Abstract
(1) Background: Dysregulation in serotonergic and noradrenergic systems may be implicated in the neurobiophysiological mechanisms underlying pain-related cognitive impairment in chronic whiplash-associated disorders (CWAD). This study aimed to unravel the role of serotonergic and noradrenergic descending pathways in cognitive functioning at rest and in response to exercise in people with CWAD. (2) Methods: 25 people with CWAD were included in this double-blind, randomized, controlled crossover study. Endogenous descending serotonergic and noradrenergic inhibitory mechanisms were modulated by using a single dose of a selective serotonin reuptake inhibitor (Citalopram) or a selective norepinephrine reuptake inhibitor (Atomoxetine). Cognitive performance was studied at rest and in response to exercise (1) without medication intake; (2) after intake of Citalopram; and (3) after intake of Atomoxetine. (3) Results: After Atomoxetine intake, selective attention improved compared with the no medication day (p < 0.05). In contrast, a single dose of Citalopram had no significant effect on cognitive functioning at rest. When performing pairwise comparisons, improvements in selective attention were found after exercise for the no medication condition (p < 0.05). In contrast, after intake of Citalopram or Atomoxetine, selective and sustained attention worsened after exercise. (4) Conclusions: A single dose of Atomoxetine improved selective attention only in one Stroop condition, and a single dose of Citalopram had no effect on cognitive functioning at rest in people with CWAD. Only without medication intake did selective attention improve in response to exercise, whereas both centrally acting medications worsened cognitive performance in response to a submaximal aerobic exercise bout in people with CWAD.
1. Introduction
People with chronic whiplash-associated disorders (CWAD) are characterized by persistent neck pain lasting more than three months, resulting from a whiplash injury [1]. Other associated symptoms reported by individuals with CWAD are psychological problems, difficulties with concentration and attention, and disability [2,3,4,5,6].
Cognitive problems associated with higher disability [6] and reduced quality of life [3] are perceived as very debilitating. Impairments in cognitive functioning are present in individuals with CWAD [3,6,7,8,9,10]. Nevertheless, the exact underlying mechanisms causing these cognitive dysfunctions remain unclear. Mild traumatic brain injury [11], chronic fatigue [12], litigation [12], pain intensity [7], and signs indicative of central sensitization (CS) or nociplastic pain [3,8,13] have been associated with cognitive disturbances in CWAD.
Notably, pain and cognition share common neural substrates and modulate one another reciprocally [14,15]. Pain can negatively affect cognitive performance [14], and associations have been revealed between decreased efficacy of endogenous pain inhibition and impaired cognitive functioning in people with chronic pain with features suggestive of CS [3,16]. In healthy persons, more efficient endogenous pain inhibition has been associated with better cognitive performance [3,8]. Hence, it can be hypothesized that dysfunctional endogenous pain inhibition, which is demonstrated in CWAD at rest and during exercise [17,18,19], precludes optimal cognitive capabilities in CWAD [20].
The neurotransmitters serotonin and norepinephrine have complex modulatory roles in pain signaling [21] and play a crucial role in endogenous pain inhibition [22]. Therefore, dysregulation of serotonin and norepinephrine systems is likely to be partly responsible for the malfunctioning of descending pain inhibitory pathways [21,23,24]. In addition, serotonin and norepinephrine systems exert profound influences on various cognitive functions such as attention, vigilance, and memory [15,25,26,27,28,29]. Accordingly, dysregulation in these monoamines may be implicated in the mechanisms underlying pain-related cognitive impairment, but research exploring this hypothesis is lacking.
Furthermore, brain-orchestrated activation of serotonergic and noradrenergic systems also plays a key role in effective exercise-induced hypoalgesia [30]. In healthy persons, a single bout of exercise results in hypoalgesia, while it may increase pain in patients with signs of CS [18,31,32]. In addition, the exercise-induced increase in serotonin and norepinephrine [33] appears to mediate the improvement of cognitive functioning after exercise [34]. A single bout of aerobic exercise has positive effects on cognitive performance in healthy individuals [9,35,36] and on attention in patients with CWAD, as our research group previously found [9].
To unravel the biological nature of pain-related cognitive impairment, the first aim was to examine the isolated effect of activating serotonergic or noradrenergic descending pathways on performance-based cognitive functioning in people with CWAD by using a single dose of a selective serotonin reuptake inhibitor (SSRI) and a selective norepinephrine reuptake inhibitor (NRI), respectively. The second aim was to investigate the effect of activating either serotonergic or noradrenergic descending pathways on post-exercise cognitive functioning in people with CWAD. We hypothesized that activation of serotonergic and/or noradrenergic descending pathways would improve cognitive functioning both at rest and in response to exercise in people with CWAD.
2. Materials and Methods
2.1. Study Design and Setting
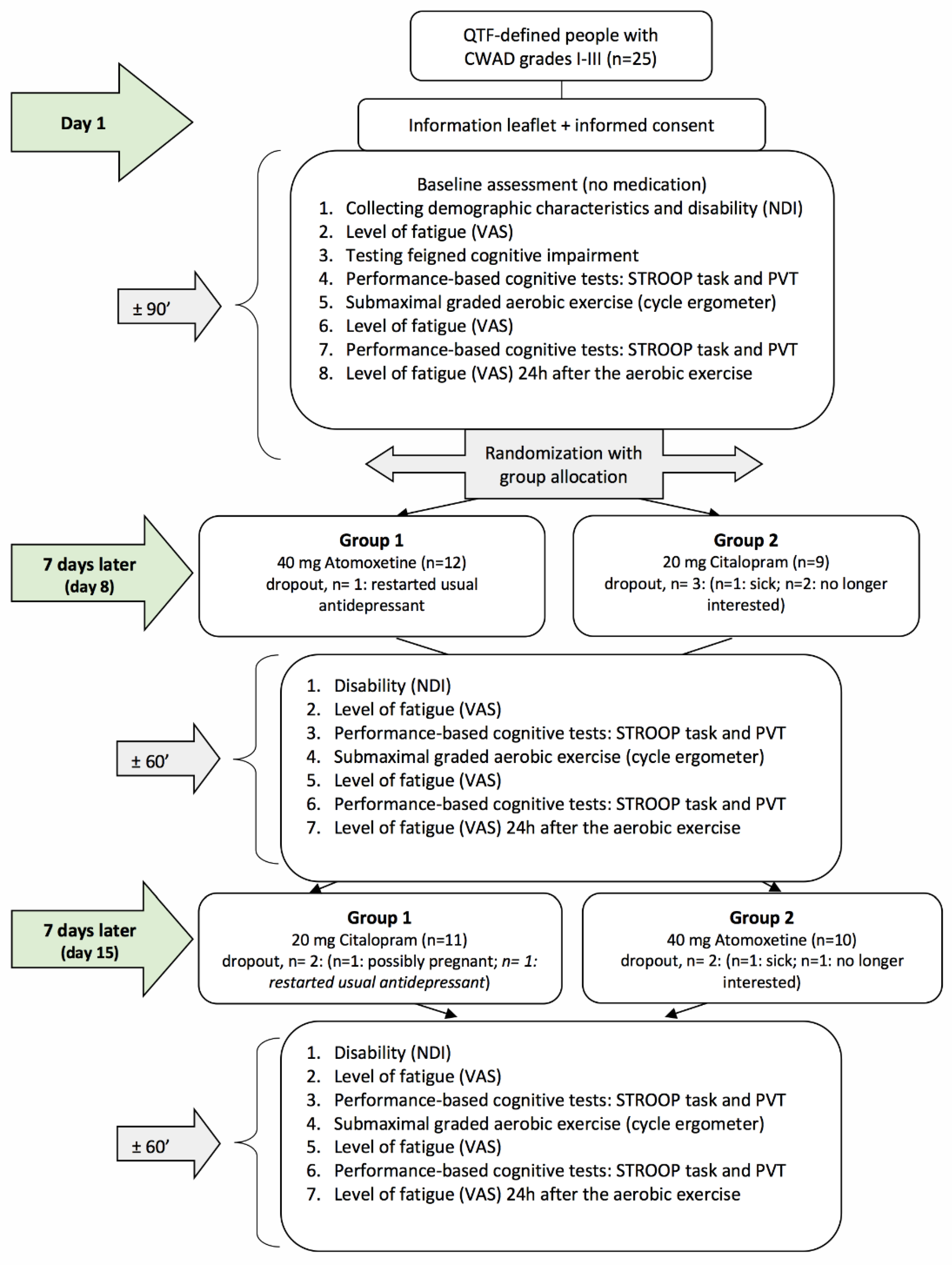
This study comprised a double-blind, randomized, controlled crossover study comparing three conditions; (1) no medication intake (baseline condition), (2) after intake of 20 mg Citalopram (SSRI), and (3) after intake of 40 mg Atomoxetine (selective NRI) (Figure 1). This study took place at the Department of Physical Medicine and Physiotherapy of the University Hospital Brussels (Belgium). The research protocol was approved by the Ethics Committee of the University Hospital Brussels/Vrije Universiteit Brussel and was in compliance with the Declaration of Helsinki. The study drugs were produced according to the Good Manufacturing Practice. All participants were thoroughly informed about the study procedures and signed a consent form prior to study enrolment. This study was registered with ClinicalTrials.gov (Identifier No. NCT01601912) and is reported in accordance with the CONSORT statement extension to randomized crossover trials [37] (Supplementary Materials).
