Clinical and Pathological Features of Osteosarcomas of the Jaws: A Retrospective Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Participant Selection Criteria
2.3. Calculation of Participant Survival
2.4. Ethical Aspects
3. Results
3.1. Characteristics of the Patients
3.2. Pathological Characteristics of Tumor Lesions
3.3. Treatment Characteristics and Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vered, M.; Wright, J.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head. Neck Pathol. 2022, 16, 63–75. [Google Scholar] [CrossRef]
- Clark, J.L.; Unni, K.K.; Dahlin, D.C.; Devine, K.D. Osteosarcoma of the Jaw. Cancer 1983, 51, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Baumhoer, D. Bone-Related Lesions of the Jaws. Surg. Pathol. Clin. 2017, 10, 693–704. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Chakravarthi, P.S.; Kattimani, V.S.; Prasad, L.K.; Satish, P.R. Juxtacortical Osteosarcoma of the Mandible: Challenges in Diagnosis and Management. Natl. J. Maxillofac. Surg. 2015, 6, 127–131. [Google Scholar] [PubMed]
- Lee, R.J.; Arshi, A.; Schwartz, H.C.; Christensen, R.E. Characteristics and Prognostic Factors of Osteosarcoma of the Jaws: A Retrospective Cohort Study. JAMA Otolaryngol. Head. Neck Surg. 2015, 141, 470–477. [Google Scholar] [CrossRef]
- Malik, F.; Gleysteen, J.P.; Agarwal, S. Osteosarcoma of the Jaw: Report of 3 Cases (Including the Rare Epithelioid Variant) with Review of Literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, e71–e80. [Google Scholar] [CrossRef] [PubMed]
- Haefliger, S.; Harder, D.; Kovac, M.; Linkeschova, K.; Eufinger, H.; Baumhoer, D. Osteosarcoma of the Mandible in a Patient with Florid Cemento-Osseous Dysplasia and Li-Fraumeni Syndrome: A Rare Coincidence. Head Neck Pathol. 2021, 15, 704–708. [Google Scholar] [CrossRef]
- Bertin, H.; Gomez-Brouchet, A.; Rédini, F. Osteosarcoma of the Jaws: An Overview of the Pathophysiological Mechanisms. Crit. Rev. Oncol. Hematol. 2020, 156, 103126. [Google Scholar] [CrossRef]
- Nissanka, E.; Amaratunge, E.; Tilakaratne, W. Clinicopathological Analysis of Osteosarcoma of Jaw Bones. Oral Dis. 2007, 13, 82–87. [Google Scholar] [CrossRef]
- August, M.; Magennis, P.; Dewitt, D. Osteogenic Sarcoma of the Jaws: Factors Influencing Prognosis. Int. J. Oral Maxillofac. Surg. 1997, 26, 198. [Google Scholar] [CrossRef]
- Granowski-LeCornu, M.; Chuang, S.K.; Kaban, L.B.; August, M. Osteosarcoma of the Jaws: Factors Influencing Prognosis. J. Oral Maxillofac. Surg. 2011, 69, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Mandelker, D. Tumor Syndromes Predisposing to Osteosarcoma. Adv. Anat. Pathol. 2018, 25, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Mandal, S.; Mallya, V.; Khurana, N.; Meher, R.; Singh, K. Osteosarcoma of the Jaw: Primary Versus Secondary—A Report of Two Cases. J. Cancer Res. Ther. 2023, 19, 2086–2089. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Meyers, P.; Huvos, A.G.; Wolden, S.; Singh, B.; Shaha, A.R.; Boyle, J.O.; Pfister, D.; Shah, J.P.; Kraus, D.H. Improved Outcomes in Patients with Osteogenic Sarcoma of the Head and Neck. Cancer 2002, 95, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Guadagnolo, B.A.; Zagars, G.K.; Raymond, A.K.; Benjamin, R.S.; Sturgis, E.M. Osteosarcoma of the Jaw/Craniofacial Region. Cancer 2009, 115, 3262–3270. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.; Toner, M.; Pierse, D.; Stassen, L.F.A. Osteosarcoma (Osteogenic Sarcoma) of the Jaws Presenting in General Dental Practice—A Series of Four Cases. Br. Dent. J. 2021, 230, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Favia, G.F. Periosteal Osteosarcoma of the Jaws: Report of 2 Cases. J. Periodontol. 2000, 71, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Broders, A.C. The Grading of Carcinoma. Minn. Med. 1925, 8, 726–730. [Google Scholar]
