Association between Glomerular Filtration Rate and β-Thalassemia Major: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Quality Assessment
2.4. Data Extraction and Analyses
2.5. Publication Bias and Sensitivity Analyses
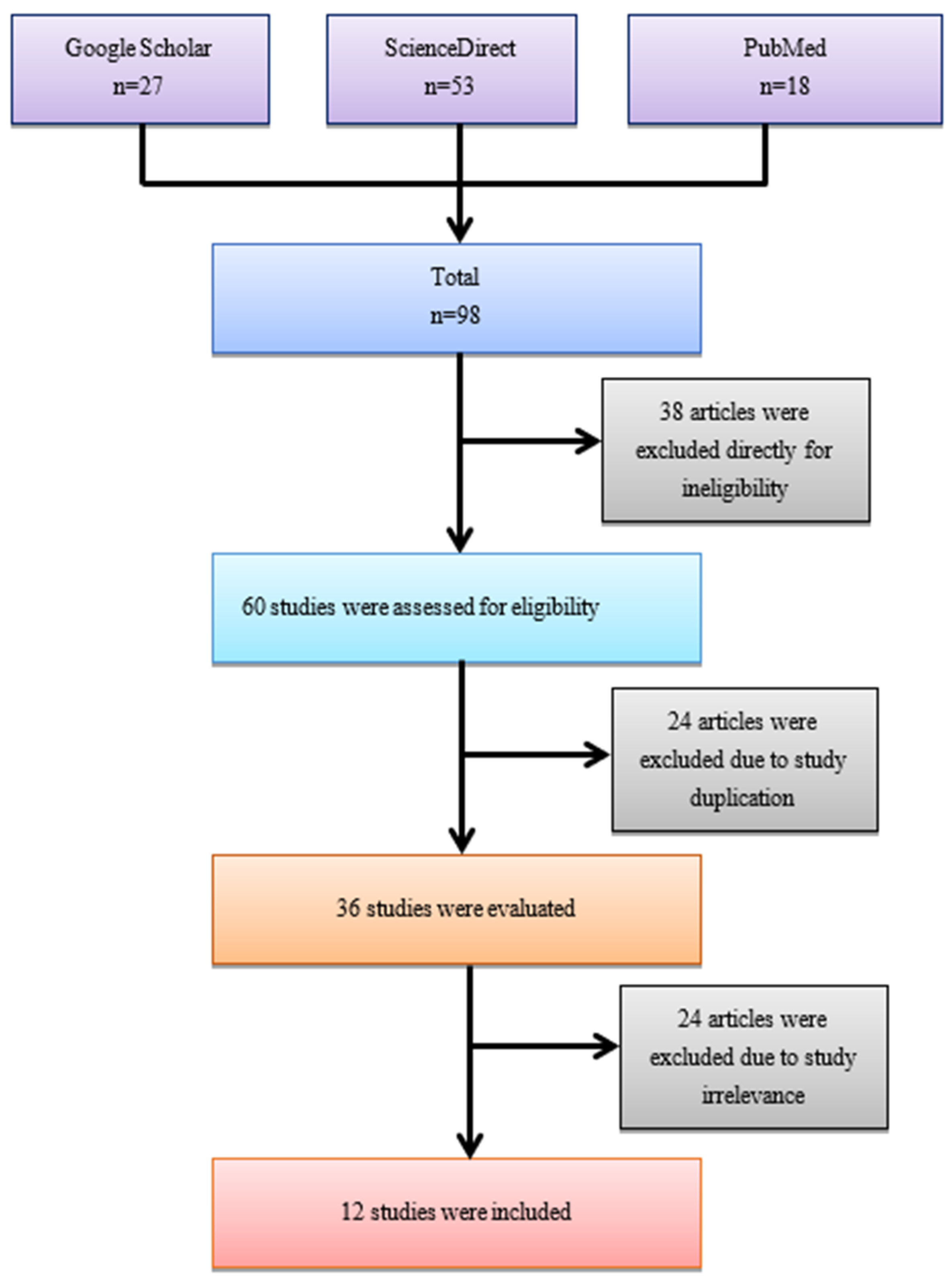
3. Results
3.1. Inclusion of the Study
3.2. Assessment of Quality
3.3. Study Characteristics
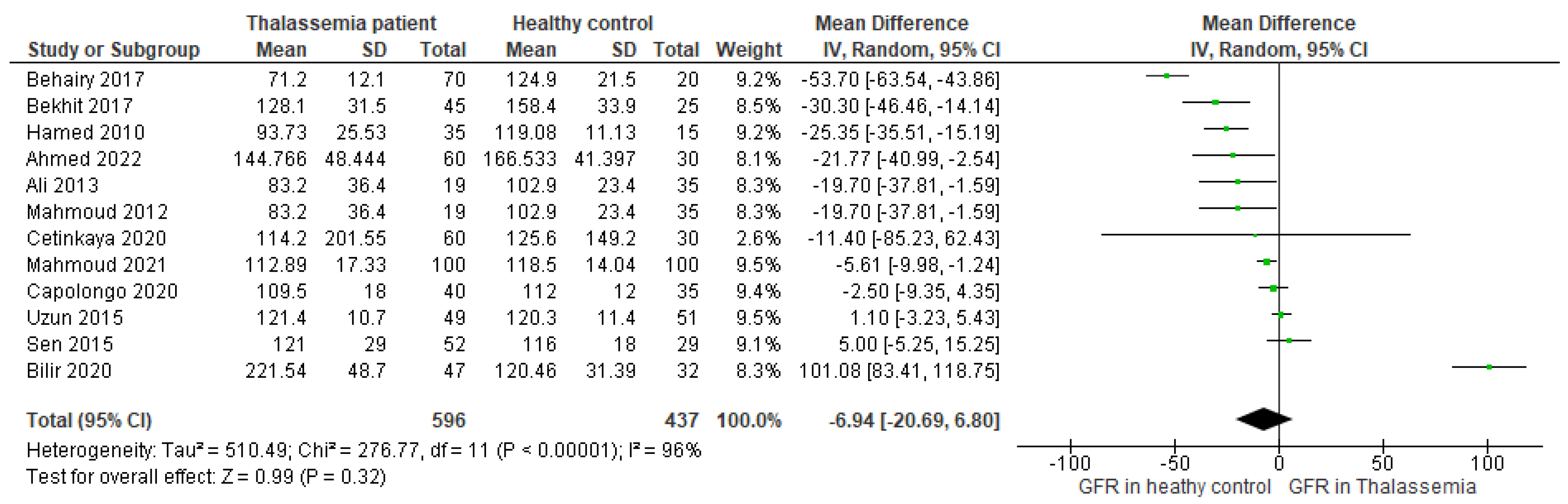
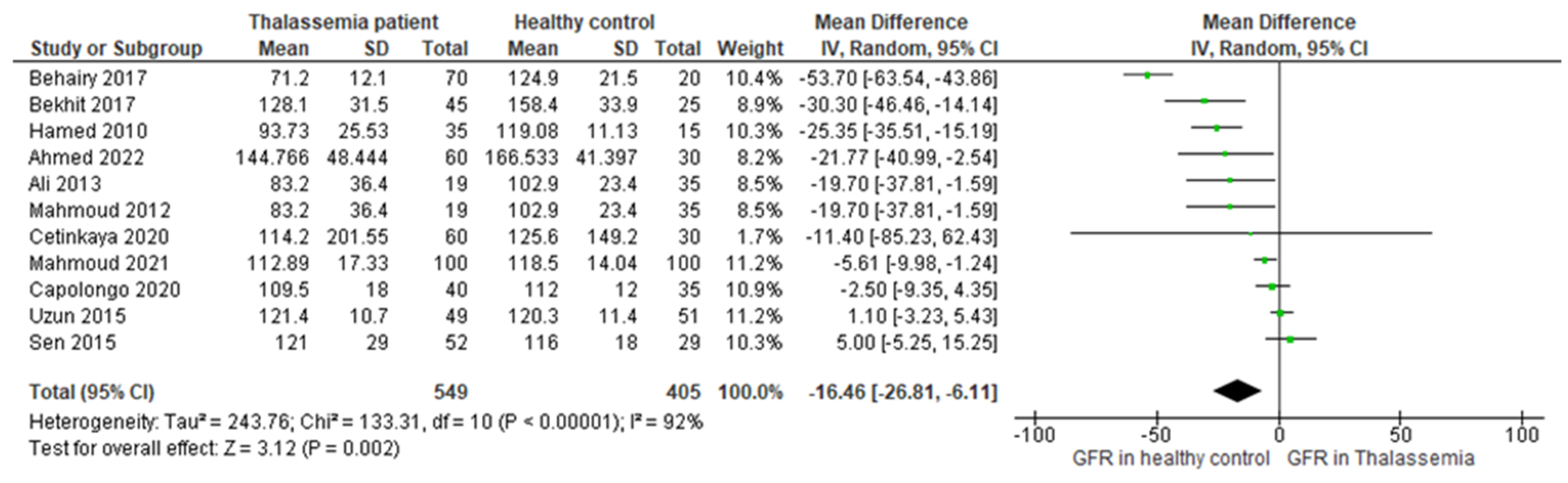
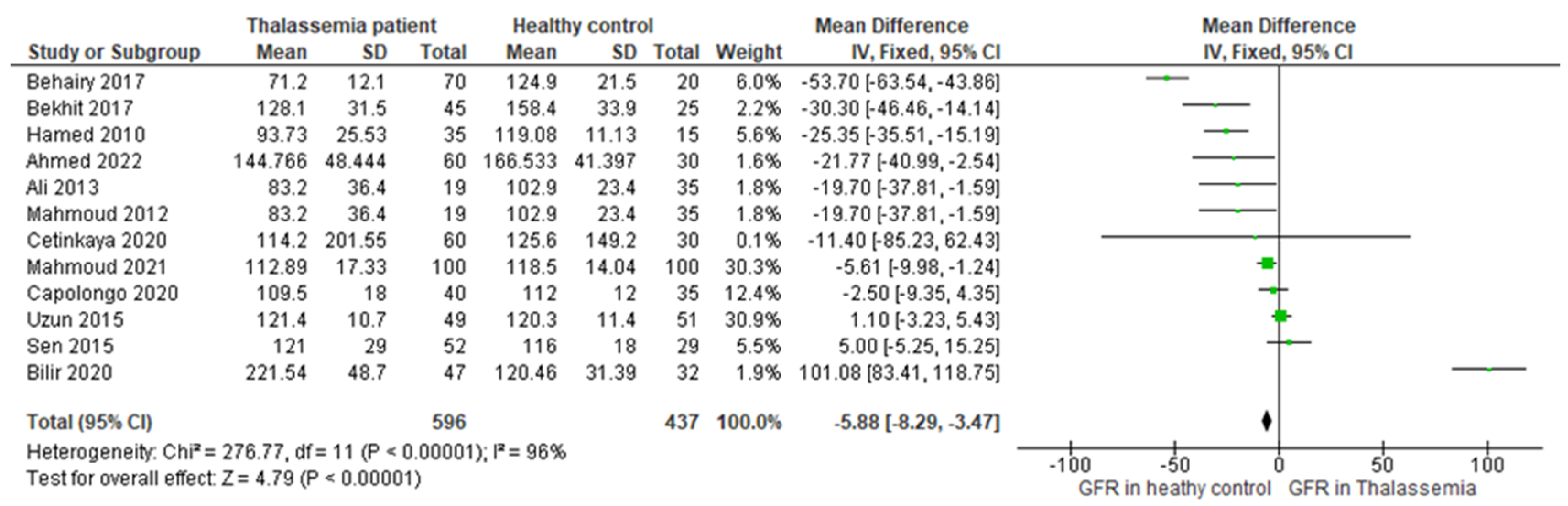
3.4. Main Outcome
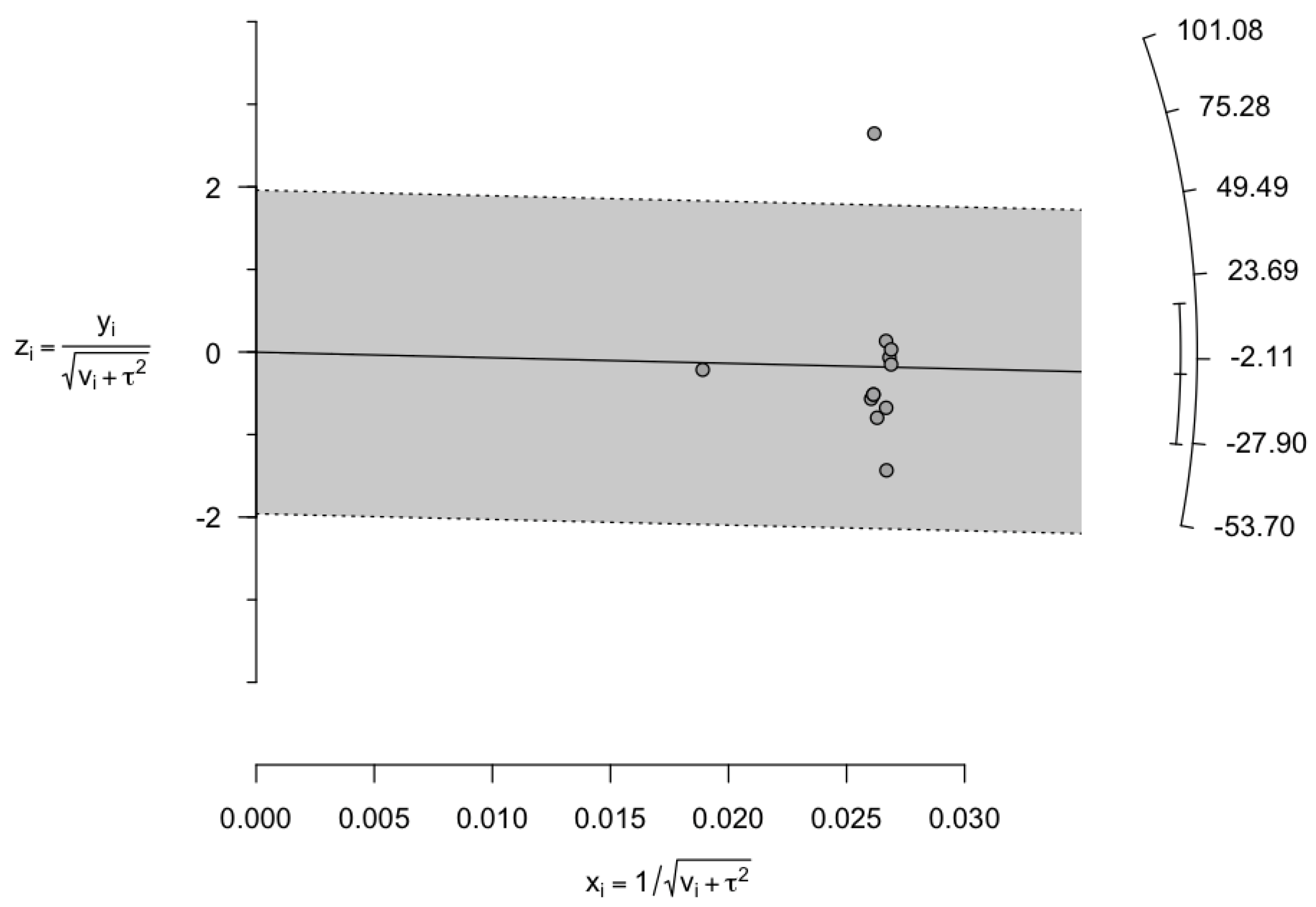
3.5. Assessment of Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helmi, N.; Bashir, M.; Shireen, A.; Ahmed, I.M. Thalassemia review: Features, dental considerations and management. Electron. Physician 2017, 9, 4003. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.J. Update on thalassemia: Clinical care and complications. Hematol. Oncol. Clin. N. Am. 2010, 24, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Aessopos, A.; Farmakis, D.; Tsironi, M.; Diamanti-Kandarakis, E.; Matzourani, M.; Fragodimiri, C.; Hatziliami, A.; Karagiorga, M. Endothelial function and arterial stiffness in sickle-thalassemia patients. Atherosclerosis 2007, 191, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Colah, R.; Gorakshakar, A.; Nadkarni, A. Global burden, distribution and prevention of β-thalassemias and hemoglobin E disorders. Expert Rev. Hematol. 2010, 3, 103–117. [Google Scholar] [CrossRef]
- Weatherall, D.; Akinyanju, O.; Fucharoen, S.; Olivieri, N.; Musgrove, P. Inherited disorders of hemoglobin. In Disease Control Priorities in Developing Countries, 2nd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Tari, K.; Valizadeh Ardalan, P.; Abbaszadehdibavar, M.; Atashi, A.; Jalili, A.; Gheidishahran, M. Thalassemia an update: Molecular basis, clinical features and treatment. Int. J. Biomed. Public Health 2018, 1, 48–58. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Coresh, J. GFR estimation: From physiology to public health. Am. J. Kidney Dis. 2014, 63, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Levey, A.S. Measured GFR as a confirmatory test for estimated GFR. J. Am. Soc. Nephrol. JASN 2009, 20, 2305–2313. [Google Scholar] [CrossRef]
- Matsushita, K.; Selvin, E.; Bash, L.D.; Franceschini, N.; Astor, B.C.; Coresh, J. Change in estimated GFR associates with coronary heart disease and mortality. J. Am. Soc. Nephrol. JASN 2009, 20, 2617. [Google Scholar] [CrossRef]