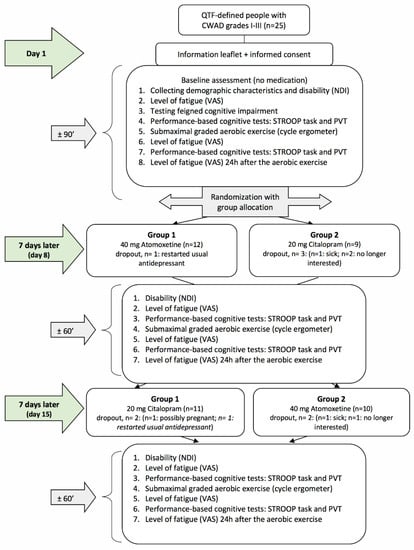
Figure 1.
Flowchart of the randomized crossover study. QTF = Quebec Task Force; CWAD = chronic whiplash-associated disorders; VAS = visual analogue scale; PVT = psychomotor vigilance task; NDI = Neck Disability Index.
The a-priori sample size calculation was performed with G*Power 3.1.5 and was based on the results of a study by Cook et al. [38] on the effect of a single submaximal exercise bout on cognitive performance in people with chronic fatigue syndrome, with and without comorbid fibromyalgia (a condition partly overlapping with CWAD), which found a small Partial η2 value of 0.04. It revealed that a sample size of 25 participants would provide 81% power with α = 0.05 to detect a statistically significant difference in cognitive performance pre-exercise versus postexercise.
2.2. Participants
People with CWAD were recruited via the Department of Physical Medicine and Physiotherapy and the Department of Emergency Medicine of the University Hospital Brussels through social media, advertisements, and a patient-support group.
Participants were eligible if they had persistent neck pain lasting at least three months resulting from a motor vehicle crash or traumatic event classifiable as WAD I, II, or III according to the Quebec Task Force criteria [39]. All participants were between 18 and 65 years old. Exclusion criteria included: (1) initial fulfillment of the WAD grade IV Quebec Task Force criteria [39]; (2) being pregnant or up to one year postnatal; (3) not being a native Dutch speaker; (4) intellectual disabilities; (5) other comorbidities that could explain the pain; (6) loss of consciousness due to the whiplash injury; (7) presence of psychiatric, metabolic, orthopedic, cardiovascular, or inflammatory disorders, and (8) presence of feigned cognitive impairment (i.e., malingering). To screen for the latter, potential participants had to complete the Rey 15-Item Memory Test for malingering during their initial study visit [40]. The description of this test can be found in a previous study of our research group [9].
Included patients were instructed to stop the use of opioid analgesics, antidepressants, and anti-epileptic medications two weeks prior to study participation. On each assessment day, the participants were asked to refrain from taking non-opioid analgesics and beta-adrenergic blocking agents; not to consume caffeine, alcohol, and nicotine; and not to undertake physical exertion. Patients were able to take non-opioid pain medication during the three-week study period but not on the assessment days.
2.3. Procedure
Baseline assessments were performed on the first test day without medication intake. Subsequently, cognitive performance was examined by two performance-based cognitive tests (Stroop task followed by Psychomotor Vigilance Task (PVT)). These tests were administered before and after a single submaximal aerobic exercise. Cognitive tests were performed 11 to 20 min postexercise because this yields the largest positive effects of a single exercise on cognitive performance [35].
After baseline assessment, an independent researcher who was not involved in the study randomly allocated the participants to one of the two groups (group 1 = day 8: selective NRI + day 15: SSRI and group 2 = day 8: SSRI + day 15: selective NRI (see Figure 1)) according to a computer-generated randomization list (http://www.randomization.com, accessed on 22 February 2023). Randomization was concealed using opaque, closed envelopes. On day 1, participants received their first single dose of medication (blinded) for day 8 and instructions regarding its administration (see Medication administration). One week after the baseline assessments, all participants were invited for the second time (day 8), and the same assessments were performed as on day 1. Afterward, they received their second single dose of medication (blinded) for day 15, as well as instructions regarding its administration (see Medication administration). One week after the second assessment, participants visited the university for the third time (day 15), and the same assessments as on day 8 were performed. Hence, the washout period was 7 days. After the final assessment, the success of participant and assessor blinding was examined by asking both to indicate group allocation, including the percentage of certainty. Reasons for study withdrawal were monitored.
2.3.1. Medication Administration
In order to ensure peak concentrations at the time of testing, all participants were instructed to take Citalopram (20 mg per os; Citalopram Sandoz®) four hours and Atomoxetine (40 mg per os; Strattera®) one and a half hours, respectively, before the scheduled start of their next appointment [41,42]. Participants were instructed not to ingest food 30 min before and after medication intake to ensure that the attainment of peak concentration was not delayed. Both pills were transparent hard-shelled capsules filled with dry white powder. The medication doses were based on the usual daily dose of Citalopram (20 mg per os) and the daily starting dose of Atomoxetine (40 mg per os) in adults.
Previous research showed that a single dose of Citalopram or Atomoxetine could alter cognitive functioning in healthy individuals and patients [43,44,45,46].
The washout period was seven days. The interval of seven days between assessment days minimizes pharmacological carry-over effects in view of the short half-life of each medication [47,48]. A single oral dose of Citalopram and Atomoxetine has, respectively, a plasma half-life time of approximately 33 h [49] and five hours [41,50].
2.3.2. Submaximal Aerobic Exercise
The acute submaximal graded aerobic exercise was performed on a cycle ergometer (Kardiomed, Alzenau, Germany), with the seat adjusted appropriately for each participant. After two minutes in the resting position, the participant’s resting heart rate was measured (heart rate monitor Polar Electro Oy, Finland). The workload started at 25 W and was increased by 25 W every minute until the participant had reached the submaximal level (i.e., target heart rate, defined as 75% of the age-predicted maximal heart rate: (220 − age) × 0.75). Participants were instructed to cycle at a constant pedaling rate of approximately 60 revolutions per minute (rpm). Heart rate was recorded at the end of every minute. The exercise was terminated when participants reached their individual target heart rates. Cooling down comprised of one minute of cycling at a workload of 25 W and a rate of 60 rpm. This aerobic power index test [51] is reliable as a submaximal exercise in people with chronic pain [52].
