Pathogenesis, Diagnostic Challenges, and Risk Factors of Pott’s Disease
Abstract
:1. Introduction
2. Materials and Methods
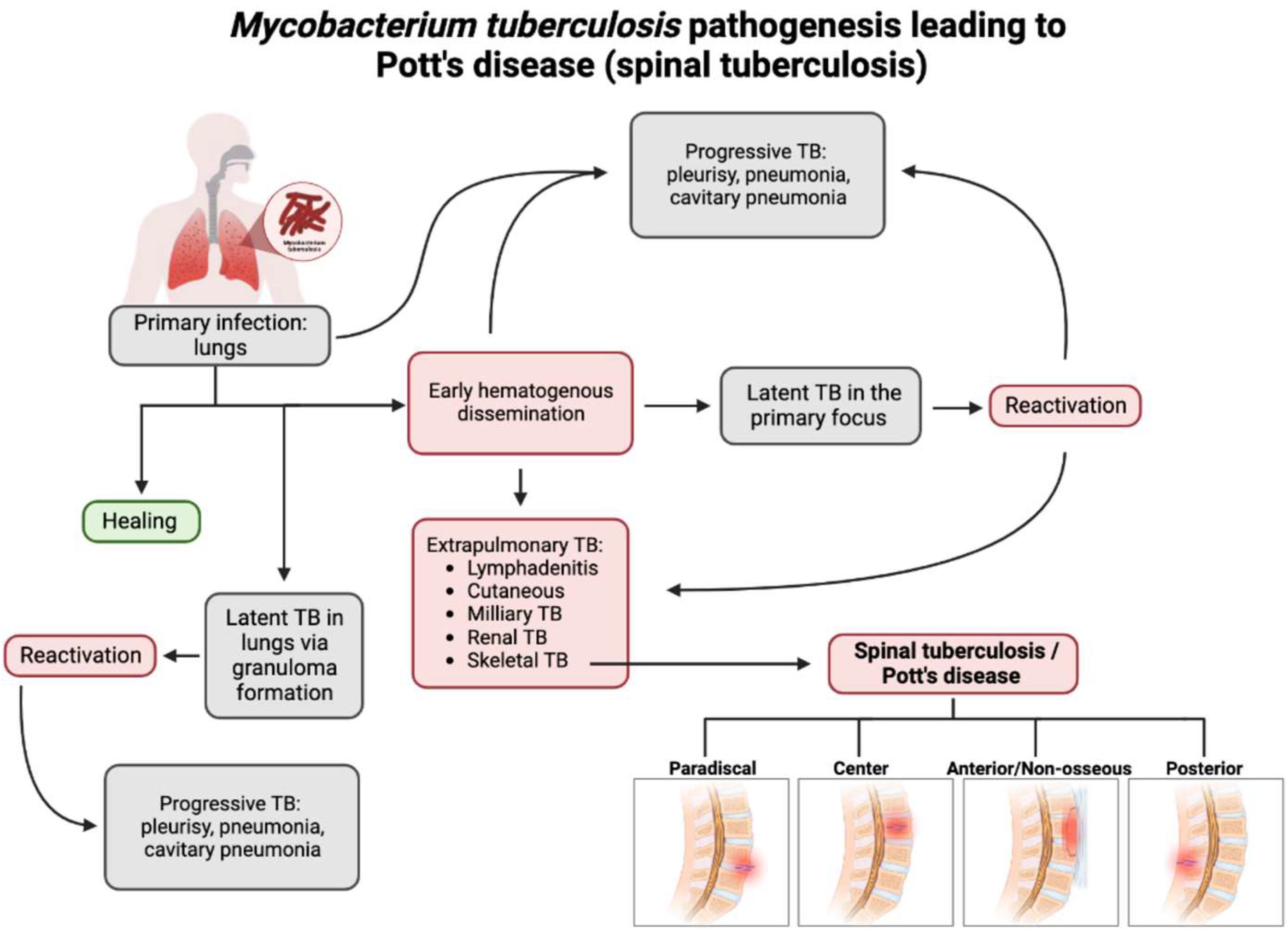
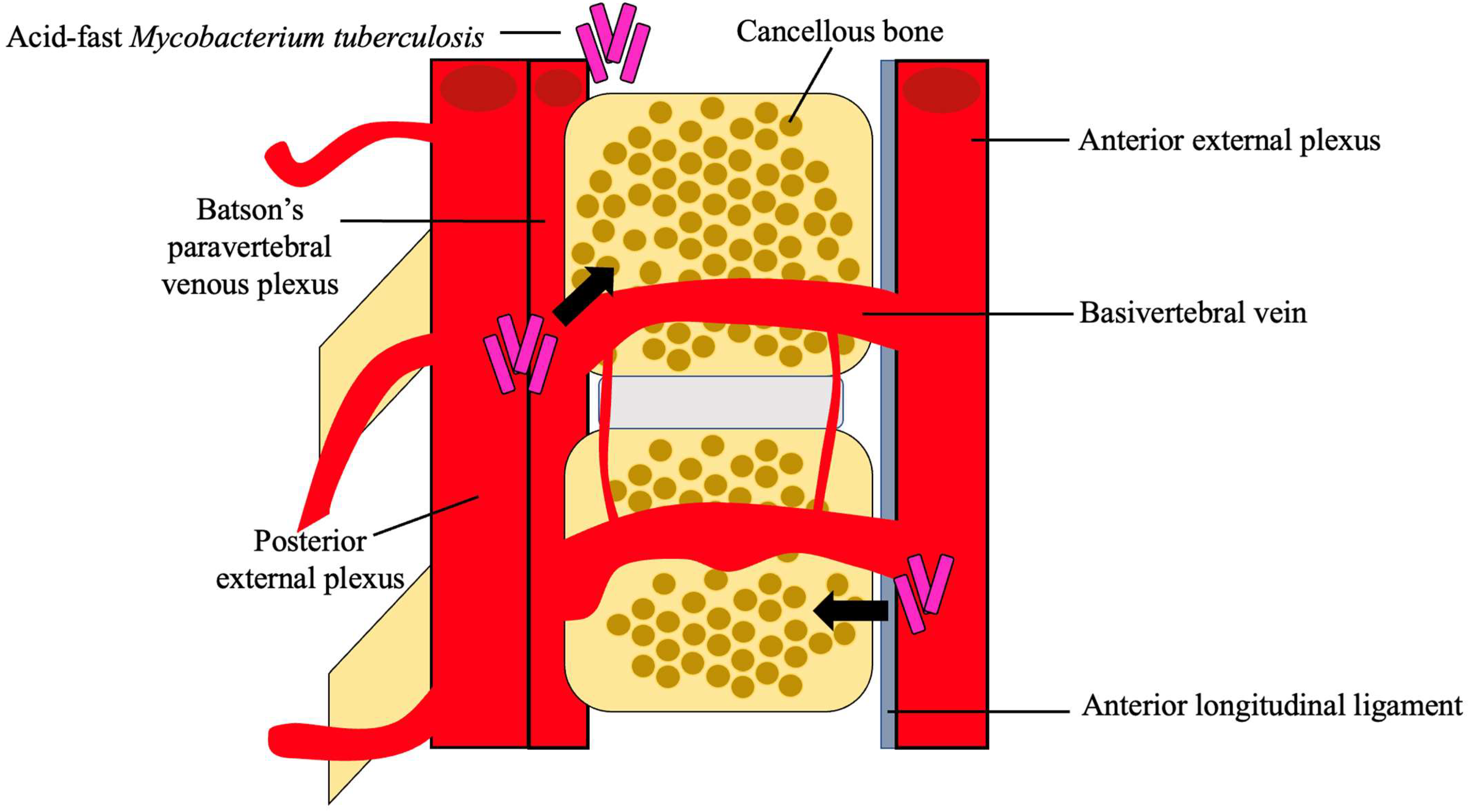
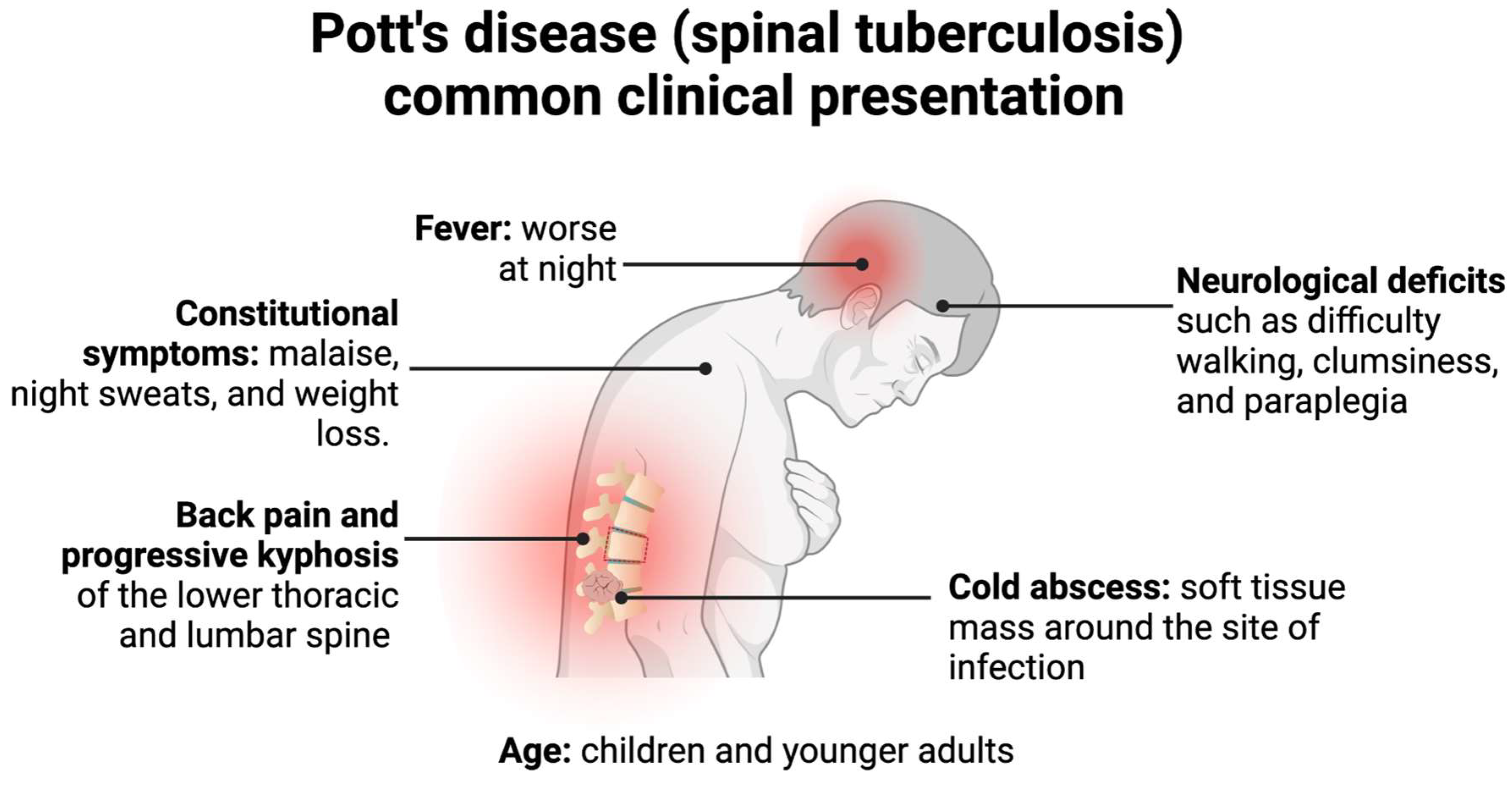
3. Pathogenesis and Clinical Presentation of Pott’s Disease
4. Diagnostic Challenges of Pott’s Disease
5. Risk Factors for Potts Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- MacNeil, A.; Glaziou, P.; Sismanidis, C.; Date, A.; Maloney, S.; Floyd, K. Global Epidemiology of Tuberculosis and Progress Toward Meeting Global Targets—Worldwide, 2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, V.K.; Subramanian, S. Pott Disease. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Garg, R.K.; Somvanshi, D.S. Spinal tuberculosis: A review. J. Spinal Cord Med. 2011, 34, 440–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, S.; Amanullah, M.F.; Ahmad, K.; Rauniyar, R.K. Pott’s Spine: Diagnostic Imaging Modalities and Technology Advancements. N. Am. J. Med. Sci. 2013, 5, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Colmenero, J.D.; Ruiz-Mesa, J.D.; Sanjuan-Jimenez, R.; Sobrino, B.; Morata, P. Establishing the diagnosis of tuberculous vertebral osteomyelitis. Eur. Spine J. 2013, 22 (Suppl. 4), 579–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamara, E.; Mehta, S.; Brust, J.C.M.; Jain, A.K. Effect of delayed diagnosis on severity of Pott’s disease. Int. Orthop. 2012, 36, 245–254. [Google Scholar] [CrossRef] [Green Version]
- McHenry, M.C.; Easley, K.A.; Locker, G.A. Vertebral osteomyelitis: Long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin. Infect Dis. 2002, 34, 1342–1350. [Google Scholar] [CrossRef]
- Pigrau-Serrallach, C.; Rodriguez-Pardo, D. Bone and joint tuberculosis. Eur. Spine J. 2013, 22 (Suppl. 4), 556–566. [Google Scholar] [CrossRef]
- Solis Garcia del Pozo, J.; Vives Soto, M.; Solera, J. Vertebral osteomyelitis: Long-term disability assessment and prognostic factors. J. Infect. 2007, 54, 129–134. [Google Scholar] [CrossRef]
