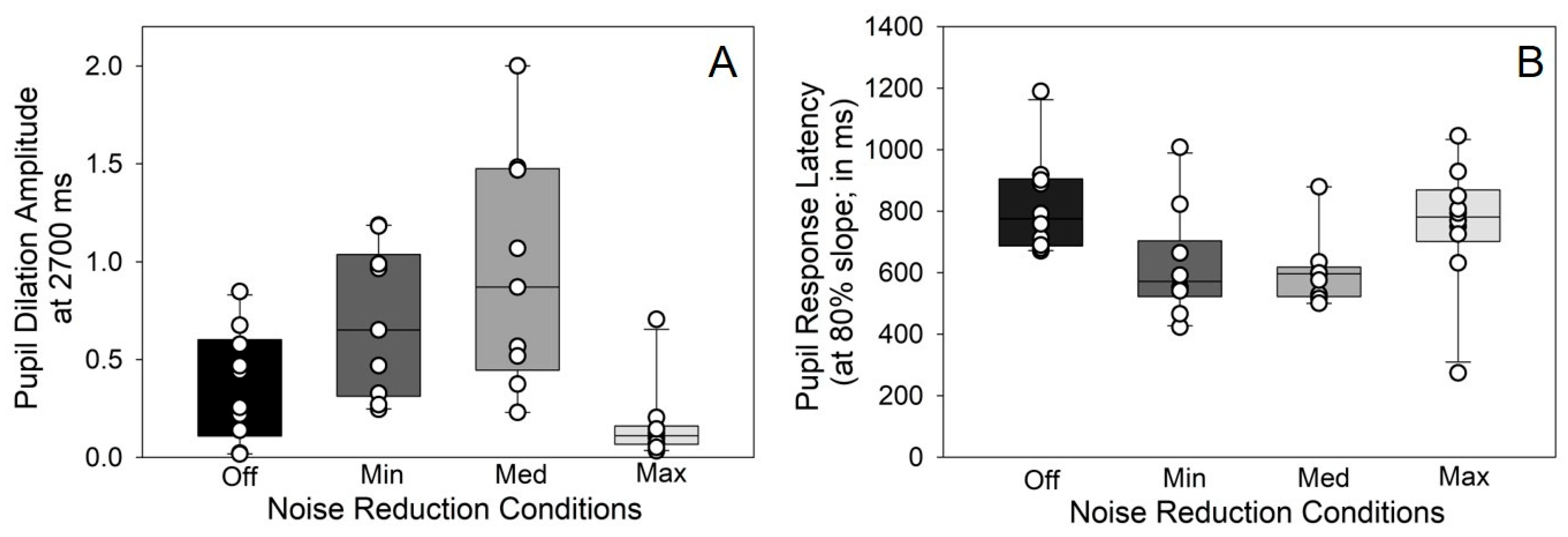
3.2. Pupillometry Data
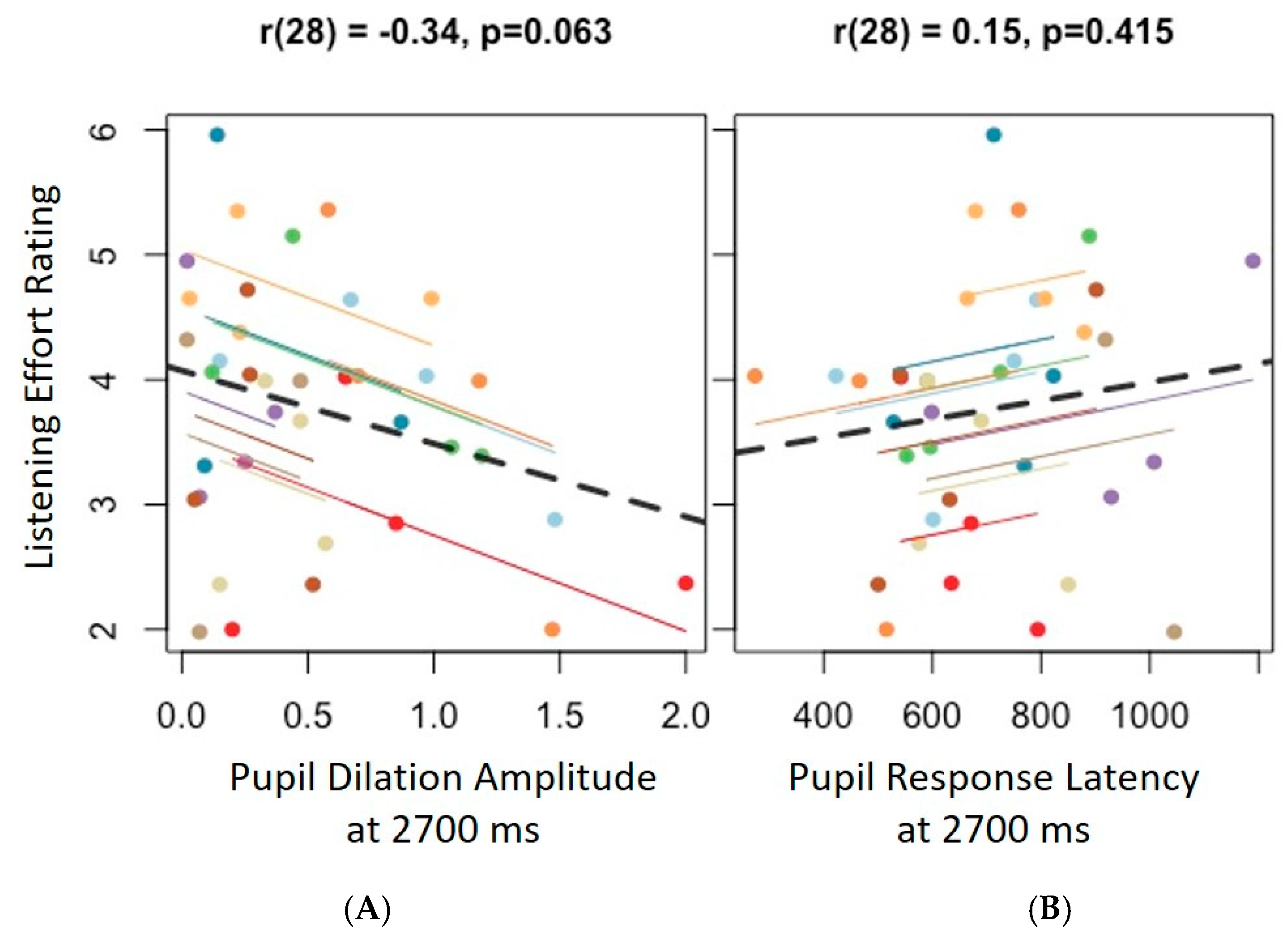
3.3. Agreement Between Self-Report and Pupillometry Data
4. Discussion
4.1. Effects of DNR on Self-Report Listening Effort
4.2. Effects of DNR on Pupil Dilation as a Measure of Listening Effort
4.3. Agreement Between Self-Report and Pupillometry Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hearing Aid |
| DNR | Digital Noise Reduction |
| CRM | Coordinate Response Measure |
| ANSI | American National Standard Institute |
| KEMAR | Knowles Electronic Manikin for Acoustic Research |
| MATLAB | Matrix Laboratory |
| FUEL | Framework of Understanding Effortful Listening |
References
- Hussein, A.B.; Lasheen, R.M.; Emara, A.A.; El Mahallawi, T. Listening Effort in Patients with Sensorineural Hearing Loss with and without Hearing Aids. Egypt. J. Otolaryngol. 2022, 38, 99. [Google Scholar] [CrossRef]
- Rudner, M.; Lunner, T.; Behrens, T.; Thorén, E.S.; Rönnberg, J. Working Memory Capacity May Influence Perceived Effort during Aided Speech Recognition in Noise. J. Am. Acad. Audiol. 2012, 23, 577–589. [Google Scholar] [CrossRef]
- Pichora-Fuller, M.K.; Kramer, S.E.; Eckert, M.A.; Edwards, B.; Hornsby, B.W.Y.; Humes, L.E.; Lemke, U.; Lunner, T.; Matthen, M.; Mackersie, C.L.; et al. Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL). Ear Hear. 2016, 37, 5S–27S. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Xu, J.; Cox, R.M. Impact of Hearing Aid Technology on Outcomes in Daily Life II: Speech Understanding and Listening Effort. Ear Hear. 2016, 37, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Picou, E.M.; Ricketts, T.A. How Directional Microphones Affect Speech Recognition, Listening Effort and Localisation for Listeners with Moderate-to-Severe Hearing Loss. Int. J. Audiol. 2017, 56, 909–918. [Google Scholar] [CrossRef]
- Kollmeier, B.; Kiessling, J. Functionality of Hearing Aids: State-of-the-Art and Future Model-Based Solutions. Int. J. Audiol. 2018, 57, S3–S28. [Google Scholar] [CrossRef]