2.4. Demographic Characteristics and Self-Reported Measures
Demographic characteristics and medication use were questioned. Additionally, the time of cognitive testing was noted.
The Neck Disability Index (NDI) was used to investigate neck pain-related disability levels (0–100) [53,54]. The NDI is valid and reliable for people with chronic neck pain [55,56].
Participants had to indicate their present levels of fatigue by drawing a vertical line on a 100-mm visual analogue scale (VAS). This VAS was filled out for each test day: before the first performance of the cognitive tests, immediately after the exercise, and 24 h after the exercise to investigate post-exertional fatigue changes. Drawing the line at 0 mm indicates no fatigue, and drawing the line at 100 mm represents unbearable fatigue [57,58].
2.5. Performance-Based Cognitive Function
To investigate cognitive function, participants performed the Stroop task and the PVT. Each test began with the presentation of written instructions. Completion of this cognitive test battery took between 20 and 30 min. The Stroop task and PVT have been described in detail in our previous studies [3,9,59].
The Stroop task [60] was used to examine selective attention, choice reaction time, and cognitive inhibition. Four different intermixed conditions were used, namely, incongruent (word and ink color are different), congruent (word and ink color are the same), category (animal names written in one color), and negative priming inverse (e.g., the word “red” displayed in green font immediately followed by the word “green” displayed in red font). Negative priming is the condition where the to-be-ignored response in the first presentation becomes the subsequent relevant dimension [61].
In order to determine cognitive inhibition ability, the Stroop reaction time of the congruent condition is subtracted from the Stroop reaction time of the incongruent condition [62]. This way, an interference score is calculated, which can be interpreted as the cognitive inhibition subcomponent of executive functioning. The Stroop interference score, mean response reaction time for correct responses, and accuracy for each condition were used in further analyses.
The PVT [63] was administered to assess vigilance or sustained attention and simple reaction time. Participants were instructed to respond to a visual stimulus (red spot on a black screen) that appeared in the middle of the screen at random interstimulus intervals varying from 2 to 10 s. Participants were instructed to press the mouse button with the thumb as quickly as possible whenever they perceived the appearance of the red spot. The trial was stored as a lapse if the participant did not respond within 500 milliseconds. The mean PVT reaction time of correct responses and the number of PVT lapses were registered and used for further analyses. The PVT has good test-retest reliability [64].
2.6. Statistical Analysis
Statistical analyses were performed using IBM®SPSS® Statistics 26.0. First, the normality of demographic variables was checked, and the residuals of the linear mixed models were checked for normal distribution. To investigate the comparability of pain-related disability and exercise characteristics between all assessment days, a random-intercept linear mixed models analysis was performed using a variance components covariance matrix and the factor “medication condition” with three levels (i.e., no medication, Citalopram, and Atomoxetine) as a fixed effect together with a random intercept for each patient. To investigate the comparability of levels of fatigue between and within all assessment days, a random-intercept linear mixed models analysis was performed using a variance components covariance matrix with the factor “medication condition” with the same three levels and factor “time” with three levels (i.e., VAS fatigue pre-exercise, postexercise, 24 h postexercise) and “medication x time” as fixed effects together with a random intercept for each patient.
In addition, a similar linear mixed models analysis with cognitive performance variables as outcome measures were applied, including “medication condition”, “time”, and “medication condition x time” as fixed effects together with a random intercept for each patient. For each outcome parameter, a linear mixed models analysis was applied to reveal group (i.e., medication condition)-by-time (i.e., pre-post aerobic exercise) interaction effects. Next, pairwise comparisons of the cognitive performance variables were performed for the factor “time” (pre-post aerobic exercise) within each medication condition and for the factor “medication condition” before the aerobic exercise using a Bonferroni correction to correct for multiple comparisons. Furthermore, randomization was included as a covariate in the model to test the absence of a sequence effect. p < 0.05 (two-sided) was considered significant.
Effect sizes of the mean differences in cognitive performance between pre-post exercise within each medication condition and effect sizes of the mean differences in cognitive performance between the no medication condition and, respectively, the Citalopram or Atomoxetine conditions before the exercise were calculated as Cohen’s d.
3. Results
3.1. Participant Flow, Group Characteristics, and Self-Reported Measures
Twenty-five people with CWAD (15 women, 10 men) were included in the study. Thirteen participants were randomly allocated to the Atomoxetine–Citalopram (group 1) sequence group and 12 participants were randomly allocated to the Citalopram–Atomoxetine (group 2) sequence group. No sequence, period, or first-order carryover effects are present in this crossover study. Demographic data and medication use are presented in Table 1. Only one participant took an anti-depressant (Redomex®), but medication intake was discontinued two weeks prior to study participation. Pain-related disability and fatigue levels are presented in Table 2. Comparable moderate pain-related disability levels were reported on all assessment days (p > 0.05). Furthermore, levels of fatigue were not significantly different pre-, immediately post-, and 24 h postexercise within each of the three assessment days nor between all assessment days (p > 0.05).

Table 1.
Demographic data in people with CWAD (n = 25) separately for each sequence.

Table 2.
Self-reported pain-related disability and fatigue levels, and exercise characteristics in people with CWAD (n = 25).
The success of assessor blinding was 100%, whereas the success of patient blinding was 96%. The dropout rate was 16%. Six patients did not complete the entire study resulting in five dropouts during the Citalopram test day and three dropouts during the Atomoxetine test day. The reasons for withdrawal from the study are presented in Figure 1. No malingering was demonstrated among the participants. Furthermore, no harm or unintended effects of the medication intake were reported.
3.2. Submaximal Aerobic Exercise
Results of submaximal aerobic exercise characteristics measured during baseline, after intake of Citalopram and Atomoxetine, are listed in Table 2. Significant differences were revealed regarding the estimated mean (95% confidence interval (CI)) resting heart rate between the no medication and the Atomoxetine condition and regarding the estimated mean (95% CI) cycle duration time and maximal workload between the Citalopram and Atomoxetine assessment days (p < 0.05).