- Mathisen, U.D.; Melsom, T.; Ingebretsen, O.C.; Jenssen, T.; Njølstad, I.; Solbu, M.D.; Toft, I.; Eriksen, B.O. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J. Am. Soc. Nephrol. JASN 2011, 22, 927. [Google Scholar] [CrossRef]
- Di Bonito, P.; Valerio, G.; Licenziati, M.; Miraglia Del Giudice, E.; Baroni, M.; Morandi, A.; Maffeis, C.; Campana, G.; Spreghini, M.; Di Sessa, A. High uric acid, reduced glomerular filtration rate and non-alcoholic fatty liver in young people with obesity. J. Endocrinol. Investig. 2020, 43, 461–468. [Google Scholar] [CrossRef]
- Arnello, F.; Ham, H.; Tondeur, M.; Piepsz, A. Evolution of single kidney glomerular filtration rate in urinary tract infection. Pediatr. Nephrol. 1999, 13, 121–124. [Google Scholar] [CrossRef]
- Bilir, Ö.A.; Kirkiz, S.; Fettah, A.; Bozkaya, İ.O.; Kara, A.; Çakar, N.; Yaralı, N. Renal function and the oxidative status among children with thalassemia major and healthy controls: A cross-sectional study. Transfus. Apher. Sci. 2020, 59, 102746. [Google Scholar] [CrossRef] [PubMed]
- Behairy, O.G.; Abd Almonaem, E.R.; Abed, N.T.; Abdel Haiea, O.M.; Zakaria, R.M.; AbdEllaty, R.I.; Asr, E.H.; Mansour, A.I.; Abdelrahman, A.M.; Elhady, H.A. Role of serum cystatin-C and beta-2 microglobulin as early markers of renal dysfunction in children with beta thalassemia major. Int. J. Nephrol. Renov. Dis. 2017, 10, 261–268. [Google Scholar] [CrossRef]
- NIH. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 25 July 2023).
- UNC. Systematic Reviews: Step 6: Assess Quality of Included Studies. Available online: https://guides.lib.unc.edu/systematic-reviews/assess-quality (accessed on 25 July 2023).
