The Clinical Utility of ABO and RHD Systems as Potential Indicators of Health Status, a Preliminary Study in Greek Population
Abstract
1. Introduction
2. Material and Methods
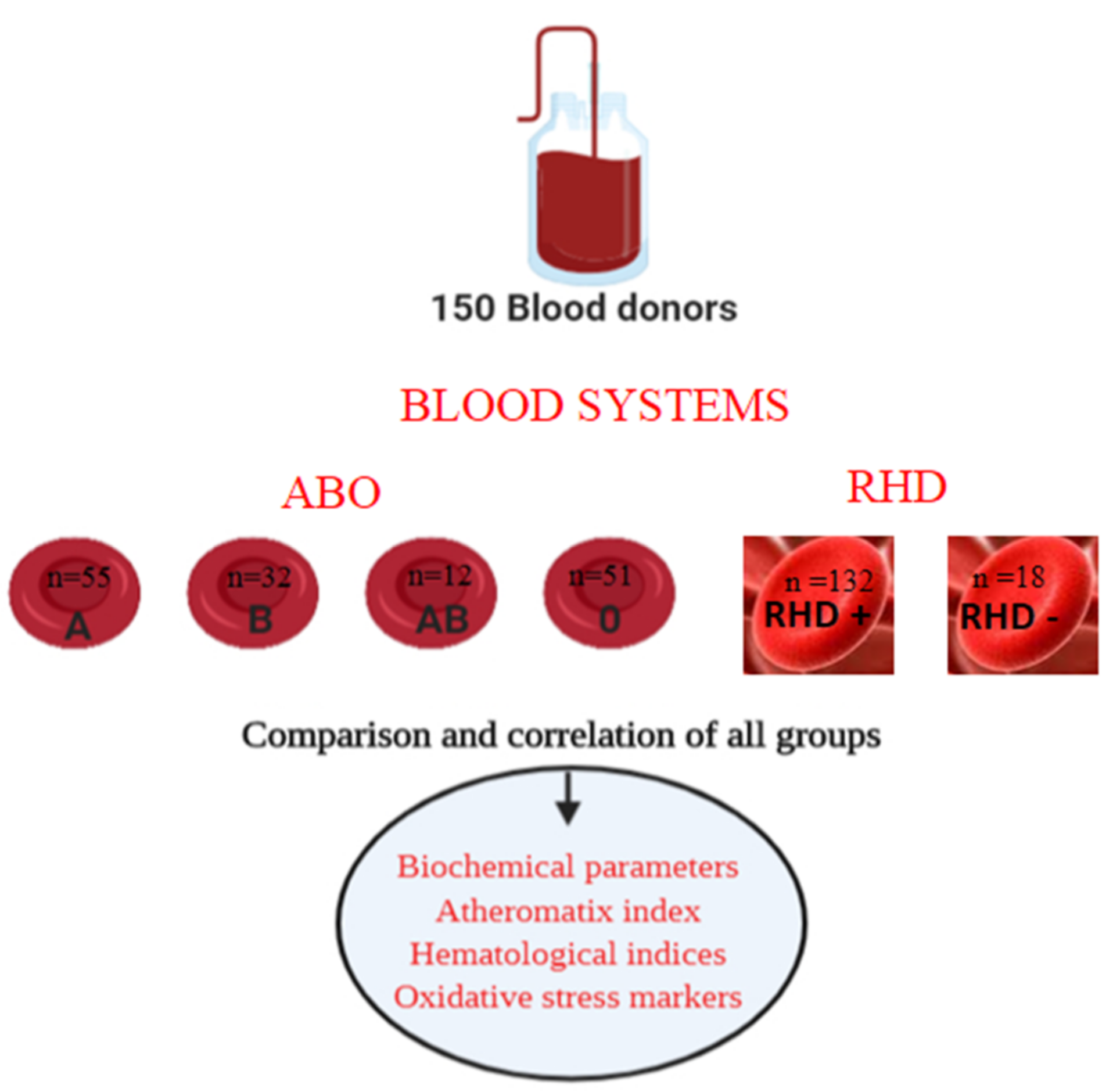
2.1. Study Design
2.2. Blood Collection
2.3. Blood Analysis
2.4. ABO Blood & Rhesus System Detection
2.5. Ethical Statement
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Correlations of Biochemical and Hematological Parameters in Different ABO Blood Groups
3.3. Correlations of Biochemical and Hematological Parameters in Different Rh-Blood Groups
3.4. Correlations of Biochemical and Hematological Parameters according to Gender in Different Blood Groups
3.5. Limitations of the Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Atheromatic Index |
| ALT | Alanine aminotransferase |
| AST | Aspartate transaminase |
| B | Blood |
| CDV | Cardiovascular disease |
| CH | Cholesterol |
| Hb | Hemoglobin |
| Ht | Hematocrit |
| Fe | Ferritin |
| FVIII | Coagulation Factor |
| HDL | High density lipoprotein |
| LDL | Low density Lipoprotein |
| U.A | Uric acid |
| VWF | von Willebrand factor |
| S.I | serum iron |
| TAS | Total Antioxidant Status |
| Tr | Triglycerides |
References
- Liumbruno, G.M.; Franchini, M. Hemostasis, cancer, and ABO blood group: The most recent evidence of association. J. Thromb. Thrombolysis 2014, 38, 160–166. [Google Scholar] [CrossRef]
- Franchini, M.; Bonfanti, C. Evolutionary aspects of ABO blood group in humans. Clin. Chim. Acta 2015, 444, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Storry, J.R.; Olsson, M.L. The ABO blood group system revisited: A review and update. Immunohematology 2009, 25, 48–59. [Google Scholar] [CrossRef]
- Eastlund, T. The histo-blood group ABO system and tissue transplantation. Transfusion 2003, 38, 975–988. [Google Scholar] [CrossRef]
- Roberts, D.F.; Tills, D.; Kopec, A.C.; Tills, R.E. The Distribution of the Human Blood Groups and Other Polymorphisms. Man 1984, 19, 674. [Google Scholar] [CrossRef]
- Roberts, D.F.; Mourant, A.E.; Kopec, A.C.; Domaniewska-Sobczak, K. Blood Groups and Diseases: A Study of Associations of Diseases with Blood Groups and Other Polymorphisms. Man 1980, 15, 385–386. [Google Scholar] [CrossRef]
- Whincup, P.H.; Danesh, J.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Rumley, A.; Lowe, G.D.O. Von Willebrand factor and coronary heart disease: Prospective study and meta-analysis. Eur. Heart J. 2002, 23, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Chasman, D.I.; Kellogg, M.; Zee, R.Y.L.; Rifai, N.; Badola, S.; Miletich, J.P.; Ridker, P.M. Novel association of ABO histo-blood group antigen with soluble ICAM-1: Results of a genome-wide association study of 6,578 women. PLoS Genet. 2008, 4, e1000118. [Google Scholar] [CrossRef]