3.3. The Isolated Effect of a Single Dose of a SSRI or a Selective NRI on Cognitive Performance at Rest in People with CWAD
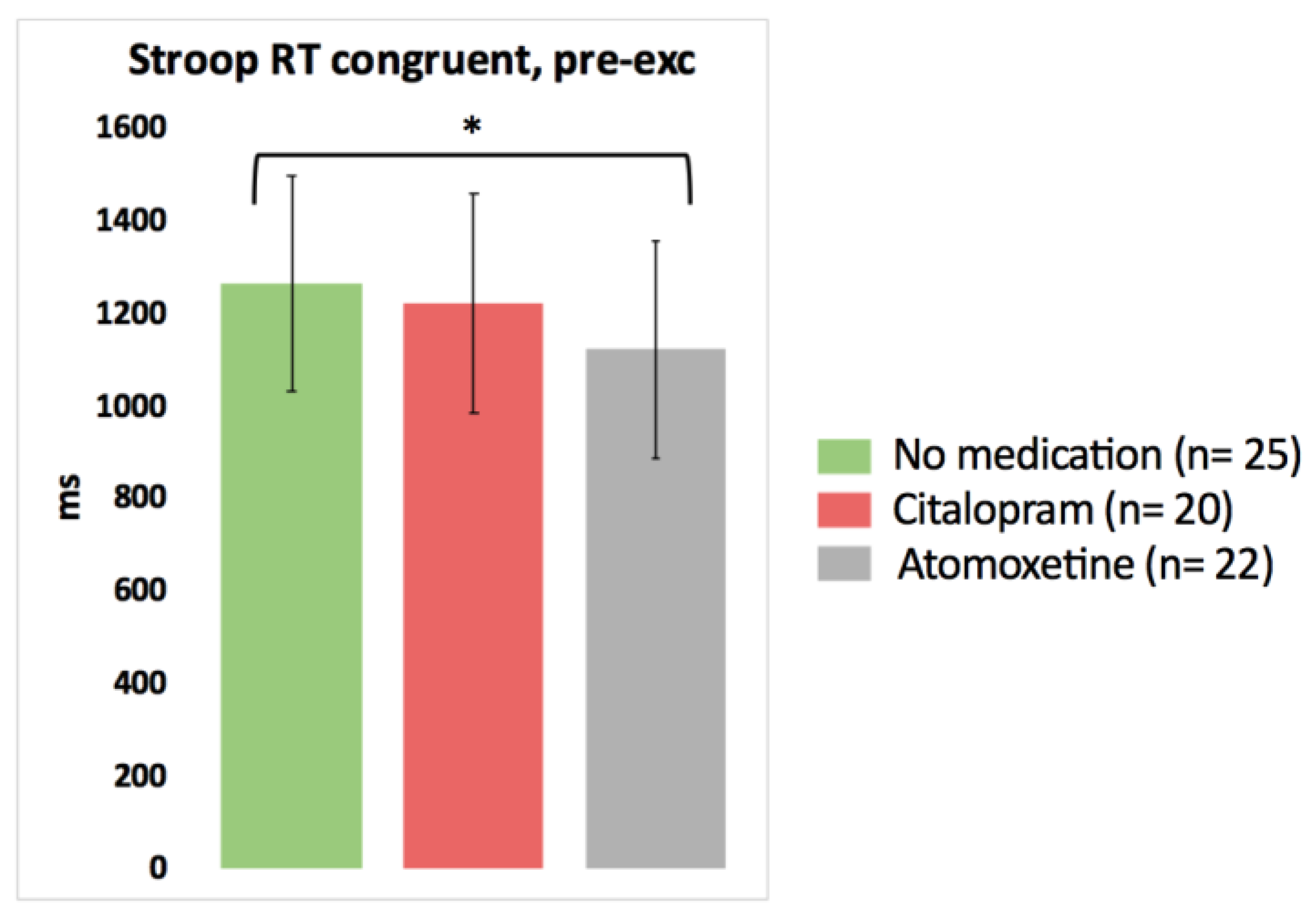
Based on the pairwise comparisons of the linear mixed models analysis, after the intake of Atomoxetine, choice reaction time significantly improved for one Stroop condition (faster Stroop reaction time congruent) compared to the no medication test day (p = 0.048, Cohen’s d = −0.37) (Figure 2; detailed statistics presented in Table 3). No other significant differences in the results of the cognitive tests pre-exercise were found between the no medication condition on the one hand and the Citalopram or Atomoxetine condition on the other hand (p > 0.05).
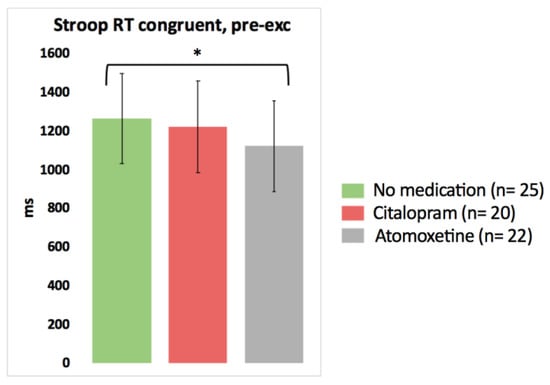
Figure 2.
The isolated effect of activated serotonergic or noradrenergic descending pathways on cognitive performance at rest in people with chronic whiplash-associated disorders. Estimated means and 95% confidence intervals are presented. * = p < 0.05; exc: exercise; RT: reaction time; only significant results are presented.

Table 3.
Cognitive performance in people with CWAD in three conditions: baseline without medication intake (n = 25), after intake of Citalopram (SSRI), and after intake of Atomoxetine (selective NRI).
3.4. The Effect of a Single Dose of a SSRI or a Selective NRI on Cognitive Performance in Response to Submaximal Aerobic Exercise in People with CWAD
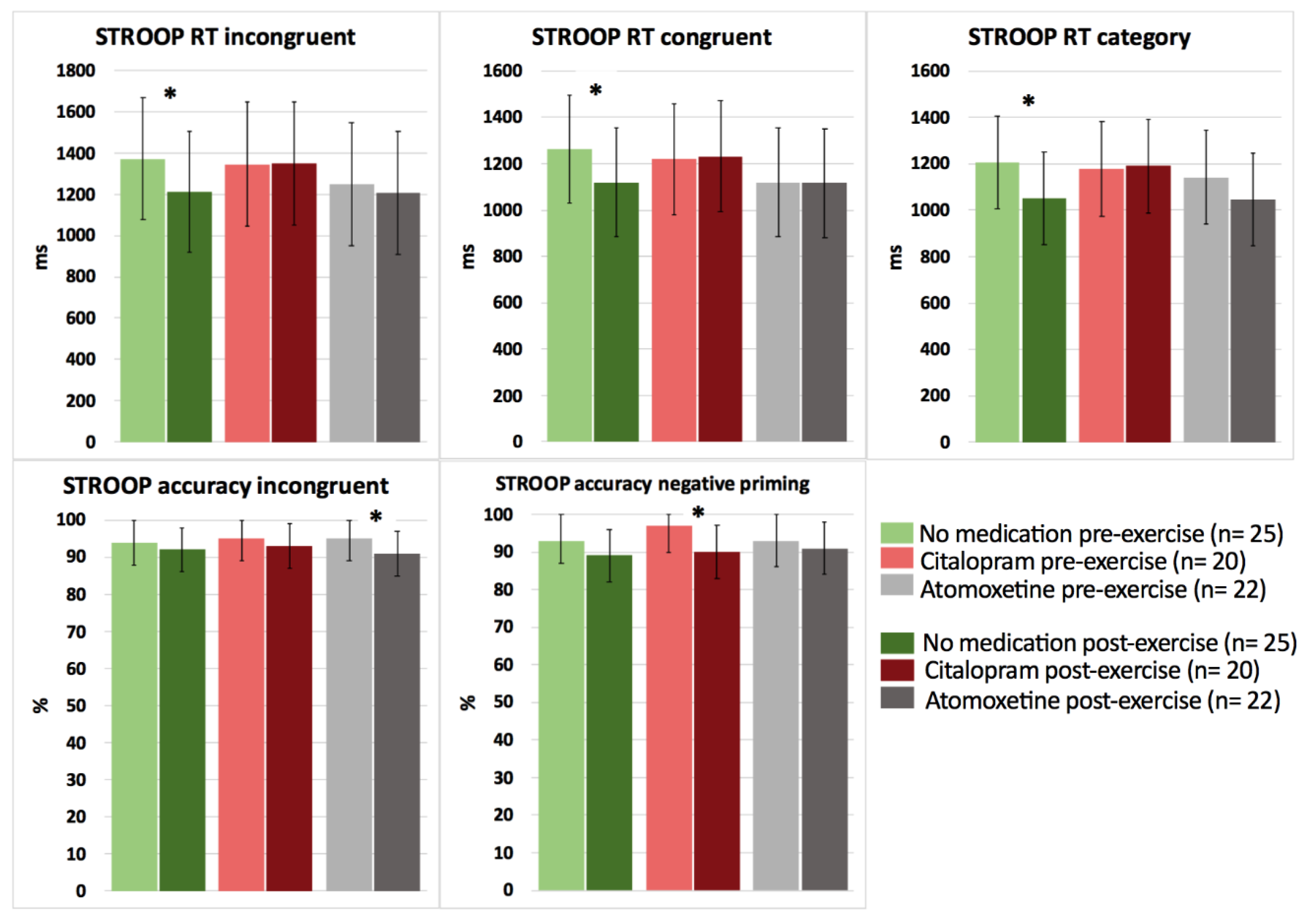
Based on the interaction effects, no significant differences were revealed between the medication conditions (no medication, Citalopram, Atomoxetine) on cognitive performance in response to a bout of acute submaximal aerobic exercise in people with CWAD (p > 0.05). However, when performing pairwise comparisons (pre-post submaximal exercise) within each medication condition, significant improvements in selective attention for Stroop reaction time incongruent (p = 0.025, d = −0.70) and choice reaction time for Stroop reaction time congruent (p = 0.018, d = −0.92) and category (p = 0.012, d = −0.65) were found after exercise for the no medication condition (Figure 3; detailed statistics presented in Table 4).