- Gadwal, S.R.; Gannon, F.H.; Fanburg-Smith, J.C.; Becoskie, E.M.; Thompson, L.D. Primary Osteosarcoma of the Head and Neck in Pediatric Patients: A Clinicopathologic Study of 22 Cases with a Review of the Literature. Cancer 2001, 91, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Baumhoer, D.; Brunner, P.; Eppenberger-Castori, S.; Smida, J.; Nathrath, M.; Jundt, G. Osteosarcomas of the Jaws Differ from their Peripheral Counterparts and Require a Distinct Treatment Approach. Experiences from the DOESAK Registry. Oral Oncol. 2014, 50, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of theExtremities Or Trunk: An Analysis of 1702 Patients Treatedon Neoadjuvant Cooperative Osteosarcoma Study GroupProtocols. J. Clin. Oncol. 2023, 41, 4323–4337. [Google Scholar] [CrossRef] [PubMed]
- Doval, D.C.; Kumar, R.V.; Kannan, V.; Sabitha, K.S.; Misra, S.; Vijay Kumar, M.; Hegde, P.; Bapsy, P.P.; Mani, K.; Shenoy, A.M.; et al. Osteosarcoma of the Jaw Bones. Br. J. Oral Maxillofac. Surg. 1997, 35, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.; Dallera, P.; Bacchini, P.; Marchetti, C.; Campobassi, A. The Istituto Rizzoli-Beretta Experience with Osteosarcoma of the Jaw. Cancer 1991, 68, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Maafs, E.; Alfeiran, A.; Mohar, A.; Barrera, J.L.; Zinser, J.; Beltran, A. Osteosarcoma of the Jaw. Head Neck 1994, 16, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Nikitakis, N.G.; Pazoki, A.; Ord, R.A. Osteogenic Sarcoma of the Jaw: A 10-Year Experience. J. Oral Maxillofac. Surg. 2007, 65, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.K.; Eisele, D.W.; Frassica, F.J.; Zahurak, M.L.; McCarthy, E.F. Osteosarcoma of the Head and Neck: A Review of the Johns Hopkins Experience. Laryngoscope 1999, 109, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Mardinger, O.; Givol, N.; Talmi, Y.P.; Taicher, S. Osteosarcoma of the Jaw: The Chaim Sheba Medical Center Experience. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 445–451. [Google Scholar] [CrossRef]
- Ogunlewe, M.O.; Ajayi, O.F.; Adeyemo, W.L.; Ladeinde, A.L.; James, O. Osteogenic Sarcoma of the Jaw Bones: A Single Institution Experience Over a 21-Year Period. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 76–81. [Google Scholar] [CrossRef]
- Junior, A.T.; de Abreu Alves, F.; Pinto, C.A.L.; Carvalho, A.L.; Kowalski, L.P.; Lopes, M.A. Clinicopathological and Immunohistochemical Analysis of Twenty-Five Head and Neck Osteosarcomas. Oral. Oncol. 2003, 39, 521–530. [Google Scholar] [CrossRef]
- Paparella, M.L.; Olvi, L.G.; Brandizzi, D.; Keszler, A.; Santini-Araujo, E.; Cabrini, R.L. Osteosarcoma of the Jaw: An Analysis of a Series of 74 Cases. Histopathology 2013, 63, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Tanzawa, H.; Uchiyama, S.; Sato, K. Statistical Observation of Osteosarcoma of the Maxillofacial Region in Japan: Analysis of 114 Japanese Cases Reported between 1930 and 1989. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Steffensen, A.; Trump, B. Clinical Features and overall Survival of Osteosarcoma of the Mandible. Int. J. Oral Maxillofac. Surg. 2022, 52, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Garrington, G.E.; Scofield, H.H.; Cornyn, J.; Hooker, S.P. Osteosarcoma of the Jaws. Analysis of 56 Cases. Cancer 1967, 20, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Ajura, A.J.; Lau, S.H. A Retrospective Clinicopathological Study of 59 Osteogenic Sarcoma of Jaw Bone Archived in a Stomatology Unit. Malays. J. Pathol. 2010, 32, 27–34. [Google Scholar]