- Barbalic, M.; Dupuis, J.; Dehghan, A.; Bis, J.C.; Hoogeveen, R.C.; Schnabel, R.B.; Nambi, V.; Bretler, M.; Smith, N.L.; Peters, A.; et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum. Mol. Genet. 2010, 19, 1863–1872. [Google Scholar] [CrossRef]
- Qi, L.; Cornelis, M.C.; Kraft, P.; Jensen, M.; van Dam, R.M.; Sun, Q.; Girman, C.J.; Laurie, C.C.; Mirel, D.B.; Hunter, D.J.; et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum. Mol. Genet. 2010, 19, 1856–1862. [Google Scholar] [CrossRef]
- Nathan, D.M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Bayoumi, N.; Vickers, M.A.; Clark, P. ABO(H) blood groups and vascular disease: A systematic review and meta-analysis. J. Thromb. Haemost. 2008, 6, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Zakai, N.A.; Judd, S.E.; Alexander, K.; Mcclure, L.A.; Kissela, B.M.; Howard, G.; Cushman, M. ABO blood type and stroke risk: The REasons for Geographic And Racial Differences in Stroke Study. J. Thromb. Haemost. 2014, 12, 564–570. [Google Scholar] [CrossRef]
- Souto, J.C.; Almasy, L.; Muñiz-Diaz, E.; Soria, J.M.; Borrell, M.; Bayén, L.; Mateo, J.; Madoz, P.; Stone, W.; Blangero, J.; et al. Functional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial thromboplastin time. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.W.; Cushman, M.; Rosamond, W.D.; Heckbert, S.R.; Tracy, R.P.; Aleksic, N.; Folsom, A.R. Coagulation factors, inflammation markers, and venous thromboembolism: The longitudinal investigation of thromboembolism etiology (LITE). Am. J. Med. 2002, 113, 636–642. [Google Scholar] [CrossRef]
- Anstee, D.J. The relationship between blood groups and disease. Blood 2010, 115, 4635–4643. [Google Scholar] [CrossRef] [PubMed]
- Oner, C.; Dogan, B.; Telatar, B.; Yagan, C.F.C.; Oguz, A. Frequency of ABO/Rhesus blood groups in patients with diabetes mellitus. J. Coll. Physicians Surg. Pak. 2016, 26, 74–75. [Google Scholar]
- Edgren, G.; Hjalgrim, H.; Rostgaard, K.; Norda, R.; Wikman, A.; Melbye, M.; Nyrén, O. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: A cohort study. Am. J. Epidemiol. 2010, 172, 1280–1285. [Google Scholar] [CrossRef]
- Xie, J.; Qureshi, A.A.; Li, Y.; Han, J. ABO blood group and incidence of skin cancer. PLoS ONE 2010, 5, e11972. [Google Scholar] [CrossRef]