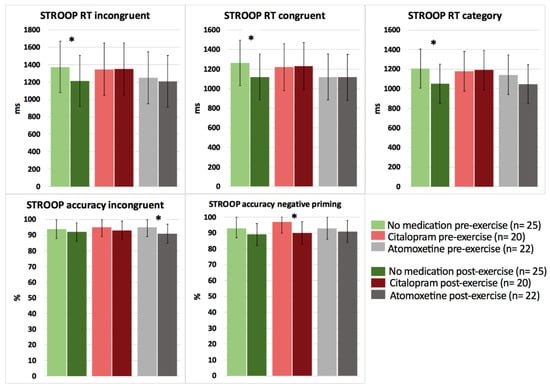
Figure 3.
The effect of a single dose of Citalopram or Atomoxetine on cognitive performance (i.e., selective attention and choice reaction time) in response to exercise in people with chronic whiplash-associated disorders. Estimated means and 95% confidence intervals are presented. * = p < 0.05; RT: reaction time; only significant results are presented.

Table 4.
Cognitive performance in people with CWAD before and after a single submaximal aerobic exercise bout in three conditions: baseline without medication intake (n = 25), after intake of Citalopram (SSRI), and after intake of Atomoxetine (selective NRI).
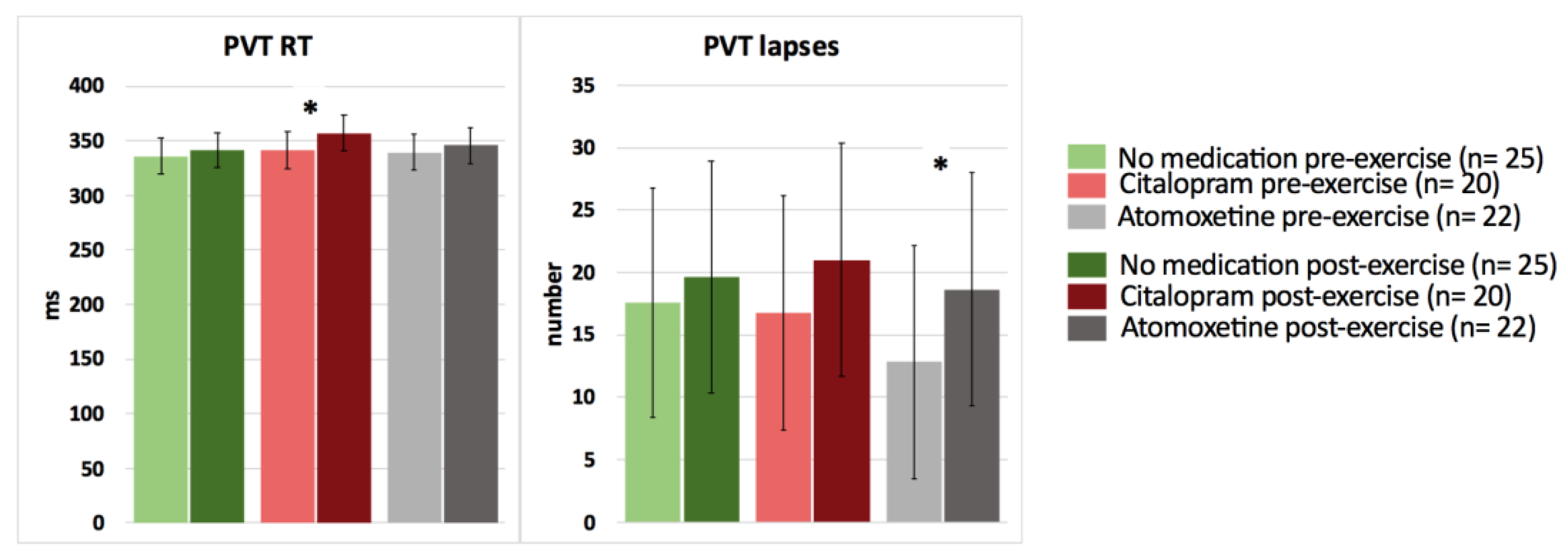
In contrast, after intake of Citalopram or Atomoxetine, both selective and sustained attention significantly worsened, and simple (PVT) reaction time significantly increased postexercise (p < 0.05) (Figure 3 and Figure 4; Table 4). More specifically, people with CWAD showed significantly decreased accuracy in Stroop reaction time incongruent after the submaximal exercise bout on the Atomoxetine assessment day (p = 0.030, d = −0.50). Additionally, participants showed a significantly higher number of lapses during the PVT after exercise compared to the pre-exercise result on the Atomoxetine assessment day (p = 0.034, d = 0.64) (Figure 4). After intake of Citalopram, Stroop accuracy negative priming significantly worsened after the aerobic exercise (p = 0.015, d = −0.65) (Figure 3). Furthermore, after Citalopram intake, simple reaction time significantly increased postexercise, thus sustained attention worsened (p = 0.021, d = 0.53) (Figure 4).
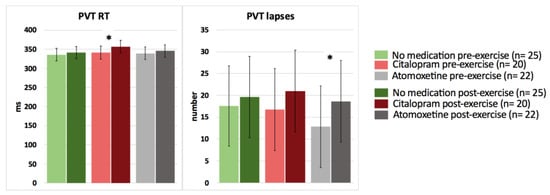
Figure 4.
The effect of a single dose of Citalopram or Atomoxetine on cognitive performance—sustained attention and simple reaction time—in response to exercise in people with chronic whiplash-associated disorders. Estimated means and 95% confidence intervals are presented. * = p < 0.05; RT: reaction time; PVT: psychomotor vigilance task; only significant results are presented.
4. Discussion
This innovative study investigated the effects of a single dose of a SSRI and a selective NRI on cognitive performance at rest and in response to exercise in people with CWAD. At rest, the intake of a single dose of Atomoxetine had a positive influence on the results of the Stroop task of only one condition by decreasing the choice reaction time during the Stroop congruent condition compared to the Stroop congruent reaction time measured without Atomoxetine intake (small effect size). The latter finding of improved selective attention is in line with our hypothesis. Nevertheless, Atomoxetine had no significant isolated effect on all other cognitive performance variables in people with CWAD. Furthermore, no significant effects of a single dose of Citalopram on cognitive functioning at rest in people with CWAD could be demonstrated. It is noteworthy that the possible reported side effects of both medications that could have an influence on cognition are drowsiness, sleeping problems, and fatigue [65]. Nonetheless, an acute dose of Citalopram or Atomoxetine did not worsen cognitive performance at rest in people with CWAD compared to the no medication condition. Additionally, levels of fatigue were similar on all assessment days.
