Giant Pancreatic Pseudocyst after Coronary Artery Bypass Graft in a Hemodialysis Patient: A Case Report
Abstract
1. Introduction
2. Case Report
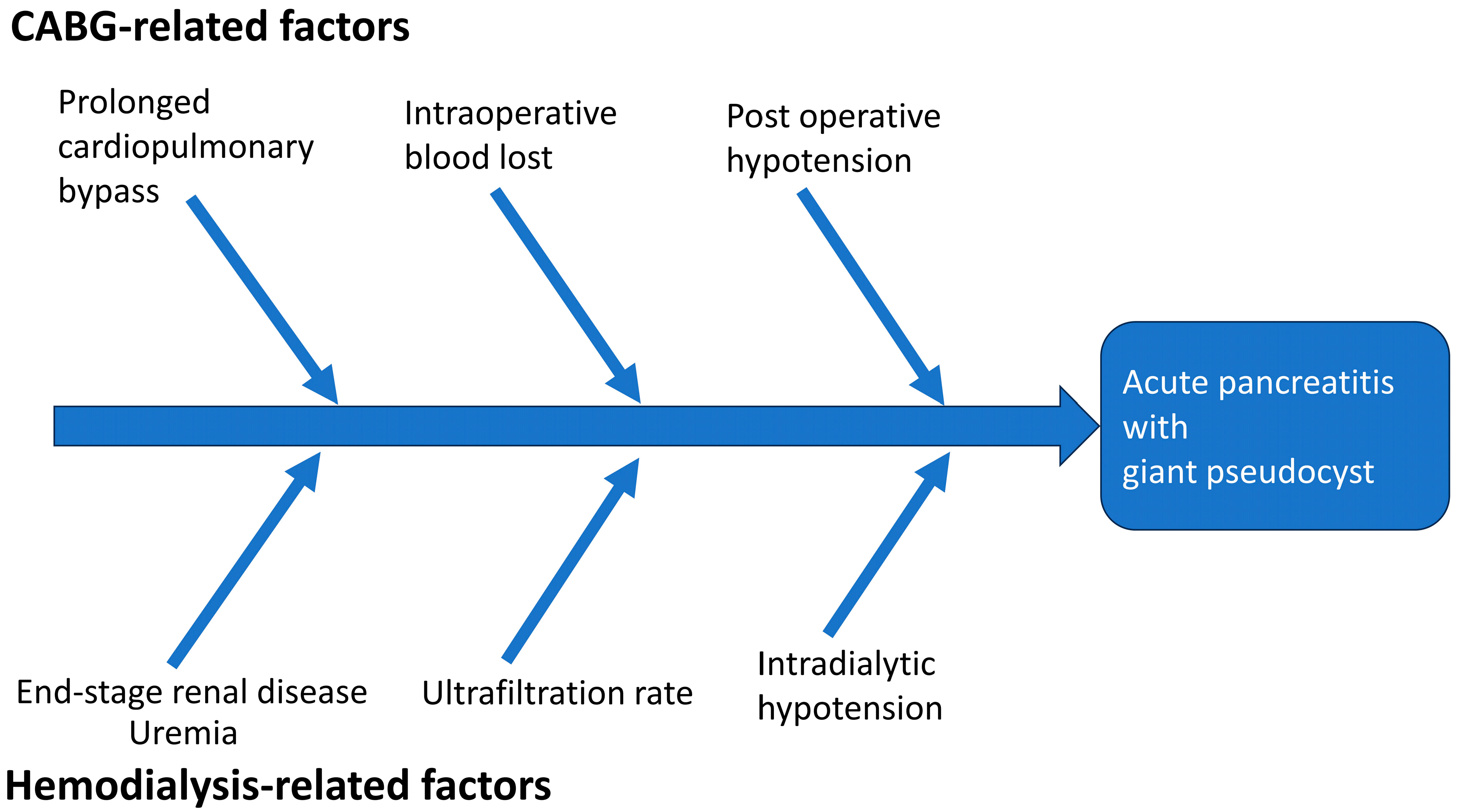
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 1998, 9, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, N.K.; Karimi Galougahi, K.; Paz, Y.; Nazif, T.; Moses, J.W.; Leon, M.B.; Stone, G.W.; Kirtane, A.J.; Karmpaliotis, D.; Bokhari, S.; et al. Diagnosis and Management of Cardiovascular Disease in Advanced and End-Stage Renal Disease. J. Am. Heart Assoc. 2016, 5, e003648. [Google Scholar] [CrossRef] [PubMed]
- K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am. J. Kidney Dis. 2005, 45, S1–S153.