- Wang, D. Diagnosis of tuberculous vertebral osteomyelitis (TVO) in a developed country and literature review. Spinal Cord 2005, 43, 531–542. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Srivastava, A.K.; Tiwari, R.K. Surgical management of Pott’s disease of the spine in pediatric patients: A single surgeon’s experience of 8 years in a tertiary care center. J. Pediatr. Neurosci. 2011, 6 (Suppl. 1), S101–S108. [Google Scholar] [CrossRef]
- Tuli, S.M. Historical aspects of Pott’s disease (spinal tuberculosis) management. Eur. Spine J. 2013, 22, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Maron, R.; Levine, D.; Dobbs, T.E.; Geisler, W.M. Two cases of pott disease associated with bilateral psoas abscesses: Case report. Spine 2006, 31, E561–E564. [Google Scholar] [CrossRef] [PubMed]
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV Coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, a017871. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liu, S.; Bao, Y.C.; Gao, R.X.; Han, C.F.; Sun, X.C.; Zhang, W.L.; Feng, S.Q. Study on the relationship between vitamin D deficiency and susceptibility to spinal tuberculosis. Int. J. Surg. 2017, 44, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Soundararajan, D.C.R.; Shetty, A.P.; Kanna, R.M. Spinal Tuberculosis: Current Concepts. Glob. Spine J. 2018, 8 (Suppl. 4), 96s–108s. [Google Scholar] [CrossRef] [Green Version]
- Khoo, L.T.; Mikawa, K.; Fessler, R.G. A surgical revisitation of Pott distemper of the spine. Spine J. 2003, 3, 130–145. [Google Scholar] [CrossRef]
- Gardini, G.; Gregori, N.; Matteelli, A.; Castelli, F. Mycobacterial skin infection. Curr. Opin. Infect. Dis. 2022, 35, 79–87. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Kanna, R.M.; Shetty, A.P. Pathophysiology and Treatment of Spinal Tuberculosis. JBJS Rev. 2014, 2, e4. [Google Scholar] [CrossRef] [PubMed]
- Weaver, P.; Lifeso, R.M. The radiological diagnosis of tuberculosis of the adult spine. Skelet. Radiol. 1984, 12, 178–186. [Google Scholar] [CrossRef]
- Jung, J.H.; Choi, S.; Kang, Y.; Cho, D.C.; Lee, S.M.; Park, T.I.; Choe, B.H.; Kim, D.; Kang, B. Development of Spinal Tuberculosis in an Adolescent With Crohn’s Disease After Infliximab Therapy: A Case Report With Literature Review. Front. Pediatr. 2021, 9, 802298. [Google Scholar] [CrossRef]