It might be possible that activation of noradrenergic transmission pre-exercise and the subsequent increased availability of norepinephrine after Atomoxetine use enhanced selective attention, but further work in this area is necessary. Citalopram intake had no significant isolated effect on cognitive performance, which is in accordance with findings in healthy persons [46,66,67].
This study provides the novel insight that the positive effects of a bout of acute aerobic exercise on selective attention and choice reaction time (medium to large effect sizes) could only be detected when no SSRI or selective NRI was taken by people with CWAD. In addition, WAD symptoms, such as pain (based on our previous study [9]) and fatigue, were not exacerbated either immediately or 24 h postexercise.
A positive influence of acute aerobic exercise on cognitive functioning was, on the one hand, hypothesized because this has been demonstrated in patients with chronic fatigue syndrome [68] and healthy people [35,36]. Furthermore, evidence is available in various chronic pain conditions that exercise therapy has a positive effect on cognitive functioning [69,70,71,72,73]. On the other hand, some evidence exists for the worsening of symptoms following physical exertion in women with CWAD [18]. Nevertheless, in a recent study, patients with CWAD did not perceive increased pain sensitivity following aerobic exercise [74].
The mechanisms that could explain the observed beneficial effects of acute aerobic exercise on cognitive functioning are presumed to be driven by physiological responses to exercise. These responses comprise changes in heart rate and plasma catecholamines, increased levels of growth factors such as brain-derived neurotrophic factor (BDNF) [35], and brain neurotransmitters such as norepinephrine, serotonin, and dopamine, mediating the exercise-induced enhancement of cognition [33,34].
The intake of a single dose of Atomoxetine resulted in a worsening of Stroop accuracy reaction time incongruent and in more errors of omission during the PVT in response to the acute exercise bout (medium effect size). Similarly, a single dose of Citalopram resulted in a worsening of Stroop accuracy reaction time (negative priming) and gave rise to diminished sustained attention after the exercise compared to the pre-exercise condition (medium effect size). The latter results are not according to our hypotheses. Because changes in, for example, BDNF, serotonin, and norepinephrine levels in response to the exercise performance were not measured, we cannot state which mechanisms accounted for the observed changes in cognitive functioning following the exercise.
Possibly, the acute medication-induced increased levels of serotonin and norepinephrine in the brain had a negative influence on the mediating effect of these monoamines on cognitive performance in response to exercise. On the other hand, it could be that the single acute dose of both centrally acting drugs was not adequate to successfully activate serotonergic and noradrenergic descending pathways in response to exercise and hence, to obtain positive effects on postexercise cognition. Therefore, the present study has limited clinical implications. It can be concluded that clinicians are advised not to use single doses of Citalopram or Atomoxetine to improve cognitive performance at rest or in response to exercise in people with CWAD.
Limitations and Recommendations for Further Research
The following limitations should be taken into account. As we could not demonstrate significant interaction effects, and a small sample size increases the risk of committing a type II error, further research with a larger sample size is warranted before firm conclusions can be drawn.
This study only investigated the effects of a single dose of Citalopram and Atomoxetine in people with CWAD. In order to ensure peak concentrations at the time of testing, all participants were instructed to take Citalopram (20 mg per os; Citalopram Sandoz®) four hours and Atomoxetine (40 mg per os; Strattera®) one and a half hours, respectively, before the scheduled start of their appointment [41,42]. However, people with chronic pain usually take these medications over a long period of time. Perhaps, these medications should be taken for a longer period of time before exerting positive effects on the influence that exercise exerts on cognitive functioning. Indeed, the onset of action of Citalopram for depression is approximately 1 to 4 weeks, and the complete response may take 8–12 weeks after initiation. Future work should examine whether such long-term administration of Citalopram has different effects, as observed here. Furthermore, this trial is not placebo-controlled. We did not include a condition with a placebo medication which could have been useful to blind participants also for the test day without medication and to enhance insights into underlying mechanisms. Originally, we intended to include a placebo group, but the ethical committee did not allow us to do that.
The improved selective attention combined with the absence of post-exertional aggravation of symptoms in response to the acute aerobic exercise in individuals with CWAD indicates the relevance of further randomized controlled trials to study the effects of graded aerobic exercise therapy on cognitive functioning.
5. Conclusions
In conclusion, a single dose of Atomoxetine improved selective attention only in one Stroop condition, and a single dose of Citalopram had no effect on cognitive functioning at rest in people with CWAD. Only without medication intake did selective attention improve in response to exercise, whereas both centrally acting medications worsened cognitive performance in response to a submaximal aerobic exercise bout in people with CWAD. Further research with larger sample sizes is warranted. Examining the influence of the long-term use of selective serotonin reuptake inhibitors and selective norepinephrine reuptake inhibitors on the physiological response to exercise training and subsequent effects on cognitive functioning in people with chronic pain is a future research avenue.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/clinpract13030063/s1, CONSORT 2010 checklist of information to include when reporting a randomized trial.
Author Contributions
Conceptualization, J.N. and K.I.; methodology, K.I., I.C., M.M., I.H. and M.D.K.; validation, I.C., J.N., M.M., M.D.K., E.R., E.H., R.P., W.V.B., I.H. and K.I.; formal analysis, M.D.K., I.C. and K.I.; investigation, I.C., J.N., M.M., M.D.K., E.R., E.H., R.P., W.V.B., I.H. and K.I.; resources, I.H., J.N. and K.I.; data curation, K.I.; writing—original draft preparation, I.C. and K.I.; writing—review and editing, I.C., J.N., M.M., M.D.K., E.R., E.H., R.P., W.V.B., I.H. and K.I.; visualization, I.C.; supervision, K.I. and J.N.; project administration, K.I.; funding acquisition, J.N., K.I. and I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Scientific Fund Willy Gepts of the University Hospital Brussels, grant number WFWG-22. Iris Coppieters, a postdoctoral researcher at Vrije Universiteit Brussel, is funded by the Research Foundation Flanders (FWO) [G007217N], Belgium. Eva Huysmans is a PhD research fellow funded by the Research Foundation Flanders (FWO) [1108619N], Belgium. Roselien Pas and Emma Rheel are funded by a Chair awarded by the Berekuyl Academy/European College for Decongestive Lymphatic Therapy, the Netherlands, to the Vrije Universiteit Brussel, Belgium. Wouter Van Bogaert is funded by the Agency for Innovation by Science and Technology (IWT)—Applied Biomedical Research Program (TBM) [150180].
Institutional Review Board Statement
The research protocol was approved by the Ethics Committee of the University Hospital Brussels/Vrije Universiteit Brussel and was in compliance with the Declaration of Helsinki. The study drugs were produced according to the Good Manufacturing Practice. All participants were thoroughly informed about the study procedures and signed a consent form prior to study enrolment. This study was registered with ClinicalTrials.gov (Identifier No. NCT01601912).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Please contact the corresponding author to obtain the research data. All requests for obtaining the research data will be considered by the research team.