- Liu, J.Y.; Birkmeyer, N.J.; Sanders, J.H.; Morton, J.R.; Henriques, H.F.; Lahey, S.J.; Dow, R.W.; Maloney, C.; DiScipio, A.W.; Clough, R.; et al. Risks of morbidity and mortality in dialysis patients undergoing coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation 2000, 102, 2973–2977. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsu, C.C.; Lin, M.H.; Sung, J.M.; Kuo, T.H. Healthcare utilization and expenditure among individuals with end-stage kidney disease in Taiwan. J. Formos. Med. Assoc. 2022, 121 (Suppl. 1), S47–S55. [Google Scholar] [CrossRef]
- Fortescue, E.B.; Kahn, K.; Bates, D.W. Development and validation of a clinical prediction rule for major adverse outcomes in coronary bypass grafting. Am. J. Cardiol. 2001, 88, 1251–1258. [Google Scholar] [CrossRef]
- Filsoufi, F.; Rahmanian, P.B.; Castillo, J.G.; Scurlock, C.; Legnani, P.E.; Adams, D.H. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann. Surg. 2007, 246, 323–329. [Google Scholar] [CrossRef]
- Rodriguez, F.; Nguyen, T.C.; Galanko, J.A.; Morton, J. Gastrointestinal complications after coronary artery bypass grafting: A national study of morbidity and mortality predictors. J. Am. Coll. Surg. 2007, 205, 741–747. [Google Scholar] [CrossRef]
- Fernández-del Castillo, C.; Harringer, W.; Warshaw, A.L.; Vlahakes, G.J.; Koski, G.; Zaslavsky, A.M.; Rattner, D.W. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N. Engl. J. Med. 1991, 325, 382–387. [Google Scholar] [CrossRef]
- Paajanen, H.; Nuutinen, P.; Harmoinen, A.; Pöyhönen, M.; Pitkänen, O.; Nordback, I.; Grönroos, J.; Nevalainen, T.J. Hyperamylasemia after cardiopulmonary bypass: Pancreatic cellular injury or impaired renal excretion of amylase? Surgery 1998, 123, 504–510. [Google Scholar] [CrossRef]
- de Waard, D.; Fagan, A.; Minnaar, C.; Horne, D. Management of patients after coronary artery bypass grafting surgery: A guide for primary care practitioners. CMAJ 2021, 193, E689–E694. [Google Scholar] [CrossRef] [PubMed]
- Lefor, A.T.; Vuocolo, P.; Parker, F.B., Jr.; Sillin, L.F. Pancreatic Complications Following Cardiopulmonary Bypass: Factors Influencing Mortality. Arch. Surg. 1992, 127, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Nys, M.; Venneman, I.; Deby-Dupont, G.; Preiser, J.-C.; Vanbelle, S.; Albert, A.; Camus, G.; Damas, P.; Larbuisson, R.; Lamy, M. Pancreatic Cellular Injury after Cardiac Surgery with Cardiopulmonary Bypass: Frequency, Time Course and Risk Fa-ctors. Shock 2007, 27, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Krejci, V.; Hiltebrand, L.B.; Sigurdsson, G.H. Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis. Crit. Care Med. 2006, 34, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Mutsuga, M.; Saito, S.; Tokuda, Y.; Nagai, K.; Umemoto, N.; Abe, T.; Usui, A. Incidence and clinical impact of silent pancreatitis after aortic arch surgery. Asian Cardiovasc. Thorac. Ann. 2023, 31, 303–311. [Google Scholar] [CrossRef]
- Elgharably, H.; Gamaleldin, M.; Ayyat, K.S.; Zaki, A.; Hodges, K.; Kindzelski, B.; Sharma, S.; Hassab, T.; Yongue, C.; Serna, S.; et al. Serious Gastrointestinal Complications After Cardiac Surgery and Associated Mortality. Ann. Thorac. Surg. 2021, 112, 1266–1274. [Google Scholar] [CrossRef]