- Kubihal, V.; Sharma, R.; Krishna Kumar, R.G.; Chandrashekhara, S.H.; Garg, R. Imaging update in spinal tuberculosis. J. Clin. Orthop. Trauma 2022, 25, 101742. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Mehta, N.; Mukherjee, R.N.; Swamy, A.M.; Siamwala, B.S.; Malik, G. Epidemiological Insights from 1,652 Patients with Spinal Tuberculosis Managed at a Single Center: A Retrospective Review of 5-Year Data. Asian Spine J. 2022, 16, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Rajasekaran, S.; Jaggi, K.R.; Myneedu, V.P. Tuberculosis of the Spine. J. Bone Jt. Surg. Am. 2020, 102, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, E.S.; Rockswold, G.L.; Bergman, T.A.; Erickson, D.L.; Seljeskog, E.L. Spinal tuberculosis: A diagnostic and management challenge. J. Neurosurg. 1995, 83, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Batirel, A.; Erdem, H.; Sengoz, G.; Pehlivanoglu, F.; Ramosaco, E.; Gulsun, S.; Tekin, R.; Mete, B.; Balkan, I.; Sevgi, D.Y.; et al. The course of spinal tuberculosis (Pott disease): Results of the multinational, multicentre Backbone-2 study. Clin. Microbiol. Infect. 2015, 21, 1008.e9–1008.e18. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Chen, Y.M.; Lee, C.W.; Chang, Y.J.; Cheng, C.Y.; Hung, J.K. Early diagnosis of spinal tuberculosis. J. Formos. Med. Assoc. 2016, 115, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Canine, C.; Medeck, S.; Hackett, A. Delayed Diagnosis of Spinal Tuberculosis in a 44-year-old Male with Acute on Chronic Low Back Pain. Clin. Pract. Cases Emerg. Med. 2019, 3, 107–111. [Google Scholar] [CrossRef]
- Dean, A.; Zyck, S.; Toshkezi, G.; Galgano, M.; Marawar, S. Challenges in the Diagnosis and Management of Spinal Tuberculosis: Case Series. Cureus 2019, 11, 3855. [Google Scholar] [CrossRef] [Green Version]
- Abdulwadou, S.; Bushra Gam, M.; Dahhan Als, K.; Greeballah, A. A Case Report of a Tragic Story of Pott’s paraplegia Cured after Four Years. Pak. J. Biol. Sci. 2020, 23, 1492–1495. [Google Scholar] [CrossRef]