- Gates, M.A.; Wolpin, B.M.; Cramer, D.W.; Hankinson, S.E.; Tworoger, S.S. ABO blood group and incidence of epithelial ovarian cancer. Int. J. Cancer 2011, 128, 482–486. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, W.K.; Hittelman, W.N.; Ro, J.Y.; Sahin, A.A.; Brown, B.W.; Mountain, C.F. Expression of blood-group antigen A—A favorable prognostic factor in non-small-cell lung cancer. N. Engl. J. Med. 1991, 324, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Borén, T.; Falk, P.; Roth, K.A.; Larson, G.; Normark, S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993, 262, 1892–1895. [Google Scholar] [CrossRef]
- Swerdlow, D.L.; Mintz, E.D.; Rodriguez, M.; Tejada, E.; Ocampo, C.; Espejo, L.; Barrett, T.J.; Petzelt, J.; Bean, N.H.; Seminario, L.; et al. Severe life-threatening cholera associated with blood group 0 in peru: Implications for the latin american epidemic. J. Infect. Dis. 1994, 170, 468–472. [Google Scholar] [CrossRef]
- Kuesap, J.; Na-Bangchang, K. The effect of ABO blood groups, hemoglobinopathy, and heme oxygenase-1 polymorphisms on malaria susceptibility and severity. Korean J. Parasitol. 2018, 56, 167–173. [Google Scholar] [CrossRef]
- Anstee, D.J.; Tanner, M.J.A. 4 Biochemical aspects of the blood group Rh (Rhesus) antigens. Baillieres. Clin. Haematol. 1993, 6, 401–422. [Google Scholar] [CrossRef]
- Cartron, J.P. RH blood group system and molecular basis of Rh-deficiency. Bailliere’s Best Pract. Res. Clin. Haematol. 1999, 12, 655–689. [Google Scholar] [CrossRef]
- Abegaz, S.B. Human ABO Blood Groups and Their Associations with Different Diseases. BioMed Res. Int. 2021, 2021, 6629060. [Google Scholar] [CrossRef]
- Groot, H.E.; Sierra, L.E.V.; Said, M.A.; Lipsic, E.; Karper, J.C.; Van Der Harst, P. Genetically determined ABO blood group and its associations with health and disease. Arter. Thromb. Vasc. Biol. 2020, 40, 830–838. [Google Scholar] [CrossRef]
- Höglund, J.; Karlsson, T.; Johansson, T.; Ek, W.E.; Johansson, Å. Characterization of the human ABO genotypes and their association to common inflammatory and cardiovascular diseases in the UK Biobank. Am. J. Hematol. 2021, 96, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Cid, J.; Nogués, N.; Montero, R.; Hurtado, M.; Briega, A.; Parra, R. Comparison of three microtube column agglutination systems for antibody screening: DG Gel, DiaMed-ID and Ortho BioVue. Transfus. Med. 2006, 16, 131–136. [Google Scholar] [CrossRef]
- Zietz, M.; Tatonetti, N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. medRxiv 2020. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. ABO blood group and thrombotic vascular disease. Thromb. Haemost. 2014, 112, 1103–1109. [Google Scholar]
- Franchini, M.; Favaloro, E.J.; Targher, G.; Lippi, G. ABO blood group, hypercoagulability, and cardiovascular and cancer risk. Crit. Rev. Clin. Lab. Sci. 2012, 49, 137–149. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015, 13, 7. [Google Scholar] [CrossRef]
