Pericardial Adhesion and Chronic Non-Specific Neck Pain following Thoracentesis: An Osteopathic Approach
Abstract
:1. Introduction
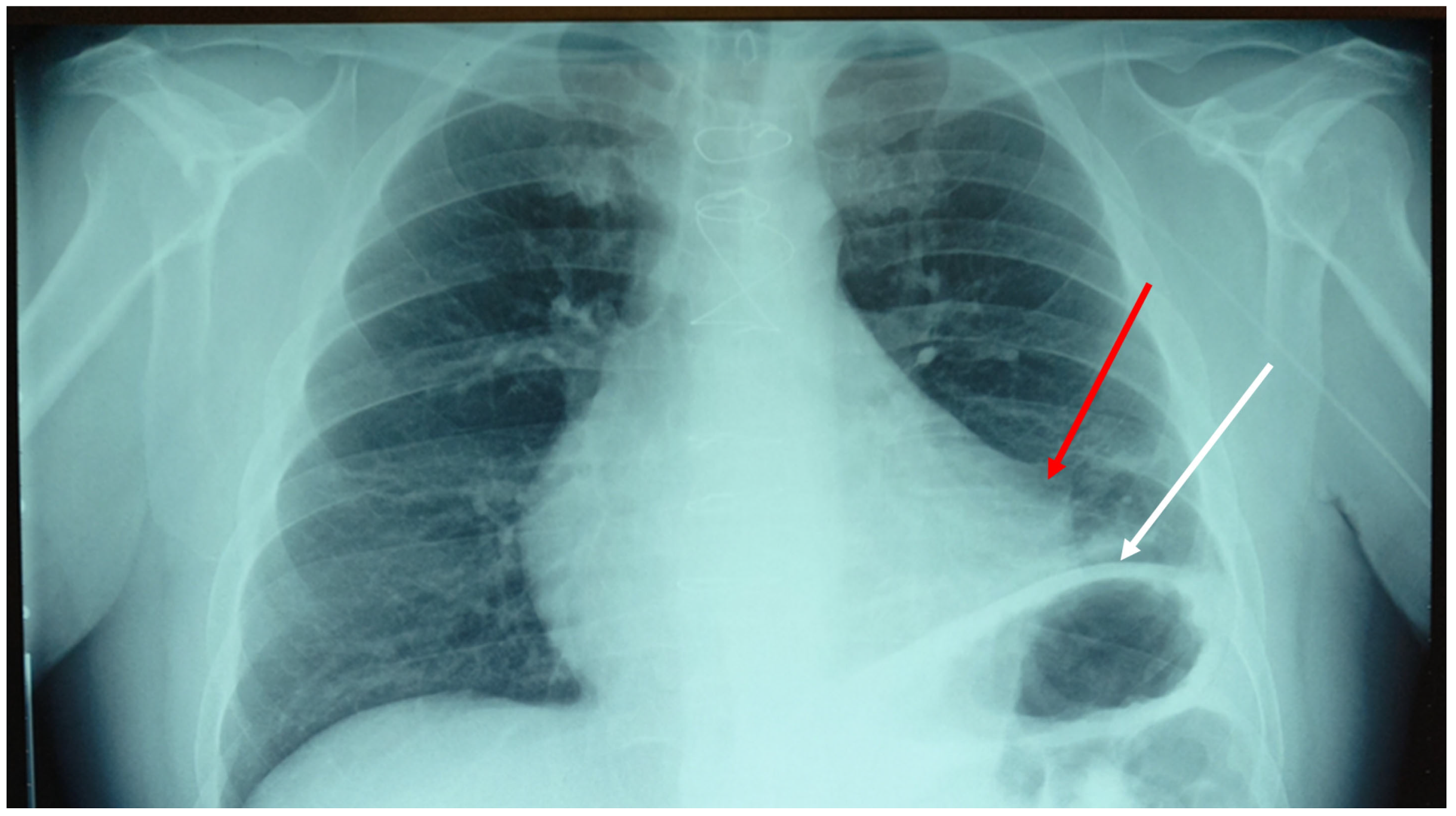
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, H.; Chen, L.; Deng, Y.; Liao, X.; Yang, Y. Ginsenoside Rh2 mitigates myocardial damage in acute myocardial infarction by regulating pyroptosis of cardiomyocytes. Clin. Exp. Hypertens. 2023, 45, 2229536. [Google Scholar] [CrossRef]
- Ricciardi, R.M.; Cipollone, A.; D’ardes, D.; Di Giacomo, D.; Pignatelli, P.; Cipollone, F.; Curia, M.C.; Magni, P.; Bucci, M. Risk factors and immunoinflammatory lechanisms leading to atherosclerosis: Focus on the role of oral microbiota dysbiosis. Microorganisms 2023, 11, 1479. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, M.; Huang, Y.; Yuan, Y.; Chen, Y.; Lu, Z.; Lin, F.; Yang, X.; Xi, D.; Chen, Y.; et al. Evaluation of pericardial thickening and adhesion using high-frequency ultrasound. J. Am. Soc. Echocardiogr. 2023, 36, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Aoyama, T. Multiple coronary artery perforation following global pericardial tamponade in a patient with prior coronary artery bypass graft surgery: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab453. [Google Scholar] [CrossRef]
- Singh, A.S.; Sivakumar, K. Case report: Pericardial adhesions from a previous coronary artery bypass surgery contain a left ventricular free wall rupture after an acute myocardial infarction to form a pseudoaneurysm. Eur. Heart J. Case Rep. 2018, 2, yty081. [Google Scholar] [CrossRef]
- Gorki, H.; Hoenicka, M.; Rupp, P.; Müller-Eising, K.; Deininger, S.; Kunert, A.; Liebold, A. Similarity of coagulation and inflammation despite different surgical revascularization strategies—A prospective randomized trial. Perfusion 2016, 31, 640–647. [Google Scholar] [CrossRef]
- Pooria, A.; Pourya, A.; Gheini, A. Postoperative complications associated with coronary artery bypass graft surgery and their therapeutic interventions. Future Cardiol. 2020, 16, 481–496. [Google Scholar] [CrossRef]
- Rizza, V.; Maranta, F.; Cianfanelli, L.; Cartella, I.; Alfieri, O.; Cianflone, D. Imaging of the diaphragm following cardiac surgery: Focus on ultrasonographic assessment. J. Ultrasound Med. 2023, 42, 2481–2490. [Google Scholar] [CrossRef]
- Petrache, I.-A.; Sharma, A.; Kumar, A.; Baderca, F.; Neagoe, O.C.; Tudorache, E.; Oancea, C.; Jifcu, M.E.; Ionica, M.; Kundnani, N.R.; et al. Nerve changes associated with post thoracotomy pain syndrome. Histol. Histopathol. 2022, 37, 999–1006. [Google Scholar] [CrossRef]