Acknowledgments
The authors would like to thank Menno Franken and Elien Vanderlinden for their assistance with data collection. The authors would like to thank Roos Colman and Wilfried Cools for their assistance with statistical data analyses.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rodriquez, A.A.; Barr, K.P.; Burns, S.P. Whiplash: Pathophysiology, diagnosis, treatment, and prognosis. Muscle Nerve 2004, 29, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Sterling, M. A proposed new classification system for whiplash associated disorders—Implications for assessment and management. Man. Ther. 2004, 9, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, I.; Ickmans, K.; Cagnie, B.; Nijs, J.; De Pauw, R.; Noten, S.; Meeus, M. Cognitive Performance Is Related to Central Sensitization and Health-related Quality of Life in Patients with Chronic Whiplash-Associated Disorders and Fibromyalgia. Pain Physician 2015, 18, E389–E401. [Google Scholar]
- Sterner, Y.; Gerdle, B. Acute and chronic whiplash disorders—A review. J. Rehabil. Med. 2004, 36, 193–209. [Google Scholar] [CrossRef]
- Wenzel, H.G.; Haug, T.T.; Mykletun, A.; Dahl, A.A. A population study of anxiety and depression among persons who report whiplash traumas. J. Psychosom. Res. 2002, 53, 831–835. [Google Scholar] [CrossRef]
- Coppieters, I.; De Pauw, R.; Kregel, J.; Malfliet, A.; Goubert, D.; Lenoir, D.; Cagnie, B.; Meeus, M. Differences Between Women with Traumatic and Idiopathic Chronic Neck Pain and Women Without Neck Pain: Interrelationships Among Disability, Cognitive Deficits, and Central Sensitization. Phys. Ther. 2017, 97, 338–353. [Google Scholar]
- Antepohl, W.; Kiviloog, L.; Andersson, J.; Gerdle, B. Cognitive impairment in patients with chronic whiplash-associated disorder--a matched control study. NeuroRehabilitation 2003, 18, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Van Oosterwijck, J.; Ickmans, K.; Baert, I.; Coppieters, I.; Roussel, N.; Struyf, F.; Pattyn, N.; Nijs, J. Interrelationships between pain processing, cortisol and cognitive performance in chronic whiplash-associated disorders. Clin. Rheumatol. 2015, 34, 545–553. [Google Scholar] [CrossRef]
- Ickmans, K.; Meeus, M.; De Kooning, M.; De Backer, A.; Kooremans, D.; Hubloue, I.; Schmitz, T.; Van Loo, M.; Nijs, J. Exercise and Cognitive Functioning in People with Chronic Whiplash-Associated Disorders: A Controlled Laboratory Study. J. Orthop. Sport. Phys. Ther. 2016, 46, 87–95. [Google Scholar] [CrossRef]
- Kessels, R.P.; Aleman, A.; Verhagen, W.I.; Van Luijtelaar, E.L. Cognitive functioning after whiplash injury: A meta-analysis. J. Int. Neuropsychol. Soc. 2000, 6, 271–278. [Google Scholar] [CrossRef]
- Konrad, C.; Geburek, A.J.; Rist, F.; Blumenroth, H.; Fischer, B.; Husstedt, I.; Arolt, V.; Schiffbauer, H.; Lohmann, H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2011, 41, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Schmand, B.; Lindeboom, J.; Schagen, S.; Heijt, R.; Koene, T.; Hamburger, H.L. Cognitive complaints in patients after whiplash injury: The impact of malingering. J. Neurol. Neurosurg. Psychiatry 1998, 64, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.-A.; Rice, A.S.C.; Rief, W.; Sluka, A.K. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016, 157, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, O.; Finn, D.P. Cognition and pain. Curr. Opin. Support. Palliat. Care 2014, 8, 130–136. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef]
- Ickmans, K.; Meeus, M.; De Kooning, M.; Lambrecht, L.; Pattyn, N.; Nijs, J. Associations Between Cognitive Performance and Pain in Chronic Fatigue Syndrome: Comorbidity with Fibromyalgia Does Matter. Pain Physician 2015, 18, E841–E852. [Google Scholar] [CrossRef]
- Daenen, L.; Nijs, J.; Roussel, N.; Wouters, K.; Van Loo, M.; Cras, P. Dysfunctional pain inhibition in patients with chronic whiplash-associated disorders: An experimental study. Clin. Rheumatol. 2013, 32, 23–31. [Google Scholar] [CrossRef]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Van Loo, M.; Paul, L. Lack of endogenous pain inhibition during exercise in people with chronic whiplash associated disorders: An experimental study. J. Pain 2012, 13, 242–254. [Google Scholar] [CrossRef]
- Banic, B.; Petersen-Felix, S.; Andersen, O.K.; Radanov, B.P.; Villiger, M.P.; Arendt-Nielsen, L.; Curatolo, M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 2004, 107, 7–15. [Google Scholar] [CrossRef]
- Linnman, C.; Appel, L.; Söderlund, A.; Frans, Ö.; Engler, H.; Furmark, T.; Gordh, T.; Långström, B.; Fredrikson, M. Chronic whiplash symptoms are related to altered regional cerebral blood flow in the resting state. Eur. J. Pain 2009, 13, 65–70. [Google Scholar] [CrossRef]
- Bannister, K.; Dickenson, A.H. What do monoamines do in pain modulation? Curr. Opin. Support. Palliat. Care 2016, 10, 143–148. [Google Scholar] [CrossRef]
- Yoshimura, M.; Furue, H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J. Pharmacol. Sci. 2006, 101, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Dickenson, A.H. The plasticity of descending controls in pain: Translational probing. J. Physiol. 2017, 595, 4159–4166. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Robbins, T.W. Noradrenergic modulation of cognition: Therapeutic implications. J. Psychopharmacol. 2013, 27, 694–718. [Google Scholar] [CrossRef]
- Cowen, P.; Sherwood, A.C. The role of serotonin in cognitive function: Evidence from recent studies and implications for understanding depression. J. Psychopharmacol. 2013, 27, 575–583. [Google Scholar] [CrossRef]
- Sara, S.J.; Bouret, S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron 2012, 76, 130–141. [Google Scholar] [CrossRef]
- Svob Strac, D.; Pivac, N.; Muck-Seler, D. The serotonergic system and cognitive function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef]
- Tully, K.; Bolshakov, V.Y. Emotional enhancement of memory: How norepinephrine enables synaptic plasticity. Mol. Brain 2010, 3, 15. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar]
- Nijs, J.; Kosek, E.; Van Oosterwijck, J.; Meeus, M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: To exercise or not to exercise? Pain Physician 2012, 15 (Suppl. S3), Es205–Es213. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Smolders, I.; Sarre, S.; DE Meirleir, K.; Keizer, H.; Serneels, M.; Ebinger, G.; Michotte, Y. Endurance training effects on neurotransmitter release in rat striatum: An in vivo microdialysis study. Acta Physiol. Scand. 1997, 159, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Kuo, Y.M. Exercise benefits brain function: The monoamine connection. Brain Sci. 2013, 3, 39–53. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Kane, C.J. Exercise and cognitive function: A randomized controlled trial examining acute exercise and free-living physical activity and sedentary effects. Mayo Clin. Proc. 2015, 90, 450–460. [Google Scholar] [CrossRef]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef]
- Cook, D.B.; Nagelkirk, P.R.; Peckerman, A.; Poluri, A.; Mores, J.; Natelson, B.H. Exercise and cognitive performance in chronic fatigue syndrome. Med. Sci. Sport. Exerc. 2005, 37, 1460–1467. [Google Scholar] [CrossRef]