- Bennett, J.H.; Thomas, G.; Evans, A.W.; Speight, P.M. Osteosarcoma of the Jaws: A 30-Year Retrospective Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Charmelo-Silva, S.; Buchanan, A.; Kalathingal, S.; Abdelsayed, R. Osteosarcoma of the Jaws: Report of 3 Cases with Emphasis on the Early Clinical and Radiographic Signs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, e57–e62. [Google Scholar] [CrossRef] [PubMed]
- Givol, N.; Buchner, A.; Taicher, S.; Kaffe, I. Radiological Features of Osteogenic Sarcoma of the Jaws. A Comparative Study of Different Radiographic Modalities. Dentomaxillofac. Radiol. 1998, 27, 313–320. [Google Scholar] [CrossRef]
- Lindqvist, C.; Teppo, L.; Sane, J.; Holmström, T.; Wolf, J. Osteosarcoma of the Mandible: Analysis of Nine Cases. J. Oral Maxillofac. Surg. 1986, 44, 759–764. [Google Scholar] [CrossRef]
- Chaudhary, M.; Chaudhary, S.D. Osteosarcoma of Jaws. J. Oral Maxillofac. Pathol. 2012, 16, 233–238. [Google Scholar] [CrossRef] [PubMed]
- ALQahtani, D.; AlSheddi, M.; Al-Sadhan, R. Epithelioid Osteosarcoma of the Maxilla: A Case Report and Review of the Literature. Int. J. Surg. Pathol. 2015, 23, 495–499. [Google Scholar] [CrossRef]
- Okada, K.; Hasegawa, T.; Yokoyama, R. Rosette-Forming Epithelioid Osteosarcoma: A Histologic Subtype with Highly Aggressive Clinical Behavior. Hum. Pathol. 2001, 32, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Unni, K.K.; Dahlin, D.C. Osteosarcoma: Pathology and Classification. Semin. Roentgenol. 1989, 24, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F.J.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A. Germ Line p53 Mutations in a Familial Syndrome of Breast Cancer, Sarcomas, and Other Neoplasms. Science 1990, 250, 1233–1238. [Google Scholar] [CrossRef]
- Tilden, W.; Saifuddin, A. An Update on Imaging of Paget’s Sarcoma. Skeletal Radiol. 2021, 50, 1275–1290. [Google Scholar] [CrossRef]
- Scranton, P.E., Jr.; DeCicco, F.A.; Totten, R.S.; Yunis, E.J. Prognostic Factors in Osteosarcoma. A Review of 20 Year’s Experience at the University of Pittsburgh Health Center Hospitals. Cancer 1975, 36, 2179–2191. [Google Scholar] [CrossRef]
- Lucas, D.R.; Unni, K.K.; McLeod, R.A.; O’Connor, M.I.; Sim, F.H. Osteoblastoma: Clinicopathologic Study of 306 Cases. Hum. Pathol. 1994, 25, 117–134. [Google Scholar] [CrossRef]
- Kimura, Y.; Tomihara, K.; Tachinami, H.; Imaue, S.; Nakamori, K.; Fujiwara, K.; Suzuki, K.; Yasuda, T.; Miwa, S.; Nakayama, E.; et al. Conventional Osteosarcoma of the Mandible Successfully Treated with Radical Surgery and Adjuvant Chemotherapy After Responding Poorly to Neoadjuvant Chemotherapy: A Case Report. J. Med. Case Rep. 2017, 11, 210. [Google Scholar] [CrossRef]
- Ferri, A.; Bianchi, B.; Ferrari, S. Mandibular Osteosarcoma: Diagnosis and Treatment. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 154–160. [Google Scholar] [CrossRef]
- Canadian Society of Otolaryngology-Head and Neck Surgery Oncology Study Group. Osteogenic Sarcoma of the Mandible and Maxilla: A Canadian Review (1980–2000). J. Otolaryngol. 2004, 33, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Thiele, O.C.; Freier, K.; Bacon, C.; Egerer, G.; Hofele, C.M. Interdisciplinary Combined Treatment of Craniofacial Osteosarcoma with Neoadjuvant and Adjuvant Chemotherapy and Excision of the Tumour: A Retrospective Study. Br. J. Oral Maxillofac. Surg. 2008, 46, 533–536. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®). Bone Cancer 2024. Available online: https://www.nccn.org/guidelines (accessed on 1 January 2024).