- Sayed, A.A.S.A.; Vijayakumar, L.S.; Chatterjee, A.; Thota, R.S. Incidence and risk factors of ipsilateral shoulder pain in patients after thoracic surgeries. Indian J. Anaesth. 2023, 67 (Suppl. S1), S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, A.; Iranami, H.; Fujii, K.; Yamazaki, A.; Doko, Y. Myofascial involvement of supra- and infraspinatus muscles contributes to ipsilateral shoulder pain after muscle-sparing thoracotomy and video-assisted thoracic surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Lindenmann, S.; Tsagkaris, C.; Farshad, M.; Widmer, J. Kinematics of the cervical spine under healthy and degenerative conditions: A systematic review. Ann. Biomed. Eng. 2022, 50, 1705–1733. [Google Scholar] [CrossRef]
- Bordoni, B.; Zanier, E. Skin, fascias, and scars: Symptoms and systemic connections. J. Multidiscip. Healthc. 2013, 7, 11–24. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.R.; Girgenti, G.T. Peritoneal adhesions in osteopathic medicine: Theory, part 1. Cureus 2023, 15, e42472. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, E.; Vieira, L.; Makita, D.K.; Rivas, L. Inhibitory tests as assessment tools for somatic dysfunctions: Mechanisms and practical applications. Cureus 2020, 12, e7700. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.R. Functional evaluation of the diaphragm with a noninvasive test. J. Osteopath. Med. 2021, 121, 835–842. [Google Scholar] [CrossRef]
- Racca, V.; Bordoni, B.; Castiglioni, P.; Modica, M.; Ferratini, M. Osteopathic manipulative treatment improves heart surgery outcomes: A randomized controlled trial. Ann. Thorac. Surg. 2017, 104, 145–152. [Google Scholar] [CrossRef]
- Rodriguez, E.R.; Tan, C.D. Structure and anatomy of the human pericardium. Prog. Cardiovasc. Dis. 2017, 59, 327–340. [Google Scholar] [CrossRef]
- Hanif, M.A.; Shrestha, B.; Hanif, H.; Shahzad, A.; Suwal, A.; Shah, S.; Oladiran, O.; Macciocca, M.; Donato, A.A. Constrictive pericarditis from an atraumatic hemopericardium after systemic thrombolysis for a massive pulmonary embolism. J. Community Hosp. Intern. Med. Perspect. 2023, 13, 46–48. [Google Scholar] [CrossRef]
- Muruganandan, S.; Mishra, E.; Singh, B. Breathlessness with pleural effusion: What do we know? Semin. Respir. Crit. Care Med. 2023, 44, 502–508. [Google Scholar] [CrossRef]
- Sutcliffe, P.; Lasrado, S. Anatomy, Head and Neck, Deep Cervical Neck Fascia; StatPearls Publishing: Treasure Island, FL, USA, 2023; Bookshelf ID: NBK541091. [Google Scholar]