- Pandita, A.; Madhuripan, N.; Pandita, S.; Hurtado, R.M. Challenges and controversies in the treatment of spinal tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 19, 100151. [Google Scholar] [CrossRef]
- Mann, T.N.; Davis, J.H.; Walzl, G.; Beltran, C.G.; du Toit, J.; Lamberts, R.P.; Chegou, N.N. Candidate Biomarkers to Distinguish Spinal Tuberculosis From Mechanical Back Pain in a Tuberculosis Endemic Setting. Front. Immunol. 2021, 12, 768040. [Google Scholar] [CrossRef]
- Lou, C.; Liu, J.; Ren, Z.; Ji, J.; Ma, H.; Dong, H.; Wang, L.; Zhang, X.; Niu, N. Analysis of the Value of Serum Biomarker LBP in the Diagnosis of Spinal Tuberculosis. Infect. Drug Resist. 2022, 15, 4915–4926. [Google Scholar] [CrossRef]
- Pai, M.; Flores, L.L.; Pai, N.; Hubbard, A.; Riley, L.W.; Colford, J.M. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2003, 3, 633–643. [Google Scholar] [CrossRef]
- Vadwai, V.; Boehme, C.; Nabeta, P.; Shetty, A.; Alland, D.; Rodrigues, C. Xpert MTB/RIF: A new pillar in diagnosis of extrapulmonary tuberculosis? J. Clin. Microbiol. 2011, 49, 2540–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortoli, E.; Russo, C.; Piersimoni, C.; Mazzola, E.; Dal Monte, P.; Pascarella, M.; Borroni, E.; Mondo, A.; Piana, F.; Scarparo, C.; et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur. Respir. J. 2012, 40, 442–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehme, C.C.; Nicol, M.P.; Nabeta, P.; Michael, J.S.; Gotuzzo, E.; Tahirli, R.; Gler, M.T.; Blakemore, R.; Worodria, W.; Gray, C.; et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet 2011, 377, 1495–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, R.N.; Ben Husien, M. Spinal tuberculosis: Review of current management. Bone Jt. J. 2018, 100, 425–431. [Google Scholar] [CrossRef]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Lu, Y.J.; Barreira-Silva, P.; Boyce, S.; Powers, J.; Cavallo, K.; Behar, S.M. CD4 T cell help prevents CD8 T cell exhaustion and promotes control of Mycobacterium tuberculosis infection. Cell Rep. 2021, 36, 109696. [Google Scholar] [CrossRef] [PubMed]