- Kremastinou, J.; Tzanakaki, G.; Karafoti, P.H.; Elton, R.A.; Weir, D.M.; Blackwell, C.C. Distribution of ABO and Lewis blood groups in Greece. Gene Geogr. 1996, 10, 201–205. [Google Scholar]
- Dentali, F.; Sironi, A.; Ageno, W.; Turato, S.; Bonfanti, C.; Frattini, F.; Crestani, S.; Franchini, M. Non-O blood type is the commonest genetic risk factor for VTE: Results from a meta-analysis of the literature. Semin. Thromb. Hemost. 2012, 38, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Sironi, A.P.; Ageno, W.; Crestani, S.; Franchini, M. ABO blood group and vascular disease: An update. Semin. Thromb. Hemost. 2014, 40, 49–59. [Google Scholar] [CrossRef]
- BA, D.M.; Sow, M.S.; Diack, A.; Dia, K.; Mboup, M.C.; Fall, P.D.; Fall, M.D. Cardiovascular disease and ABO blood-groups in Africans. Are blood-group A individuals at higher risk of ischemic disease?: A pilot study. Egypt. Heart J. 2017, 69, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Ke, X.; Xiong, L.; Shi, Y.; Li, J.; Tan, X.; Ye, S. Analysis of circulating cholesterol levels as a mediator of an association between ABO blood group and coronary heart disease. Circ. Cardiovasc. Genet. 2014, 7, 43–48. [Google Scholar] [CrossRef]
- Wiggins, K.L.; Smith, N.L.; Glazer, N.L.; Rosendaal, F.R.; Heckbert, S.R.; Psaty, B.M.; Rice, K.M.; Lumley, T. ABO genotype and risk of thrombotic events and hemorrhagic stroke. J. Thromb. Haemost. 2009, 7, 263–269. [Google Scholar] [CrossRef]
- Ewald, D.R.; Sumner, S.C.J. Blood type biochemistry and human disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 517–535. [Google Scholar] [CrossRef]
- El-Sayed, M.I.K.; Amin, H.K. ABO blood groups in correlation with hyperlipidemia, diabetes mellitus type II, and essential hypertension. Asian J. Pharm. Clin. Res. 2015, 236–243. [Google Scholar]
- Fagherazzi, G.; Gusto, G.; Clavel-Chapelon, F.; Balkau, B.; Bonnet, F. ABO and Rhesus blood groups and risk of type 2 diabetes: Evidence from the large E3N cohort study. Diabetologia 2015, 58, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Carl, H.; Soumya, R.; Srinivas, P.; Vani, R. Oxidative stress in erythrocytes of banked ABO blood. Hematology 2016, 21, 630–634. [Google Scholar] [CrossRef][Green Version]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Ornstein, D.L.; Woloshin, S.; Schwartz, L.M. Association of age, sex, and race with body iron stores in adults: Analysis of NHANES III data. Am. Heart J. 2000, 140, 98–104. [Google Scholar] [CrossRef]