- Desjardins, J.L.; Doherty, K.A. The Effect of Hearing Aid Noise Reduction on Listening Effort in Hearing-Impaired Adults. Ear Hear. 2014, 35, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Radun, J.; Tervahartiala, I.-K.; Kontinen, V.; Keränen, J.; Hongisto, V. Do Active Noise-Cancelling Headphones’ Influence Performance, Stress, or Experience in Office Context? Build. Environ. 2024, 266, 112102. [Google Scholar] [CrossRef]
- Yun, H.J.; Jo, S.; Kim, J.-S.; Jo, M.; Kim, D.; Shin, J.; Moon, I.J. Investigating the Neurophysiological Effects of Active Noise Cancellation on Concentration in Noisy Environments Using Functional Near-Infrared Spectroscopy. Hear. Res. 2025, 467, 109408. [Google Scholar] [CrossRef]
- Valderrama, J.T.; Mejia, J.; Wong, A.; Chong-White, N.; Edwards, B. The Value of Headphone Accommodations in Apple Airpods Pro for Managing Speech-in-Noise Hearing Difficulties of Individuals with Normal Audiograms. Int. J. Audiol. 2024, 63, 447–457. [Google Scholar] [CrossRef]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef] [PubMed]
- McGarrigle, R.; Munro, K.J.; Dawes, P.; Stewart, A.J.; Moore, D.R.; Barry, J.G.; Amitay, S. Listening Effort and Fatigue: What Exactly Are We Measuring? A British Society of Audiology Cognition in Hearing Special Interest Group ‘White Paper’. Int. J. Audiol. 2014, 53, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Xu, J.; Cox, R.; Pendergraft, P. A Comparison of Two Methods for Measuring Listening Effort As Part of an Audiologic Test Battery. Am. J. Audiol. 2015, 24, 419–431. [Google Scholar] [CrossRef]
- Houben, R.; Van Doorn-Bierman, M.; Dreschler, W.A. Using Response Time to Speech as a Measure for Listening Effort. Int. J. Audiol. 2013, 52, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.-B.; Kressner, A.A.; Relaño-Iborra, H.; Bækgaard, P.; Dau, T.; Wendt, D. Investigating the Reliability of Pupillometry as a Measure of Individualized Listening Effort. Trends Hear. 2023, 27, 23312165231153288. [Google Scholar] [CrossRef]