- Marsoner, K.; Voetsch, A.; Lierzer, C.; Sodeck, G.H.; Fruhwald, S.; Dapunt, O.; Mischinger, H.J.; Kornprat, P. Gastrointestinal complications following on-pump cardiac surgery-A propensity matched analysis. PLoS ONE 2019, 14, e0217874. [Google Scholar] [CrossRef]
- Musleh, G.S.; Patel, N.C.; Grayson, A.D.; Pullan, D.M.; Keenan, D.J.; Fabri, B.M.; Hasan, R. Off-pump coronary artery bypass surgery does not reduce gastrointestinal complications. Eur. J. Cardiothorac. Surg. 2003, 23, 170–174. [Google Scholar] [CrossRef]
- Fitzgerald, T.; Kim, D.; Karakozis, S.; Alam, H.; Provido, H.; Kirkpatrick, J. Visceral ischemia after cardiopulmonary bypass. Am. Surg. 2000, 66, 623–626. [Google Scholar] [CrossRef]
- Yilmaz, A.T.; Arslan, M.; Demirkilç, U.; Ozal, E.; Kuralay, E.; Bingöl, H.; Oz, B.S.; Tatar, H.; Oztürk, O.Y. Gastrointestinal complications after cardiac surgery. Eur. J. Cardiothorac. Surg. 1996, 10, 763–767. [Google Scholar] [CrossRef]
- Hou, S.W.; Lee, Y.K.; Hsu, C.Y.; Lee, C.C.; Su, Y.C. Increased risk of acute pancreatitis in patients with chronic hemodialysis: A 4-year follow-up study. PLoS ONE 2013, 8, e71801. [Google Scholar] [CrossRef][Green Version]
- Sirinek, K.R.; O’Dorisio, T.M.; Gaskill, H.V.; Levine, B.A. Chronic renal failure: Effect of hemodialysis on gastrointestinal hormones. Am. J. Surg. 1984, 148, 732–735. [Google Scholar] [CrossRef]
- Yu, A.W.; Nawab, Z.M.; Barnes, W.E.; Lai, K.N.; Ing, T.S.; Daugirdas, J.T. Splanchnic erythrocyte content decreases during hemodialysis: A new compensatory mechanism for hypovolemia. Kidney Int. 1997, 51, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Ookawara, S.; Ito, K.; Ueda, Y.; Minato, S.; Kaneko, S.; Hirata, M.; Kitano, T.; Miyazawa, H.; Hirai, K.; Morishita, Y. Factors affecting intradialytic hepatic oxygenation: Associations between ultrafiltration rate and changes in systemic blood pressure. Int. J. Artif. Organs 2023, 46, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Joglar, F.M.; Saadé, M. Outcome of pancreatitis in CAPD and HD patients. Perit. Dial. Int. 1995, 15, 264–266. [Google Scholar] [CrossRef]
- Sunkara, T.; Caughey, M.E.; Rawla, P.; Yarlagadda, K.S.; Gaduputi, V. Severe Acute Pancreatitis as an Index Clinical Manifestation of Parathyroid Adenoma. Cureus 2018, 10, e2445. [Google Scholar] [CrossRef]
- Frick, T.W. The role of calcium in acute pancreatitis. Surgery 2012, 152, S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.; Locatelli, F.; Rodriguez, M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin. J. Am. Soc. Nephrol. 2011, 6, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Misgar, R.A.; Mathew, V.; Pandit, K.; Chowdhury, S. Primary hyperparathyroidism presenting as recurrent acute pancreatitis: A case report and review of literature. Indian J. Endocrinol. Metab. 2011, 15, 54–56. [Google Scholar] [CrossRef]
- Mixter, C.G.; Keynes, W.M.; Cope, O. Further Experience with Pancreatitis as a Diagnostic Clue to Hyperparathyroidism. N. Engl. J. Med. 1962, 266, 265–272. [Google Scholar] [CrossRef]