- Sharin, F.; Pai, A.; Mair, M. Management of Osteosarcoma of the Head and Neck. Curr. Opin. Otolaryngol. Head Neck Surg. 2023, 31, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Monden, N.; Chikuie, N.; Taruya, T.; Hamamoto, T.; Ishino, T.; Ueda, T.; Takeno, S. A Case of Primary Osteosarcoma of the Mandible that Responded to Preoperative Chemotherapy: p16 as a Potential Prognostic Factor. Acta Med. Okayama 2022, 76, 229–233. [Google Scholar]
- Woll, P.J.; Reichardt, P.; Le Cesne, A.; Bonvalot, S.; Azzarelli, A.; Hoekstra, H.J.; Leahy, M.; Van Coevorden, F.; Verweij, J.; Hogendoorn, P.C.W.; et al. Adjuvant Chemotherapy with Doxorubicin, Ifosfamide, and Lenograstim for Resected Soft-Tissue Sarcoma (EORTC 62931): A Multicentre Randomised Controlled Trial. Lancet Oncol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Julieron, M.; Brouchet, A.; Italiano, A.; Schouman, T.; Marcy, P.Y.; Odin, G.; Lacout, A.; Dassonville, O.; Peyrottes-Birstwisles, I.; et al. Osteosarcomas of the Mandible: Are they Different from Other Tumor Sites? Crit. Rev. Oncol. Hematol. 2012, 82, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Friebele, J.C.; Peck, J.; Pan, X.; Abdel-Rasoul, M.; Mayerson, J.L. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am. J. Orthop. 2015, 44, 547–553. [Google Scholar] [PubMed]
- Boussouni, S.; Touré, G. Chondroblastic Osteosarcoma of the Mandible in a Patient on Risedronate: A Rare Case of Neoadjuvant Chemotherapy Failure. Cureus 2021, 13, e19929. [Google Scholar] [CrossRef] [PubMed]
- Smeele, L.E.; Kostense, P.J.; van der Waal, I.; Snow, G.B. Effect of Chemotherapy on Survival of Craniofacial Osteosarcoma: A Systematic Review of 201 Patients. J. Clin. Oncol. 1997, 15, 363–367. [Google Scholar] [CrossRef]
- Khadembaschi, D.; Jafri, M.; Praveen, P.; Parmar, S.; Breik, O. Does Neoadjuvant Chemotherapy Provide a Survival Benefit in Maxillofacial Osteosarcoma: A Systematic Review and Pooled Analysis. Oral Oncol. 2022, 135, 106133. [Google Scholar] [CrossRef]
- Bouaoud, J.; Beinse, G.; Epaillard, N.; Amor-Sehlil, M.; Bidault, F.; Brocheriou, I.; Hervé, G.; Spano, J.; Janot, F.; Boudou-Rouquette, P.; et al. Lack of Efficacy of Neoadjuvant Chemotherapy in Adult Patients with Maxillo-Facial High-Grade Osteosarcomas: A French Experience in Two Reference Centers. Oral Oncol. 