- Sarangi, L.; Johnson, J. Feasibility of Using Wearable Sensors to Measure Listening-Related Stress. Perspect. ASHA SIGs 2024, 9, 1631–1645. [Google Scholar] [CrossRef]
- Colby, S.; McMurray, B. Cognitive and Physiological Measures of Listening Effort During Degraded Speech Perception: Relating Dual-Task and Pupillometry Paradigms. J. Speech Lang. Hear. Res. 2021, 64, 3627–3652. [Google Scholar] [PubMed]
- Alhanbali, S.; Dawes, P.; Millman, R.E.; Munro, K.J. Measures of Listening Effort Are Multidimensional. Ear Hear. 2019, 40, 1084–1097. [Google Scholar] [CrossRef]
- Naylor, G.; Koelewijn, T.; Zekveld, A.A.; Kramer, S.E. The Application of Pupillometry in Hearing Science to Assess Listening Effort. Trends Hear. 2018, 22, 2331216518799437. [Google Scholar] [CrossRef]
- Van Der Meer, E.; Beyer, R.; Horn, J.; Foth, M.; Bornemann, B.; Ries, J.; Kramer, J.; Warmuth, E.; Heekeren, H.R.; Wartenburger, I. Resource Allocation and Fluid Intelligence: Insights from Pupillometry. Psychophysiology 2010, 47, 158–169. [Google Scholar] [CrossRef]
- Zekveld, A.A.; Koelewijn, T.; Kramer, S.E. The Pupil Dilation Response to Auditory Stimuli: Current State of Knowledge. Trends Hear. 2018, 22, 2331216518777174. [Google Scholar] [CrossRef]
- Zekveld, A.A.; Kramer, S.E.; Festen, J.M. Pupil Response as an Indication of Effortful Listening: The Influence of Sentence Intelligibility. Ear Hear. 2010, 31, 480–490. [Google Scholar] [CrossRef]
- Winn, M.B.; Edwards, J.R.; Litovsky, R.Y. The Impact of Auditory Spectral Resolution on Listening Effort Revealed by Pupil Dilation. Ear Hear. 2015, 36, e153–e165. [Google Scholar] [CrossRef]
- Abramowitz, J.C.; Goupell, M.J.; DeRoy Milvae, K. Cochlear–Implant Simulated Signal Degradation Exacerbates Listening Effort in Older Listeners. Ear Hear. 2024, 45, 441–450. [Google Scholar] [CrossRef]
- Wendt, D.; Hietkamp, R.K.; Lunner, T. Impact of Noise and Noise Reduction on Processing Effort: A Pupillometry Study. Ear Hear. 2017, 38, 690–700. [Google Scholar] [CrossRef]