- Latorre, I.; Martínez-Lacasa, X.; Font, R.; Lacoma, A.; Puig, J.; Tural, C.; Lite, J.; Prat, C.; Cuchi, E.; Ausina, V.; et al. IFN-γ response on T-cell based assays in HIV-infected patients for detection of tuberculosis infection. BMC Infect. Dis. 2010, 10, 348. [Google Scholar] [CrossRef]
- Montales, M.T.; Chaudhury, A.; Beebe, A.; Patil, S.; Patil, N. HIV-Associated TB Syndemic: A Growing Clinical Challenge Worldwide. Front. Public Health 2015, 3, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Mishra, M.; Dabla, P.K.; Sharma, S. Vitamin D status in pediatric osteoarticular tuberculosis. J. Clin. Orthop. Trauma 2015, 6, 227–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panwar, A.; Garg, R.K.; Malhotra, H.S.; Jain, A.; Singh, A.K.; Prakash, S.; Kumar, N.; Garg, R.; Mahdi, A.A.; Verma, R.; et al. 25-Hydroxy Vitamin D, Vitamin D Receptor and Toll-like Receptor 2 Polymorphisms in Spinal Tuberculosis: A Case-Control Study. Medicine 2016, 95, e3418. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Barber, K.D.; Barber, D.L. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a018424. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Shaka, M.F.; Hussen Kabthymer, R.; Meshesha, M.D.; Borde, M.T. Vitamin D deficiency among apparently healthy children and children with common medical illnesses in Sub-Saharan Africa: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 75, 103403. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Deng, A.; Guo, C.F.; Wang, Y.X.; Chen, L.Q.; Wang, Y.F.; Wu, J.H.; Liu, J.Y. Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Arch. Med. Res. 2010, 41, 46–49. [Google Scholar] [CrossRef]
- Yadav, U.; Kumar, P.; Rai, V. FokI polymorphism of the vitamin D receptor (VDR) gene and susceptibility to tuberculosis: Evidence through a meta-analysis. Infect. Genet. Evol. 2021, 92, 104871. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Li, H.M.; Liu, Y.; Zhang, R.J.; Ding, J.Y.; Shen, C.L. Vitamin D receptor gene polymorphisms and osteoarthritis: A meta-analysis. Rheumatology 2021, 60, 538–548. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Riggs, B.L.; DeLuca, H.F. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 1980, 51, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, W.C. Risk of long-term infection-related death in clinical osteoporotic vertebral fractures: A hospital-based analysis. PLoS ONE 2017, 12, e0182614. [Google Scholar] [CrossRef] [Green Version]