2019, 95, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, K.S.; Rathod, P.; Dalvi, R.; Pawar, A.; Thottiyen, S.; Pandya, S.; Sharma, M.; Patel, S.; Warikoo, V.; Pandya, S. Osteosarcoma of Head and Neck Region: Tertiary Cancer Care Center Experience. Indian J. Otolaryngol. Head Neck. Surg. 2024, 76, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, M.; Kelley, M.R. Human Apurinic Endonuclease 1 (APE1) Expression and Prognostic Significance in Osteosarcoma: Enhanced Sensitivity of Osteosarcoma to DNA Damaging Agents using Silencing RNA APE1 Expression Inhibition. Mol. Cancer Ther. 2004, 3, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Uozaki, H.; Horiuchi, H.; Ishida, T.; Iijima, T.; Imamura, T.; Machinami, R. Overexpression of Resistance-Related Proteins (Metallothioneins, Glutathione-S-Transferase Pi, Heat Shock Protein 27, and Lung Resistance-Related Protein) in Osteosarcoma. Relationship with Poor Prognosis. Cancer 1997, 79, 2336–2344. [Google Scholar] [CrossRef]
- He, H.; Ni, J.; Huang, J. Molecular Mechanisms of Chemoresistance in Osteosarcoma (Review). Oncol. Lett. 2014, 7, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Wopat, H.; Harrod, T.; Brem, R.F.; Kaltman, R.; Anderson, K.; Robien, K. Body Composition and Chemotherapy Toxicity among Women Treated for Breast Cancer: A Systematic Review. J. Cancer Surviv. 2023. [Google Scholar] [CrossRef] [PubMed]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin Alleviates Acute Doxorubicin Cardiotoxicity in Rats Via Suppression of Oxidative Stress, Inflammation and Apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Corbeau, A.; Heemsbergen, W.D.; Kuipers, S.C.; Godart, J.; Creutzberg, C.L.; Nout, R.A.; de Boer, S.M. Predictive Factors for Toxicity After Primary Chemoradiation for Locally Advanced Cervical Cancer: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.; Athanasou, N.; Gerrand, C.; Judson, I.; Lewis, I.; Morland, B.; Peake, D.; Seddon, B.; Whelan, J. UK Guidelines for the Management of Bone Sarcomas. Sarcoma 2010, 2010, 317462. [Google Scholar] [CrossRef]
- Jasnau, S.; Meyer, U.; Potratz, J.; Jundt, G.; Kevric, M.; Joos, U.K.; Jürgens, H.; Bielack, S.S.; Cooperative Osteosarcoma Study Group, C. Craniofacial Osteosarcoma Experience of the Cooperative German-Austrian-Swiss Osteosarcoma Study Group. Oral Oncol. 2008, 44, 286–294. [Google Scholar] [CrossRef]