- Lewis, G.A.; Bidelman, G.M. Autonomic Nervous System Correlates of Speech Categorization Revealed Through Pupillometry. Front. Neurosci. 2020, 13, 1418. [Google Scholar] [CrossRef]
- Winn, M.B. Rapid Release From Listening Effort Resulting From Semantic Context, and Effects of Spectral Degradation and Cochlear Implants. Trends Hear. 2016, 20, 2331216516669723. [Google Scholar] [CrossRef]
- Eddins, D.A.; Liu, C. Psychometric Properties of the Coordinate Response Measure Corpus with Various Types of Background Interference. J. Acoust. Soc. Am. 2012, 131, EL177–EL183. [Google Scholar] [CrossRef]
- Bolia, R.S.; Nelson, W.T.; Ericson, M.A.; Simpson, B.D. A Speech Corpus for Multitalker Communications Research. J. Acoust. Soc. Am. 2000, 107, 1065–1066. [Google Scholar] [CrossRef]
- Shetty, H.N.; Raju, S.; Singh S, S. The Relationship between Age, Acceptable Noise Level, and Listening Effort in Middle-Aged and Older-Aged Individuals. J. Otol. 2023, 18, 220–229. [Google Scholar] [CrossRef]
- Stronks, H.C.; Tops, A.L.; Quach, K.W.; Briaire, J.J.; Frijns, J.H.M. Listening Effort Measured With Pupillometry in Cochlear Implant Users Depends on Sound Level, But Not on the Signal to Noise Ratio When Using the Matrix Test. Ear Hear. 2024, 45, 1461–1473. [Google Scholar] [CrossRef]
- Humes, L.E.; Kidd, G.R.; Fogerty, D. Exploring Use of the Coordinate Response Measure in a Multitalker Babble Paradigm. J. Speech Lang. Hear. Res. 2017, 60, 741–754. [Google Scholar] [CrossRef]
- Jensen, J.; Pedersen, M.S. Analysis of Beamformer Directed Single-Channel Noise Reduction System for Hearing Aid Applications. In Proceedings of the 2015 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), South Brisbane, QLD, Australia, 19–24 April 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 5728–5732. [Google Scholar]
- ANSI S3.22-2003; Specification of Hearing Aid Characteristics. American National Standards Institute: Washington, DC, USA, 2003.