- Seong, J.M.; Yoon, Y.S.; Lee, K.S.; Bae, N.Y.; Gi, M.Y.; Yoon, H. Gender difference in relationship between serum ferritin and 25-hydroxyvitamin D in Korean adults. PLoS ONE 2017, 12, e0177722. [Google Scholar] [CrossRef]
- Bachman, E.; Feng, R.; Travison, T.; Li, M.; Olbina, G.; Ostland, V.; Ulloor, J.; Zhang, A.; Basaria, S.; Ganz, T.; et al. Testosterone suppresses hepcidin in men: A potential mechanism for testosterone-induced erythrocytosis. J. Clin. Endocrinol. Metab. 2010, 95, 4743–4747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, F.; Zhang, H.; Gao, Y.; Tan, A.; Zhang, S.; Xiao, Q.; Zhang, B.; Huang, L.; Ye, B.; et al. The Association between the Levels of Serum Ferritin and Sex Hormones in a Large Scale of Chinese Male Population. PLoS ONE 2013, 8, e75908. [Google Scholar] [CrossRef]
- Chao, K.C.; Chang, C.C.; Chiou, H.Y.; Chang, J.S. Serum ferritin is inversely correlated with testosterone in boys and young male adolescents: A cross-sectional study in Taiwan. PLoS ONE 2015, 10, e0144238. [Google Scholar] [CrossRef] [PubMed]
- Kwenti, T.E.; Kwenti, T.D.B. Anaemia and its association with month and blood phenotype in blood donors in Fako division, Cameroon. BMC Hematol. 2016, 16, 29. [Google Scholar] [CrossRef]
- Iwanaga, S.; Sakano, N.; Taketa, K.; Takahashi, N.; Wang, D.H.; Takahashi, H.; Kubo, M.; Miyatake, N.; Ogino, K. Comparison of serum ferritin and oxidative stress biomarkers between Japanese workers with and without metabolic syndrome. Obes. Res. Clin. Pract. 2014, 8, e271–e282. [Google Scholar] [CrossRef] [PubMed]
- Borza, C.; Muntean, D.; Dehelean, C.; Savoiu, G.; Serban, C.; Simu, G.; Andoni, M.; Butur, M.; Drag, S. Oxidative Stress and Lipid Peroxidation–A Lipid Metabolism Dysfunction. Lipid Metab. 2013, 34, 23–38. [Google Scholar]
- Lawton, K.A.; Berger, A.; Mitchell, M.; Milgram, K.E.; Evans, A.M.; Guo, L.; Hanson, R.W.; Kalhan, S.C.; Ryals, J.A.; Milburn, M.V. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008, 9, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Perttula, K.; Pajouh, S.K.; Hubbard, A.; Nomura, D.K.; Rappaport, S.M. Untargeted Lipidomic Profiling of Human Plasma Reveals Differences due to Race, Gender and Smoking Status. J. Postgenom. Drug Biomark. Dev. 2014, 4, 1000131. [Google Scholar] [CrossRef]
- Dahlén, T.; Clements, M.; Zhao, J.; Olsson, M.L.; Edgren, G. An agnostic study of associations between abo and rhd blood group and phenome-wide disease risk. Elife 2021, 10, e65658. [Google Scholar] [CrossRef] [PubMed]
| Blood Group | |||||
|---|---|---|---|---|---|
| A (n = 55) Mean (±SD) | B (n = 32) Mean (±SD) | O (n = 51) Mean (±SD) | AB (n = 12) Mean (±SD) | Non O (n = 99) Mean (±SD) | |
