Scope and Limits of Teriparatide Use in Delayed and Nonunions: A Case Series
Abstract
:1. Introduction
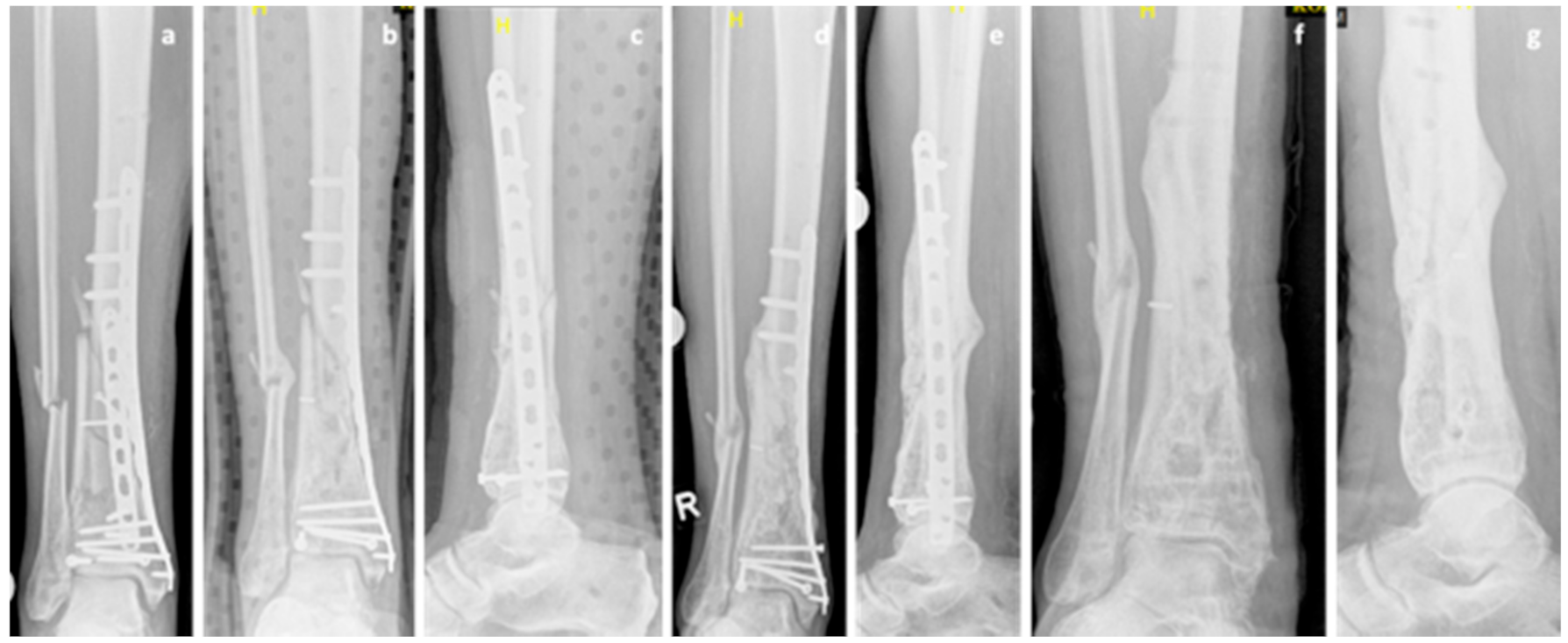
2. Case Series
3. Discussion
3.1. Safety and Feasibility of Teriparatide Application
3.2. Spectrum of Teriparatide Indications in Nonunions and Delayed Unions
3.3. Nonunions and Delayed Unions with Bisphosphonate Treatment
3.4. Lack of Definitions for Nonunions and Delayed Unions as Well as of Standardized Protocols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santolini, E.; West, R.; Giannoudis, P.V. Risk factors for long bone fracture non-union: A stratification approach based on the level of the existing scientific evidence. Injury 2015, 46 (Suppl. 8), S8–S19. [Google Scholar] [CrossRef]
- Canintika, A.F.; Dilogo, I.H. Teriparatide for treating delayed union and nonunion: A systematic review. J. Clin. Orthop. Trauma 2020, 11, S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, T.W.; Kakar, S.; Einhorn, T.A. New technologies for the enhancement of skeletal repair. Injury 2007, 38 (Suppl. 1), S49–S62. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Gudipati, S.; Harwood, P.; Kanakaris, N.K. Long bone non-unions treated with the diamond concept: A case series of 64 patients. Injury 2015, 46 (Suppl. 8), S48–S54. [Google Scholar] [CrossRef]
- Frölke, J.P.; Patka, P. Definition and classification of fracture non-unions. Injury 2007, 38 (Suppl. 2), S19–S22. [Google Scholar] [CrossRef]
- Corrales, L.A.; Morshed, S.; Bhandari, M.; Miclau, T., 3rd. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J. Bone Joint Surg. Am. 2008, 90, 1862–1868. [Google Scholar] [CrossRef]
- Marongiu, G.; Dolci, A.; Verona, M.; Capone, A. The biology and treatment of acute long-bones diaphyseal fractures: Overview of the current options for bone healing enhancement. Bone Rep. 2020, 12, 100249. [Google Scholar] [CrossRef]
- Marongiu, G.; Contini, A.; Cozzi Lepri, A.; Donadu, M.; Verona, M.; Capone, A. The Treatment of Acute Diaphyseal Long-bones Fractures with Orthobiologics and Pharmacological Interventions for Bone Healing Enhancement: A Systematic Review of Clinical Evidence. Bioengineering 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Whelan, D.B.; Bhandari, M.; Stephen, D.; Kreder, H.; McKee, M.D.; Zdero, R.; Schemitsch, E.H. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J. Trauma 2010, 68, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, J.T.; Bauer, D.C. Clinical use of bone turnover markers to monitor pharmacologic fracture prevention therapy. Curr. Osteoporos. Rep. 2012, 10, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.P.; Haddad, F.S. The Unified Classification System (UCS): Improving our understanding of periprosthetic fractures. Bone Joint J. 2014, 96, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Gustilo, R.B.; Anderson, J.T. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: Retrospective and prospective analyses. J. Bone Joint Surg. Am. 1976, 58, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Black, D.M.; Greenspan, S.L.; Ensrud, K.E.; Palermo, L.; McGowan, J.A.; Lang, T.F.; Garnero, P.; Bouxsein, M.L.; Bilezikian, J.P.; Rosen, C.J. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N. Engl. J. Med. 2003, 349, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Hodsman, A.B.; Fraher, L.J.; Watson, P.H.; Ostbye, T.; Stitt, L.W.; Adachi, J.D.; Taves, D.H.; Drost, D. A randomized controlled trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 1997, 82, 620–628. [Google Scholar] [CrossRef]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef]