- Jürgens, T.; Ihly, P.; Tchorz, J.; Nishiyama, T.; Tanaka, C.; Suzuki, D.; Shinden, S.; Kitama, T.; Ogawa, K.; Zaar, J.; et al. Closedness of Acoustic Coupling and Audiological Measures Are Associated with Individual Speech-in-Noise Benefit From Noise Reduction in Hearing Aids. Trends Hear. 2025, 29, 23312165251325983. [Google Scholar] [CrossRef]
- Bradley, J.V. Complete Counterbalancing of Immediate Sequential Effects in a Latin Square Design. J. Am. Stat. Assoc. 1958, 53, 525–528. [Google Scholar] [CrossRef]
- Cuve, H.C.; Stojanov, J.; Roberts-Gaal, X.; Catmur, C.; Bird, G. Validation of Gazepoint Low-Cost Eye-Tracking and Psychophysiology Bundle. Behav. Res. 2022, 54, 1027–1049. [Google Scholar] [CrossRef]
- Rahme, M.; Parsa, V.; Farahani, M.; Folkeard, P.; Scollie, S.; Johnsrude, I.S. Evaluating the Validity of Gazepoint GP3 HD in Assessing Listening Effort: A Pupillometry Study. Am. J. Audiol. 2025, 34, 742–753. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef]
- Neher, T.; Grimm, G.; Hohmann, V.; Kollmeier, B. Do Hearing Loss and Cognitive Function Modulate Benefit From Different Binaural Noise-Reduction Settings? Ear Hear. 2014, 35, e52–e62. [Google Scholar] [CrossRef]
- Winn, M.B.; Wendt, D.; Koelewijn, T.; Kuchinsky, S.E. Best Practices and Advice for Using Pupillometry to Measure Listening Effort: An Introduction for Those Who Want to Get Started. Trends Hear. 2018, 22, 2331216518800869. [Google Scholar] [CrossRef]
- Fiedler, L.; Seifi Ala, T.; Graversen, C.; Alickovic, E.; Lunner, T.; Wendt, D. Hearing Aid Noise Reduction Lowers the Sustained Listening Effort During Continuous Speech in Noise—A Combined Pupillometry and EEG Study. Ear Hear. 2021, 42, 1590–1601. [Google Scholar] [CrossRef]
- Wang, Y.; Naylor, G.; Kramer, S.E.; Zekveld, A.A.; Wendt, D.; Ohlenforst, B.; Lunner, T. Relations Between Self-Reported Daily-Life Fatigue, Hearing Status, and Pupil Dilation During a Speech Perception in Noise Task. Ear Hear. 2018, 39, 573–582. [Google Scholar] [CrossRef]
- Wendt, D.; Dau, T.; Hjortkjær, J. Impact of Background Noise and Sentence Complexity on Processing Demands during Sentence Comprehension. Front. Psychol. 2016, 7, 345. [Google Scholar] [CrossRef]
- Van Der Wel, P.; Van Steenbergen, H. Pupil Dilation as an Index of Effort in Cognitive Control Tasks: A Review. Psychon. Bull. Rev. 2018, 25, 2005–2015. [Google Scholar] [CrossRef]
- McGarrigle, R.; Rakusen, L.; Mattys, S. Effortful Listening under the Microscope: Examining Relations between Pupillometric and Subjective Markers of Effort and Tiredness from Listening. Psychophysiology 2021, 58, e13703. [Google Scholar] [CrossRef]
- Picou, E.M.; Ricketts, T.A. The Relationship between Speech Recognition, Behavioural Listening Effort, and Subjective Ratings. Int. J. Audiol. 2018, 57, 457–467. [Google Scholar] [CrossRef]
- Keur-Huizinga, L.; Huizinga, N.A.; Zekveld, A.A.; Versfeld, N.J.; Van De Ven, S.R.B.; Van Dijk, W.A.J.; De Geus, E.J.C.; Kramer, S.E. Effects of Hearing Acuity on Psychophysiological Responses to Effortful Speech Perception. Hear. Res. 2024, 448, 109031. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarangi, L.; Johnson, J.; Bidelman, G.M. Effects of Digital Noise Reduction Processing on Subjective and Objective (Pupillometry) Assays of Listening Effort. Audiol. Res. 2025, 15, 122. https://doi.org/10.3390/audiolres15050122
Sarangi L, Johnson J, Bidelman GM. Effects of Digital Noise Reduction Processing on Subjective and Objective (Pupillometry) Assays of Listening Effort. Audiology Research. 2025; 15(5):122. https://doi.org/10.3390/audiolres15050122
Chicago/Turabian StyleSarangi, Lipika, Jani Johnson, and Gavin M. Bidelman. 2025. "Effects of Digital Noise Reduction Processing on Subjective and Objective (Pupillometry) Assays of Listening Effort" Audiology Research 15, no. 5: 122. https://doi.org/10.3390/audiolres15050122
APA StyleSarangi, L., Johnson, J., & Bidelman, G. M. (2025). Effects of Digital Noise Reduction Processing on Subjective and Objective (Pupillometry) Assays of Listening Effort. Audiology Research, 15(5), 122. https://doi.org/10.3390/audiolres15050122