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Prodhan, A.S.U.; Khandker, S.S.; Reshetnyak, T.; Kotyla, P.J.; Hassan, R.; Hossan, T. Prevalence of antiphospholipid antibodies in Behçet’s disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227836. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Georgy, D.B.; Meabed, M.H.; Botrous, O.E. The association between plasma microRNA-451 expression levels and chronic kidney disease in children with β-thalassemia major. Iran. J. Kidney Dis. 2022, 16, 188. [Google Scholar]
- Ali, B.A.; Mahmoud, A.M. Frequency of glomerular dysfunction in children with beta thalassaemia major. Sultan Qaboos Univ. Med. J. 2014, 14, e88. [Google Scholar] [CrossRef][Green Version]
- Bekhit, O.E.; El Dash, H.H.; Ahmed, M.S. Early detection of kidney dysfunction in Egyptian patients with beta-thalassemia major. Egypt. Pediatr. Assoc. Gaz. 2017, 65, 85–89. [Google Scholar] [CrossRef]
- Cetinkaya, P.U.; Azik, F.M.; Karakus, V.; Huddam, B.; Yilmaz, N. β2-microglobulin, neutrophil gelatinase-associated lipocalin, and endocan values in evaluating renal functions in patients with β-thalassemia major. Hemoglobin 2020, 44, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; Elian, D.M.; Abd El Hady, N.M.; Abdallah, H.M.; Abdelsattar, S.; Khalil, F.O.; Abd El Naby, S.A. Assessment of subclinical renal glomerular and tubular dysfunction in children with beta thalassemia major. Children 2021, 8, 100. [Google Scholar] [CrossRef]
- Şen, V.; Ece, A.; Uluca, Ü.; Söker, M.; Güneş, A.; Kaplan, İ.; Tan, İ.; Yel, S.; Mete, N.; Sahin, C. Urinary early kidney injury molecules in children with beta-thalassemia major. Ren. Fail. 2015, 37, 607–613. [Google Scholar] [CrossRef]
- Uzun, E.; Balcı, Y.I.; Yüksel, S.; Aral, Y.Z.; Aybek, H.; Akdağ, B. Glomerular and tubular functions in children with different forms of beta thalassemia. Ren. Fail. 2015, 37, 1414–1418. [Google Scholar] [CrossRef]
- Capolongo, G.; Zacchia, M.; Beneduci, A.; Costantini, S.; Cinque, P.; Spasiano, A.; De Luca, G.; Di Pietro, M.E.; Ricchi, P.; Trepiccione, F. Urinary metabolic profile of patients with transfusion-dependent β-thalassemia major undergoing deferasirox therapy. Kidney Blood Press. Res. 2020, 45, 455–466. [Google Scholar] [CrossRef]
- Hamed, E.A.; ElMelegy, N.T. Renal functions in pediatric patients with beta-thalassemia major: Relation to chelation therapy: Original prospective study. Ital. J. Pediatr. 2010, 36, 39. [Google Scholar] [CrossRef]
- Mahmoud, A.; Ali, B. Cystatin C as an Early Marker of Glomerular Dysfunction in Children with Beta Thalassemia Major. Bull. Egypt. Soc. Physiol. Sci. 2012, 32, 265–278. [Google Scholar] [CrossRef]
- Saghir, S.; Riaz, A.; Hasan, A.; Bhatti, Y.A.; Ghuman, A.A.; Shakil, M. Cystatin C an early marker of Glomerular dysfunction in thalassemia major. Prof. Med. J. 2020, 27, 300–308. [Google Scholar] [CrossRef][Green Version]
- Cai, Q.; Dekker, L.H.; Bakker, S.J.; de Borst, M.H.; Navis, G.J. Dietary patterns based on estimated glomerular filtration rate and kidney function decline in the general population: The lifelines cohort study. Nutrients 2020, 12, 1099. [Google Scholar] [CrossRef]