- Spitzer, W.O.; Skovron, M.L.; Salmi, L.R.; Cassidy, J.D.; Duranceau, J.; Suissa, S.; Zeiss, E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: Redefining “whiplash” and its management. Spine 1995, 20 (Suppl. 8), 1S–73S. [Google Scholar]
- Rey, A. L’examen Clinique en Psychologie (The Clinical Examination in Psychology); University Press of France: Paris, France, 1964. [Google Scholar]
- Sauer, J.M.; Ring, B.J.; Witcher, J.W. Clinical pharmacokinetics of atomoxetine. Clin. Pharmacokinet. 2005, 44, 571–590. [Google Scholar] [CrossRef]
- Sangkuhl, K.; Klein, T.E.; Altman, R.B. PharmGKB summary: Citalopram pharmacokinetics pathway. Pharm. Genom. 2011, 21, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R.J.; Rittman, T.; Rae, C.; Passamonti, L.; Jones, S.P.; Vatansever, D.; Rodríguez, P.V.; Ye, Z.; Nombela, C.; Hughes, L.E.; et al. Atomoxetine and citalopram alter brain network organization in Parkinson’s disease. Brain Commun. 2019, 1, fcz013. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R.J.; Rittman, T.; Passamonti, L.; Ye, Z.; Sami, S.; Jones, S.P.; Nombela, C.; Vázquez Rodríguez, P.; Vatansever, D.; Rae, C.L.; et al. Atomoxetine Enhances Connectivity of Prefrontal Networks in Parkinson’s Disease. Neuropsychopharmacology 2016, 41, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Hampshire, A.; Müller, U.; Rubia, K.; Del Campo, N.; Craig, K.; Regenthal, R.; Suckling, J.; Roiser, J.P.; Grant, J.E.; et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biol. Psychiatry 2009, 65, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Muller, U.; Blackwell, A.D.; Clark, L.; Robbins, T.W.; Sahakian, B.J. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 2006, 311, 861–863. [Google Scholar] [CrossRef]
- Chalon, S.; Desager, J.; DeSante, K.A.; Frye, R.F.; Witcher, J.; Long, A.J.; Sauer, J.; Golnez, J.; Smith, B.P.; Thomasson, H.R.; et al. Effect of hepatic impairment on the pharmacokinetics of atomoxetine and its metabolites. Clin. Pharmacol. Ther. 2003, 73, 178–191. [Google Scholar] [CrossRef]
- Rocha, A.; Marques, M.P.; Coelho, E.B.; Lanchote, V.L. Enantioselective analysis of citalopram and demethylcitalopram in human and rat plasma by chiral LC-MS/MS: Application to pharmacokinetics. Chirality 2007, 19, 793–801. [Google Scholar] [CrossRef]
- Milne, R.J.; Goa, K.L. Citalopram: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depressive illness. Drugs 1991, 41, 450–477. [Google Scholar] [CrossRef]
- Clemow, D.B.; Bushe, C.J. Atomoxetine in patients with ADHD: A clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients. J. Psychopharmacol. 2015, 29, 1221–1230. [Google Scholar] [CrossRef]
- Telford, R.D.; Minikin, B.R.; Hahn, A.G.; Hooper, L.A. A simple method for the assessment of general fitness: The tri-level profile. Aust. J. Sci. Med. Sport. 1989, 21, 6–9. [Google Scholar]
- Wallman, K.E.; Morton, A.R.; Goodman, C.; Grove, J.R. Physiological responses during a submaximal cycle test in chronic fatigue syndrome. Med. Sci. Sport. Exerc. 2004, 36, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Jorritsma, W.; De Vries, G.E.; Geertzen, J.H.B.; Dijkstra, P.U.; Reneman, M.F. Neck Pain and Disability Scale and the Neck Disability Index: Reproducibility of the Dutch Language Versions. Eur. Spine J. 2010, 19, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, W.; de Vries, G.E.; Dijkstra, P.U.; Geertzen, J.H.B.; Reneman, M.F. Neck Pain and Disability Scale and Neck Disability Index: Validity of Dutch language versions. Eur. Spine J. 2012, 21, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar]
- Nijs, J.; Thielemans, A. Kinesiophobia and symptomatology in chronic fatigue syndrome: A psychometric study of two questionnaires. Psychol. Psychother. 2008, 81 Pt 3, 273–283. [Google Scholar] [CrossRef]
- Ickmans, K.; Meeus, M.; Kos, D.; Clarys, P.; Meersdom, G.; Lambrecht, L.; Pattyn, N.; Nijs, J. Cognitive performance is of clinical importance, but is unrelated to pain severity in women with chronic fatigue syndrome. Clin. Rheumatol. 2013, 32, 1475–1485. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Tipper, S.P. The negative priming effect: Inhibitory priming by ignored objects. Q. J. Exp. Psychol. A 1985, 37, 571–590. [Google Scholar] [CrossRef]
- Westerhausen, R.; Kompus, K.; Hugdahl, K. Impaired cognitive inhibition in schizophrenia: A meta-analysis of the Stroop interference effect. Schizophr. Res. 2011, 133, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Dinges, D.; Powell, J. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Dorrian, J.; Rogers, N.; Dinges, D. Psychomotor Vigilance Performance: Neurocognitive Assay Sensitive to Sleep Loss; Marcel Dekker: New York, NY, USA, 2005; pp. 39–70. [Google Scholar]
- Richter, T.; Paluch, Z.; Alusik, S. The non-antidepressant effects of citalopram: A clinician’s perspective. Neuro Endocrinol. Lett. 2014, 35, 7–12. [Google Scholar] [PubMed]
- Nandam, L.S.; Hester, R.; Wagner, J.; Cummins, T.D.; Garner, K.; Dean, A.J.; Kim, B.N.; Nathan, P.J.; Mattingley, J.B.; Bellgrove, M.A. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol. Psychiatry 2011, 69, 902–904. [Google Scholar] [CrossRef]
- Almeida, S.; Glahn, D.; Argyropoulos, S.; Frangou, S. Acute citalopram administration may disrupt contextual information processing in healthy males. Eur. Psychiatry 2010, 25, 87–91. [Google Scholar] [CrossRef]
- LaManca, J.J.; Sisto, S.A.; DeLuca, J.; Johnson, S.K.; Lange, G.; Pareja, J.; Cook, S.; Natelson, B.H. Influence of exhaustive treadmill exercise on cognitive functioning in chronic fatigue syndrome. Am. J. Med. 1998, 105, 59S–65S. [Google Scholar] [CrossRef]
- Luoto, S.; Taimela, S.; Hurri, H.; Alaranta, H. Mechanisms explaining the association between low back trouble and deficits in information processing. A controlled study with follow-up. Spine 1999, 24, 255–261. [Google Scholar] [CrossRef]
- Wallman, K.E.; Morton, A.R.; Goodman, C.; Grove, R.; Guilfoyle, A.M. Randomised controlled trial of graded exercise in chronic fatigue syndrome. Med. J. Aust. 2004, 180, 444–448. [Google Scholar] [CrossRef]
- Munguia-Izquierdo, D.; Legaz-Arrese, A. Exercise in warm water decreases pain and improves cognitive function in middle-aged women with fibromyalgia. Clin. Exp. Rheumatol. 2007, 25, 823–830. [Google Scholar]
- Etnier, J.L.; Karper, W.B.; Gapin, J.I.; Barella, L.A.; Chang, Y.K.; Murphy, K.J. Exercise, fibromyalgia, and fibrofog: A pilot study. J. Phys. Act. Health 2009, 6, 239–246. [Google Scholar] [CrossRef]
- Munguia-Izquierdo, D.; Legaz-Arrese, A. Assessment of the effects of aquatic therapy on global symptomatology in patients with fibromyalgia syndrome: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2008, 89, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Ritchie, C.; Pedler, A.; McCamley, K.; Roberts, K.; Sterling, M. Exercise induced hypoalgesia is elicited by isometric, but not aerobic exercise in individuals with chronic whiplash associated disorders. Scand. J. Pain 2017, 15, 14–21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).