- Adriaensen, D.; Brouns, I.; Timmermans, J.P. Sensory input to the central nervous system from the lungs and airways: A prominent role for purinergic signalling via P2X2/3 receptors. Auton. Neurosci. 2015, 191, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Han, M.; Zhu, J.X.; Sun, N.; Tang, J.; Huo, F.; Li, J.; Xu, F.; Du, J. Metabotropic glutamate subtype 7 and 8 receptors oppositely modulate cardiac nociception in the rat nucleus tractus solitarius. Neuroscience 2012, 220, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Bontinck, J.; Chys, M.; Coppieters, I.; Meeus, M.; Cagnie, B. Exploration of somatosensory function of patients with acute nonspecific neck pain, through quantitative sensory testing and self-reported symptoms. Clin. J. Pain. 2023, 39, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Zanier, E. Anatomic connections of the diaphragm: Influence of respiration on the body system. Multidiscip. Healthc. 2013, 6, 281–291. [Google Scholar] [CrossRef] [PubMed]
- de Zoete, R.M.; Armfield, N.R.; McAuley, J.H.; Chen, K.; Sterling, M. Comparative effectiveness of physical exercise interventions for chronic non-specific neck pain: A systematic review with network meta-analysis of 40 randomised controlled trials. Br. J. Sports Med. 2020, 55, 730–742. [Google Scholar] [CrossRef]
- Hidalgo, B.; Hall, T.; Bossert, J.; Dugeny, A.; Cagnie, B.; Pitance, L. The efficacy of manual therapy and exercise for treating non-specific neck pain: A systematic review. J. Back Musculoskelet. Rehabil. 2017, 30, 1149–1169. [Google Scholar] [CrossRef]
- Gevers-Montoro, C.; Provencher, B.; Descarreaux, M.; Ortega de Mues, A.; Piché, M. Neurophysiological mechanisms of chiropractic spinal manipulation for spine pain. Eur. J. Pain 2021, 25, 1429–1448. [Google Scholar] [CrossRef]
- Sbardella, S.; La Russa, C.; Bernetti, A.; Mangone, M.; Guarnera, A.; Pezzi, L.; Paoloni, M.; Agostini, F.; Santilli, V.; Saggini, R.; et al. Muscle Energy Technique in the Rehabilitative Treatment for Acute and Chronic Non-Specific Neck Pain: A Systematic Review. Healthcare 2021, 9, 746. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, B.; Escher, A. Pericardial Adhesion and Chronic Non-Specific Neck Pain following Thoracentesis: An Osteopathic Approach. Clin. Pract. 2023, 13, 1313-1318. https://doi.org/10.3390/clinpract13060117
Bordoni B, Escher A. Pericardial Adhesion and Chronic Non-Specific Neck Pain following Thoracentesis: An Osteopathic Approach. Clinics and Practice. 2023; 13(6):1313-1318. https://doi.org/10.3390/clinpract13060117
Chicago/Turabian StyleBordoni, Bruno, and Allan Escher. 2023. "Pericardial Adhesion and Chronic Non-Specific Neck Pain following Thoracentesis: An Osteopathic Approach" Clinics and Practice 13, no. 6: 1313-1318. https://doi.org/10.3390/clinpract13060117
APA StyleBordoni, B., & Escher, A. (2023). Pericardial Adhesion and Chronic Non-Specific Neck Pain following Thoracentesis: An Osteopathic Approach. Clinics and Practice, 13(6), 1313-1318. https://doi.org/10.3390/clinpract13060117