- Jalali, A.; Khalilian, H.; Ahmadzadeh, A.; Sarvestani, S.; Rahim, F.; Zandian, K.; Asar, S. Renal function in transfusion-dependent pediatric beta-thalassemia major patients. Hematology 2011, 16, 249–254. [Google Scholar] [CrossRef]
- Koren, G.; Kochavi-Atiya, Y.; Bentur, Y.; Olivieri, N. The effects of subcutaneous deferoxamine administration on renal function in thalassemia major. Int. J. Hematol. 1991, 54, 371–375. [Google Scholar] [PubMed]
- Ghobrial, E.E.; Abdel-Aziz, H.A.; Kaddah, A.M.; Mubarak, N.A. Urinary transforming growth factor β-1 as a marker of renal dysfunction in sickle cell disease. Pediatr. Neonatol. 2016, 57, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Haymann, J.-P.; Stankovic, K.; Levy, P.; Avellino, V.; Tharaux, P.-L.; Letavernier, E.; Grateau, G.; Baud, L.; Girot, R.; Lionnet, F. Glomerular hyperfiltration in adult sickle cell anemia: A frequent hemolysis associated feature. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 756. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, J.; Tarhini, A.; Taher, A.T. Renal complications in thalassemia. Thalass. Rep. 2018, 8, 7481. [Google Scholar] [CrossRef]
- de Dreuzy, E.; Bhukhai, K.; Leboulch, P.; Payen, E. Current and future alternative therapies for beta-thalassemia major. Biomed. J. 2016, 39, 24–38. [Google Scholar] [CrossRef]
- Milo, G.; Nevo, R.F.G.; Pazgal, I.; Gafter-Gvili, A.; Shpilberg, O.; Gafter, U.; Erman, A.; Stark, P. GFR in Patients with β-Thalassemia Major. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 1350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwartz, G.J.; Furth, S.L. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr. Nephrol. 2007, 22, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.; Nyman, U.; Björk, J.; Lindström, V.; Rippe, B.; Sterner, G.; Christensson, A. Simple cystatin C–based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan–Barratt prediction equations for children. Clin. Chem. 2005, 51, 1420–1431. [Google Scholar] [CrossRef]
- Michels, W.M.; Grootendorst, D.C.; Verduijn, M.; Elliott, E.G.; Dekker, F.W.; Krediet, R.T. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1003. [Google Scholar] [CrossRef] [PubMed]

| Study ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Overall Score (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed 2022 | Y | Y | Y | Y | Y | N | U | Y | Y | 77.7 |
| Ali 2013 | Y | Y | Y | Y | Y | N | NA | Y | Y | 87.5 |
| Bilir 2020 | Y | Y | Y | Y | Y | N | U | Y | Y | 77.7 |
| Behairy 2017 | Y | Y | Y | Y | Y | N | U | Y | Y | 77.7 |
| Bekhit 2017 | Y | Y | Y | Y | Y | N | U | Y | Y | 77.7 |
| Capolongo 2020 | Y | Y | Y | Y | Y | N | U | Y | Y | 77.7 |
| Cetinkaya 2020 | Y | Y | Y | Y | Y | N | U | Y | Y | 77.7 |
| Hamed 2010 | Y | Y | Y | Y | Y | N | Y | Y | Y | 88.8 |
| Mahmoud 2012 | Y | Y | Y | Y | Y | N | NA | Y | Y | 87.5 |
| Mahmoud 2021 | Y | Y | Y | Y | Y | N | NA | Y | Y | 87.5 |
| Sen 2015 | Y | Y | Y | U | Y | N | U | Y | Y | 66.6 |
| Uzun 2015 | Y | Y | Y | Y | Y | N | U | Y | Y | 77.7 |