| Sex, Male (%) | 60 | 45 | 50 | 50 | 55 |
| Age | 42 (±12.08) | 43 (±9.55) | 46 (±9.26) | 44 (±8.54) | 42.5 (±10.45) |
| BMI | 26.8 (±2.51) | 26.3 (±2.15) | 26.4 (±1.87) | 26.2 (±1.90) | 26.6 (±1.85) |
| Smoking, Yes (%) | 28 | 19 | 11 | 20 | 23 |
| Rhesus Type | ||
|---|---|---|
| RhD+ (n = 132) Mean ± SD | RhD (n = 18) Mean ± SD | |
| Sex, Male (%) | 48 | 55 |
| Age | 42.3 (± 11.07) | 43.2 (± 9.18) |
| BMI | 26.9 (± 2.12) | 25.9 (± 2.14) |
| Smoking, Yes (%) | 24 | 13 |
| Blood Group Mean (SD +/−) | Statistical Significant p-Value | |||||
|---|---|---|---|---|---|---|
| Biochemical Parameters | A | B | O | AB | Non O | |
| Triglycerides (mg/dL) | 105.45 (+34.08) | 116.14 (±38.21) | 129.65 * (+29.54) | 92.25 (+19.65) | 119.12 (+23.58) | O-A,B,AB 0.05 0.03 0.02 |
| HDL (mg/dL) | 53.24 (+13.21) | 56.14 (+11.54) | 50.45 (+12.58) | 48.13 (+9.54) | 56.86 (+13.45) | ns |
| LDL (mg/dL) | 121.42 (+26.05) | 126.45 (+24.35) | 131.14 * (+29.54) | 123.01 (+23.65) | 122.32 (+22.55) | 0.04 0.02 0.01 |
| Total Cholesterol (mg/dL) | 184.52 (±52.65) | 200.36 (+49.56) | 229.26 * (+65.85) | 210.45 (+64.88) | 212.24 (+55.85) | 0.05 0.02 0.02 |
| Iron (μg/dL) | 101.12 (±36.34) | 108.35 (±43.48) | 108.23 (±39.56) | 119.14 * (35.65) | 101.12 (41.54) | AB-A,B,O 0.05 0.05 0.05 |
| Ferritin (ng/mL) | 107.15 (+54.85) | 70.35 (+29.54) | 158.45 * (+48.54) | 100.32 (+32.45) | 85.12 (+49.85) | 0.02 0.01 0.01 |
| Uric acid (mg/dL) | 5.01 (+1.44) | 5.26 (+1.23) | 6.03 * (+1.65) | 5.25 (+1.34) | 5.13 (+1.38) | 0.01 0.02 0.05 |
| Atheromatic index | 3.88 (±0.94) | 4.01 (±0.85) | 4.42 * (±0.98) | 4.11 (±0.98) | 3.95 (±0.96) | 0.02 0.03 0.05 |
| AST (U/L) | 16.01 (+2.43) | 17.12 (+2.87) | 18.14 (+2.65) | 17.13 (+2.54) | 17.74 (+2.61) | ns |
| ALT (U/L) | 19.14 (+3.65) | 18.32 (+3.45) | 20.45 (+3.44) | 18.78 (+3.65) | 19.65 (+3.52) | ns |
| Glucose (mg/dL) | 96.47 (+13.54) | 98.41 (+13.12) | 100.01 * (+12.54) | 103.65 (+10.54) | 98.31 (+8.65) | 0.05 0.05 0.05 |
| TAS (μM) | 244.14 (+28.65) | 240.71 (+32.54) | 239.25 (+31.45) | 219.14 * (+29.45) | 245.45 (+30.14) | AB-A,B,O 0.05 0.05 0.05 |
| Hematological Parameters | A | B | O | AB | Non O | |
| Ht (%) | 44.12 (+3.8) | 45.14 (+2.9) | 45.01 (+3.45) | 43.33 (+2.85) | 45.03 (+2.97) | ns |
| Hb (g/dL) | 15.02 (+1.2) | 15.05 (+1.05) | 15.32 (+1.26) | 14.22 (+1.45) | 15.18 (+1.12) | ns |
| Rhesus Type Mean (SD +/−) | |||
|---|---|---|---|
| Biochemical Parameters | RhD+ | RhD− | Statistical Significant p-Value |
| Triglycerides (mg/dL) | 128.12 * (±54.85) | 116.47 (±48.61) | 0.05 |
| HDL (mg/dL) | 53.05 (±12.54) | 54.47 (±11.54) | ns |