- Brener, Z.Z.; Bergman, M. Necrotizing pancreatitis due to hypercalcemia in a hemodialysis patient with pica. Clin. Kidney J. 2014, 7, 399–401. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Lab Finding | at Admission | Two Days Later | Reference Range |
|---|---|---|---|
| WBC (/uL) | 8000 | 7600 | 3900–10,600 |
| Hemoglobin (g/dL) | 9.4 | 9.4 | 13.5–17.5 |
| Hematocrit (%) | 31.6 | 31.6 | 41–53 |
| Amylase (U/L) | 387 | 86 | 28–100 |
| Lipase (U/L) | 1048 | 99 | 11–82 |
| AST (U/L) | 24 | 13–40 | |
| Glucose (mg/dL) | 117 | 74–100 | |
| LDH (U/L) | 414 | 135–260 | |
| Calcium (mg/dL) | 9.5 | 10.2 | 8.6–10.3 |
| Triglyceride (mg/dL) | 168 | <150 | |
| BUN (mg/dL) | 33.5 | 41 | 7–25 |
| Albumin (g/dL) | 3.07 | 3.5–5.4 | |
| Intact-PTH (pg/mL) | 49.5 | 15–65 |
| Study | Year | Country | Operation Type | Number | Incidence of AP | Note |
|---|---|---|---|---|---|---|
| Ohno et al. [15] | 2023 | Japan | Aortic arch surgery | 353 | 4% (n = 14) | None required drainage |
| Elgharably et al. [16] | 2021 | US | All cardiac surgery | 29,909 | 0.13% (n = 38) | |
| Marsoner et al. [17] | 2019 | Austria | On-pump cardiac surgery | 4883 | 0.84% (n = 41) | Longer on-pump time in patients with GI complications (OR 1.006, 95%CI 1.001–1.011) |
| Musleh et al. [18] | 2003 | UK | CABG (on- and off-pump) | 2327 | 0.13% (n = 3) | Off-pump and on-pump techniques had similar rates of GI complications |
| Fitzgerald et al. [19] | 2000 | US | All cardiac surgery | 14,521 | 0.19% (n = 27) | More visceral ischemia with longer pump times and ESRD patients |
| Yilmaz et al. [20] | 1996 | Turkey | All cardiac surgery | 3158 | 0.63% (n = 2) | |
| Lefor et al. [12] | 1992 | US | CABG | 5621 | 0.44% (n = 25) | None had pancreatic pseudocysts |
| Castillo et al. [9] | 1991 | US | Cardiac surgery patients with CPB | 300 | 7.7% (n = 23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.-J.; Hsieh, C.-Y.; Su, Y.-J.; Huang, C.-C.; Huang, W.-H.; Weng, C.-H.; Yen, T.-H.; Hsu, C.-W. Giant Pancreatic Pseudocyst after Coronary Artery Bypass Graft in a Hemodialysis Patient: A Case Report. Clin. Pract. 2023, 13, 1236-1243. https://doi.org/10.3390/clinpract13050111
Chan M-J, Hsieh C-Y, Su Y-J, Huang C-C, Huang W-H, Weng C-H, Yen T-H, Hsu C-W. Giant Pancreatic Pseudocyst after Coronary Artery Bypass Graft in a Hemodialysis Patient: A Case Report. Clinics and Practice. 2023; 13(5):1236-1243. https://doi.org/10.3390/clinpract13050111
Chicago/Turabian StyleChan, Ming-Jen, Chun-Yih Hsieh, Yi-Jiun Su, Chien-Chang Huang, Wen-Hung Huang, Cheng-Hao Weng, Tzung-Hai Yen, and Ching-Wei Hsu. 2023. "Giant Pancreatic Pseudocyst after Coronary Artery Bypass Graft in a Hemodialysis Patient: A Case Report" Clinics and Practice 13, no. 5: 1236-1243. https://doi.org/10.3390/clinpract13050111
APA StyleChan, M.-J., Hsieh, C.-Y., Su, Y.-J., Huang, C.-C., Huang, W.-H., Weng, C.-H., Yen, T.-H., & Hsu, C.-W. (2023). Giant Pancreatic Pseudocyst after Coronary Artery Bypass Graft in a Hemodialysis Patient: A Case Report. Clinics and Practice, 13(5), 1236-1243. https://doi.org/10.3390/clinpract13050111