- Cranney, A.; Papaioannou, A.; Zytaruk, N.; Hanley, D.; Adachi, J.; Goltzman, D.; Murray, T.; Hodsman, A. Parathyroid hormone for the treatment of osteoporosis: A systematic review. CMAJ 2006, 175, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, F.; Kurinomaru, N.; Hijioka, A. Weekly Teriparatide for Delayed Unions of Atypical Subtrochanteric Femur Fractures. Biol. Ther. 2014, 4, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Mitani, Y. Effective treatment of a steroid-induced femoral neck fracture nonunion with a once-weekly administration of teriparatide in a rheumatoid patient: A case report. Arch. Osteoporos. 2013, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochi, K.; Ikari, K.; Naomi, A.; Momohara, S. Administration of teriparatide treatment for a challenging case of nonunion of periprosthetic fracture after total knee arthroplasty. Arch. Osteoporos. 2013, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Tachiiri, H.; Okuda, Y.; Yamasaki, T.; Kusakabe, T. Weekly teriparatide administration for the treatment of delayed union: A report of two cases. Arch. Osteoporos. 2014, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Pola, E.; Pambianco, V.; Colangelo, D.; Formica, V.M.; Autore, G.; Nasto, L.A. Teriparatide anabolic therapy as potential treatment of type II dens non-union fractures. World J. Orthop. 2017, 8, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Bednar, D.A. Teriparatide treatment of a glucocorticoid-associated resorbing nonunion of a type III odontoid process fracture: A case report. J. Spinal. Disord. Tech. 2013, 26, E319–E322. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Ando, M.; Sasaki, S. Effective treatment of delayed union of a lumbar vertebral fracture with daily administration of teriparatide in a patient with diffuse idiopathic skeletal hyperostosis. Eur. Spine. J. 2015, 24 (Suppl. 4), 573–576. [Google Scholar] [CrossRef]
- Rubery, P.T.; Bukata, S.V. Teriparatide may accelerate healing in delayed unions of type III odontoid fractures: A report of 3 cases. Clin. Spine Surg. 2010, 23, 151–155. [Google Scholar] [CrossRef]
- Chintamaneni, S.; Finzel, K.; Gruber, B.L. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporos. Int. 2010, 21, 1059–1063. [Google Scholar] [CrossRef]
- Nauth, A.; Lee, M.; Gardner, M.J.; Brinker, M.R.; Warner, S.J.; Tornetta, P., 3rd; Leucht, P. Principles of Nonunion Management: State of the Art. J. Orthop. Trauma 2018, 32 (Suppl. 1), S52–S57. [Google Scholar] [CrossRef]
- Jain, A.K.; Sinha, S. Infected nonunion of the long bones. Clin. Orthop. Relat. Res. 2005, 57–65. [Google Scholar] [CrossRef]
- Coppola, C.; Del Buono, A.; Maffulli, N. Teriparatide in Fracture Non-Unions. Transl. Med. UniSa. 2015, 12, 47–53. [Google Scholar] [PubMed]
- Yue, B.; Ng, A.; Tang, H.; Joseph, S.; Richardson, M. Delayed healing of lower limb fractures with bisphosphonate therapy. Ann. R. Coll. Surg. Engl. 2015, 97, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastirr, I.; Reichardt, M.; Andresen, R.; Radmer, S.; Schröder, G.; Westphal, T.; Mittlmeier, T.; Schober, H.C. Therapy of aseptic nonunions with parathyroid hormone. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 169–173. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismailidis, P.; Suhm, N.; Clauss, M.; Mündermann, A.; Cadosch, D. Scope and Limits of Teriparatide Use in Delayed and Nonunions: A Case Series. Clin. Pract. 2021, 11, 47-57. https://doi.org/10.3390/clinpract11010009
Ismailidis P, Suhm N, Clauss M, Mündermann A, Cadosch D. Scope and Limits of Teriparatide Use in Delayed and Nonunions: A Case Series. Clinics and Practice. 2021; 11(1):47-57. https://doi.org/10.3390/clinpract11010009
Chicago/Turabian StyleIsmailidis, Petros, Norbert Suhm, Martin Clauss, Annegret Mündermann, and Dieter Cadosch. 2021. "Scope and Limits of Teriparatide Use in Delayed and Nonunions: A Case Series" Clinics and Practice 11, no. 1: 47-57. https://doi.org/10.3390/clinpract11010009
APA StyleIsmailidis, P., Suhm, N., Clauss, M., Mündermann, A., & Cadosch, D. (2021). Scope and Limits of Teriparatide Use in Delayed and Nonunions: A Case Series. Clinics and Practice, 11(1), 47-57. https://doi.org/10.3390/clinpract11010009