| LDL (mg/dL) | 125.88 * (±27.85) | 118.33 (±23.47) | 0.05 |
| Total Cholesterol (mg/dL) | 234.84 * (±54.57) | 201.67 (±45.95) | 0.05 |
| Iron (μg/dL) | 110.24 (±42.74) | 102.12 (±41.85) | ns |
| Ferritin (ng/mL) | 125.5 * (±45.47) | 113.2 (±54.74) | 0.05 |
| Uric acid (mg/dL) | 5.52 (±1.41) | 5.35 (±1.08) | ns |
| Atheromatic index | 4.21 (±0.85) | 3.81 (±0.73) | ns |
| AST (U/L) | 17.05 (±2.45) | 17.24 (±2.12) | ns |
| ALT (U/L) | 19.14 (±3.02) | 19.19 (±2.98) | ns |
| Glucose (mg/dL) | 104.5 (±32.45) | 97.5 (±29.45) | ns |
| TAS (m M) | 258.35 (±35.45) | 241.5 (±39.45) | ns |
| Hematological Parameters | + | − | |
| Ht (%) | 45.2 (±3.14) | 43.4 (±3.25) | ns |
| Hb (g/dL) | 15.85 (±1.45) | 14.24 (±1.23) | ns |
| Blood Group | ||||||
|---|---|---|---|---|---|---|
| Male Mean (SD +/−) | ||||||
| Biochemical Parameters | A | B | O | AB | Non O | Statistical Significant p-Value |
| Triglycerides (mg/dL) | 128.54 (±32.75) | 209.12 * (±29.65) | 128.47 (±34.56) | 102.75 (±28.65) | 168.24 (±30.05) | B-A,O,AB 0.02 0.02 0.03 |
| HDL (mg/dL) | 48.25 (±12.25) | 45.35 (±11.56) | 49.36 (±10.85) | 45.25 (±11.98) | 47.51 (±12.56) | ns |
| LDL (mg/dL) | 123.47 (±15.65) | 143.58 * (±14.32) | 132.58 (±15.45) | 118.25 (±16.74) | 135.78 (±15.57) | 0.05 0.05 0.05 |
| Total Cholesterol (mg/dL) | 260.5 * 6 (±58.54) | 235.85 (±56.85) | 228.78 (±51.65) | 200.16 (±54.35) | 250.36 (±56.58) | 0.05 0.05 0.05 |
| Iron (μg/dL) | 111.85 (±40.54) | 105.25 (±34.54) | 102.36 (±32.54) | 105.5 (±37.46) | 109.45 (±37.53) | ns |
| Ferritin (ng/mL) | 168.02 * (±58.65) | 57.36 (±61.32) | 330.25 (±52.85) | 145.24 (±55.65) | 110.14 (±53.26) | 0.05 0.05 0.05 |
| Uric acid(mg/dL) | 6.02 (±1.23) | 6.05 * (±1.36) | 6.48 (±1.65) | 5.9 (±1.54) | 5.91 (±1.46) | ns 0.05 ns |
| Atheromatic index | 4.1 (±0.85) | 5.3 * (±0.96) | 4.6 (±0.78) | 4.29 (±0.79) | 4.7 (±0.98) | 0.05 0.03 0.02 |
| Glucose (mg/dL) | 93.25 (±14.54) | 97.38 (±16.54) | 98.48 (±17.65) | 94.15 (±16.65) | 94.87 (±15.65) | ns |
| TAS (mM) | 242.35 (±21.54) | 242.85 * (±22.65) | 258.12 (±24.65) | 189.25 (±21.36) | 243.32 (±22.65) | ns 0.05 0.05 |
| Hematological Parameters | A | B | O | AB | Non O | |
| Ht (%) | 44.74 (±3.22) | 44.25 (±3.08) | 45.01 (±3.65) | 44.55 (±3.12) | 44.12 (±3.11) | ns |
| Hb (g/dL) | 15.02 (±1.56) | 14.28 (±1.36) | 15.01 (±1.47) | 14.95 (±1.32) | 14.4 (±1.45) | ns |
| Female Mean (SD +/−) | ||||||
| Biochemical Parameters | A | B | O | AB | Non O | Statistical Significant p-Value |
| Triglycerides (mg/dL) | 102.45 (±47.94) | 101.32 (±45.85) | 105.12 (±44.69) | 81.75 * (±44.65) | 103.14 (±46.91) | AB-A,O,AB 0.05 0.03 0.02 |