| Study ID | Location | Study Type | Number of Participants | Participants’ Demographics | GFR Measurement Method | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Male (%) | Female (%) | BMI | Age | Case Type | Control Type | |||||
| Ahmed 2022 | Egypt | Case–control | 60 | 30 | 64 | 36 | NR | 9.35 ± 6.14 | β-TM a | Healthy control | Simple height-independent formula | [19] |
| Ali 2013 | Egypt | Cross-sectional | 19 | 35 | 63.2 * | 36.8 * | NR | 9.80 ± 1.70 * | β-TM | Healthy control | Schwartz formula | [20] |
| Bilir 2020 | Turkey | Cross-sectional | 47 | 32 | 58.23 | 41.77 | 18.57 ± 2.32 | 12.03 ± 4.47 | β-TM | Healthy control | Schwartz formula | [13] |
| Behairy 2017 | Egypt | Case–control | 70 | 20 | 64.5 | 35.5 | 21.9 ± 5.35 | 9.5 ± 3.66 | β-TM | Healthy control | Schwartz formula | [14] |
| Bekhit 2017 | Egypt | Case–control | 45 | 25 | 46.66 * | 53.34 * | NR | 8.30 ± 3.50 * | β-TM | Healthy control | Schwartz formula | [21] |
| Capolongo 2020 | Italy | Case–control | 40 | 35 | NR | NR | 23.35 ± 2.35 | 33.5 ± 13 | β-TM | Healthy control | Schwartz formula and CKD-EPI | [26] |
| Cetinkaya 2020 | Turkey | Case–control | 60 | 30 | 60 | 40 | NR | 21.5 ± 23 | β-TM | Healthy control | Schwartz formula | [22] |
| Hamed 2010 | Egypt | Case–control | 35 | 15 | 66.67 | 33.33 | 16.69 ± 2.75 | 8.56 ± 3.9 | TDβ-TM | Healthy control | Schwartz formula | [27] |
| Mahmoud 2012 | Egypt | Cross-sectional | 19 | 35 | 61.33 | 38.67 | 17.75 ± 2.6 | 9.7 ± 1.4 | β-TM | Healthy control | Schwartz formula | [28] |
| Mahmoud 2021 | Egypt | Case–control | 100 | 100 | 59 | 41 | NR | 9 ± 9.75 | β-TM | Healthy control | Schwartz formula | [23] |
| Sen 2015 | Turkey | Cross-sectional | 52 | 29 | 55.55 | 44.45 | NR | 8.95 ± 4.2 | β-TM | Healthy control | Schwartz formula | [24] |
| Uzun 2015 | Turkey | Case–control | 49 | 51 | 52 | 48 | NR | 9.95 ± 4.1 | β-TM | Healthy control | Schwartz formula | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandker, S.S.; Jannat, N.; Sarkar, D.; Pranto, A.H.; Hoque, I.A.; Zaman, J.; Uddin, M.N.; Suez, E. Association between Glomerular Filtration Rate and β-Thalassemia Major: A Systematic Review and Meta-Analysis. Thalass. Rep. 2023, 13, 195-205. https://doi.org/10.3390/thalassrep13030018
Khandker SS, Jannat N, Sarkar D, Pranto AH, Hoque IA, Zaman J, Uddin MN, Suez E. Association between Glomerular Filtration Rate and β-Thalassemia Major: A Systematic Review and Meta-Analysis. Thalassemia Reports. 2023; 13(3):195-205. https://doi.org/10.3390/thalassrep13030018
Chicago/Turabian StyleKhandker, Shahad Saif, Nurani Jannat, Deepannita Sarkar, Alif Hasan Pranto, Ismoth Ara Hoque, Jemema Zaman, Md. Nizam Uddin, and Ehsan Suez. 2023. "Association between Glomerular Filtration Rate and β-Thalassemia Major: A Systematic Review and Meta-Analysis" Thalassemia Reports 13, no. 3: 195-205. https://doi.org/10.3390/thalassrep13030018
APA StyleKhandker, S. S., Jannat, N., Sarkar, D., Pranto, A. H., Hoque, I. A., Zaman, J., Uddin, M. N., & Suez, E. (2023). Association between Glomerular Filtration Rate and β-Thalassemia Major: A Systematic Review and Meta-Analysis. Thalassemia Reports, 13(3), 195-205. https://doi.org/10.3390/thalassrep13030018