- Weitzmann, M.N. Bone and the Immune System. Toxicol. Pathol. 2017, 45, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Nhamoyebonde, S.; Leslie, A. Biological differences between the sexes and susceptibility to tuberculosis. J. Infect. Dis. 2014, 209 (Suppl. 3), S100–S106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zumla, A. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Lancet Infect. Dis. 2010, 10, 303–304. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Rajaram, M.V.S.; Wu, Q.; Moliva, J.I.; Torrelles, J.B.; Turner, J.; Schlesinger, L.S. Identification of an Increased Alveolar Macrophage Subpopulation in Old Mice That Displays Unique Inflammatory Characteristics and Is Permissive to Mycobacterium tuberculosis Infection. J. Immunol. 2019, 203, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glassman, I.; Nguyen, K.H.; Giess, J.; Alcantara, C.; Booth, M.; Venketaraman, V. Pathogenesis, Diagnostic Challenges, and Risk Factors of Pott’s Disease. Clin. Pract. 2023, 13, 155-165. https://doi.org/10.3390/clinpract13010014
Glassman I, Nguyen KH, Giess J, Alcantara C, Booth M, Venketaraman V. Pathogenesis, Diagnostic Challenges, and Risk Factors of Pott’s Disease. Clinics and Practice. 2023; 13(1):155-165. https://doi.org/10.3390/clinpract13010014
Chicago/Turabian StyleGlassman, Ira, Kevin H. Nguyen, Jane Giess, Cheldon Alcantara, Michelle Booth, and Vishwanath Venketaraman. 2023. "Pathogenesis, Diagnostic Challenges, and Risk Factors of Pott’s Disease" Clinics and Practice 13, no. 1: 155-165. https://doi.org/10.3390/clinpract13010014
APA StyleGlassman, I., Nguyen, K. H., Giess, J., Alcantara, C., Booth, M., & Venketaraman, V. (2023). Pathogenesis, Diagnostic Challenges, and Risk Factors of Pott’s Disease. Clinics and Practice, 13(1), 155-165. https://doi.org/10.3390/clinpract13010014