| HDL (mg/dL) | 61.74 (±13.54) | 58.65 (±11.65) | 59.45 (±12.85) | 51.24 * (±13.58) | 59.15 (±13.3) | 0.04 0.05 0.05 |
| LDL (mg/dL) | 120.24 (±15.65) | 113.45 (±13.85) | 117.65 (±12.65) | 127.24 * (±14.93) | 117.45 (±14.35) | 0.05 0.05 0.05 |
| Total Cholesterol (mg/dL) | 196.14 (±22.25) | 200.21 (±19.65) | 226.52 (± 24.98) | 220.74 * (±23.65) | 200.32 (±22.35) | 0.05 0.05 0.05 |
| Iron (μg/dL) | 101.12 (±39.65) | 105.25 (±37.56) | 110.36 (±43.61) | 133.54 (±44.78) | 104.24 (±41.35) | ns |
| Ferritin (ng/mL) | 40.56 (±53.53) | 127.12 * (±51.12) | 72.45 (±23.12) | 55.31 (±22.11) | 88.12 (±35.65) | 0.05 0.05 0.05 |
| Uric acid(mg/dL) | 4.81 (±1.12) | 5.38 * (±1.25) | 5.23 (±1.36) | 3.94 (±1.19) | 4.71 (±1.38) | 0.05 0.05 0.05 |
| Atheromatic index | 3.51 (±0.89) | 3.72 (±0.79) | 3.59 (±0.94) | 3.94 * (±0.79) | 3.72 (±0.84) | 0.05 0.05 0.05 |
| Glucose (mg/dL) | 96.54 (±12.36) | 111.14 (±19.65) | 102.54 (±14.65) | 108.54 (±16.35) | 104.45 (±16.42) | ns |
| TAS (mM) | 237.14 (±27.65) | 245.65 * (±29.43) | 239.55 (±27.42) | 234.84 (±23.35) | 242.15 (±25.65) | ns |
| Hematological Parameters | A | B | O | AB | Non O | |
| Ht (%) | 46.02 (±3.11) | 45.21 (±2.65) | 45.22 (±2.11) | 44.32 (±3.15) | 45.36 (±2.95) | ns |
| Hb (g/dL) | 15.12 (±1.32) | 15.14 (±1.11) | 15.32 (±1.65) | 14.85 (±1.02) | 15.21 (±1.10) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lymperaki, E.; Stalika, E.; Tzavelas, G.; Tormpantoni, E.; Samara, D.; Vagdatli, E.; Tsamesidis, I. The Clinical Utility of ABO and RHD Systems as Potential Indicators of Health Status, a Preliminary Study in Greek Population. Clin. Pract. 2022, 12, 406-418. https://doi.org/10.3390/clinpract12030045
Lymperaki E, Stalika E, Tzavelas G, Tormpantoni E, Samara D, Vagdatli E, Tsamesidis I. The Clinical Utility of ABO and RHD Systems as Potential Indicators of Health Status, a Preliminary Study in Greek Population. Clinics and Practice. 2022; 12(3):406-418. https://doi.org/10.3390/clinpract12030045
Chicago/Turabian StyleLymperaki, Evgenia, Evangelia Stalika, George Tzavelas, Efthymia Tormpantoni, Diana Samara, Eleni Vagdatli, and Ioannis Tsamesidis. 2022. "The Clinical Utility of ABO and RHD Systems as Potential Indicators of Health Status, a Preliminary Study in Greek Population" Clinics and Practice 12, no. 3: 406-418. https://doi.org/10.3390/clinpract12030045
APA StyleLymperaki, E., Stalika, E., Tzavelas, G., Tormpantoni, E., Samara, D., Vagdatli, E., & Tsamesidis, I. (2022). The Clinical Utility of ABO and RHD Systems as Potential Indicators of Health Status, a Preliminary Study in Greek Population. Clinics and Practice, 12(3), 406-418. https://doi.org/10.3390/clinpract12030045