- Yamaguchi, D.T. “Ins” and “Outs” of Mesenchymal Stem Cell Osteogenesis in Regenerative Medicine. World J. Stem Cells 2014, 6, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Söder, S.; Sander, J.; Ries, J.; Geppert, C.; Kesting, M.; Wehrhan, F. Craniofacial Osteosarcoma-Pilot Study on the Expression of Osteobiologic Characteristics and Hypothesis on Metastasis. Front. Oncol. 2020, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Unni, K.K.; Wollan, P.C.; Lund, B.A. Chondrosarcoma of the Jaw and Facial Bones. Cancer 1995, 76, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Bertin, H.; Peries, S.; Amiaud, J.; Van Acker, N.; Perrot, B.; Bouvier, C.; Aubert, S.; Marie, B.; Larousserie, F.; De Pinieux, G.; et al. Characterization of the Tumor Microenvironment in Jaw Osteosarcomas, Towards Prognostic Markers and New Therapeutic Targets. Cancers 2023, 15, 1004. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, H.; Schreuder, W.H.; de Lange, J. Osteosarcoma: A Comparison of Jaw Versus Nonjaw Localizations and Review of the Literature. Sarcoma 2013, 2013, 316123. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, A.S.; Narang, R.S.; Mahajan, S. Osteosarcoma: A Case Report and Evaluation. J. Oral Maxillofac. Pathol. 2021, 25, 374–375. [Google Scholar] [PubMed]
- van Es, R.J.; Keus, R.B.; van der Waal, I.; Koole, R.; Vermey, A. Osteosarcoma of the Jaw Bones. Long-Term Follow Up of 48 Cases. Int. J. Oral Maxillofac. Surg. 1997, 26, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.J.; Sercarz, J.A.; Tran, L.; Dodd, L.G.; Selch, M.; Calcaterra, T.C. Osteogenic Sarcoma of the Head and Neck. the UCLA Experience. Arch. Otolaryngol. Head Neck. Surg. 1991, 117, 761–766. [Google Scholar] [CrossRef]
- Rodrigues, I.; Botelho, J.; Machado, V.; Proença, L.; Mendes, J.J.; Zagalo, C. Profiling Oral Health Status, Values, and Related Quality of Life in Patients with Oral Cancer: A Pilot Study. Front. Oral Health. 2023, 4, 1268657. [Google Scholar] [CrossRef]
- Vermaire, J.A.; Partoredjo, A.S.K.; de Groot, R.J.; Brand, H.S.; Speksnijder, C.M. Mastication in Health-Related Quality of Life in Patients Treated for Oral Cancer: A Systematic Review. Eur. J. Cancer Care. 2022, 31, e13744. [Google Scholar] [CrossRef]
- Rana, M.; Kanatas, A.; Herzberg, P.Y.; Khoschdell, M.; Kokemueller, H.; Gellrich, N.; Rana, M. Prospective Study of the Influence of Psychological and Medical Factors on Quality of Life and Severity of Symptoms among Patients with Oral Squamous Cell Carcinoma. Br. J. Oral Maxillofac. Surg. 2015, 53, 364–370. [Google Scholar] [CrossRef] [PubMed]
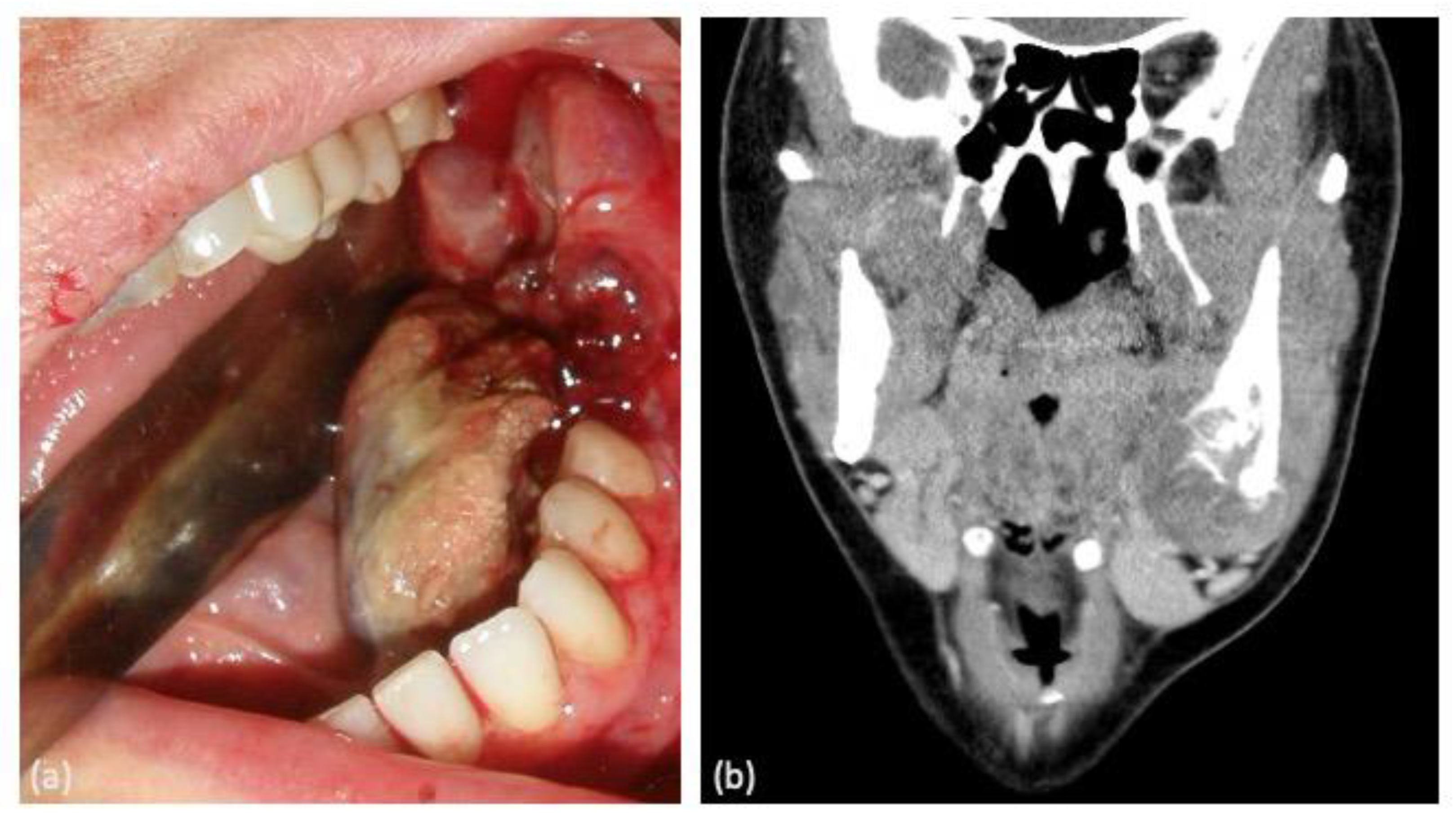

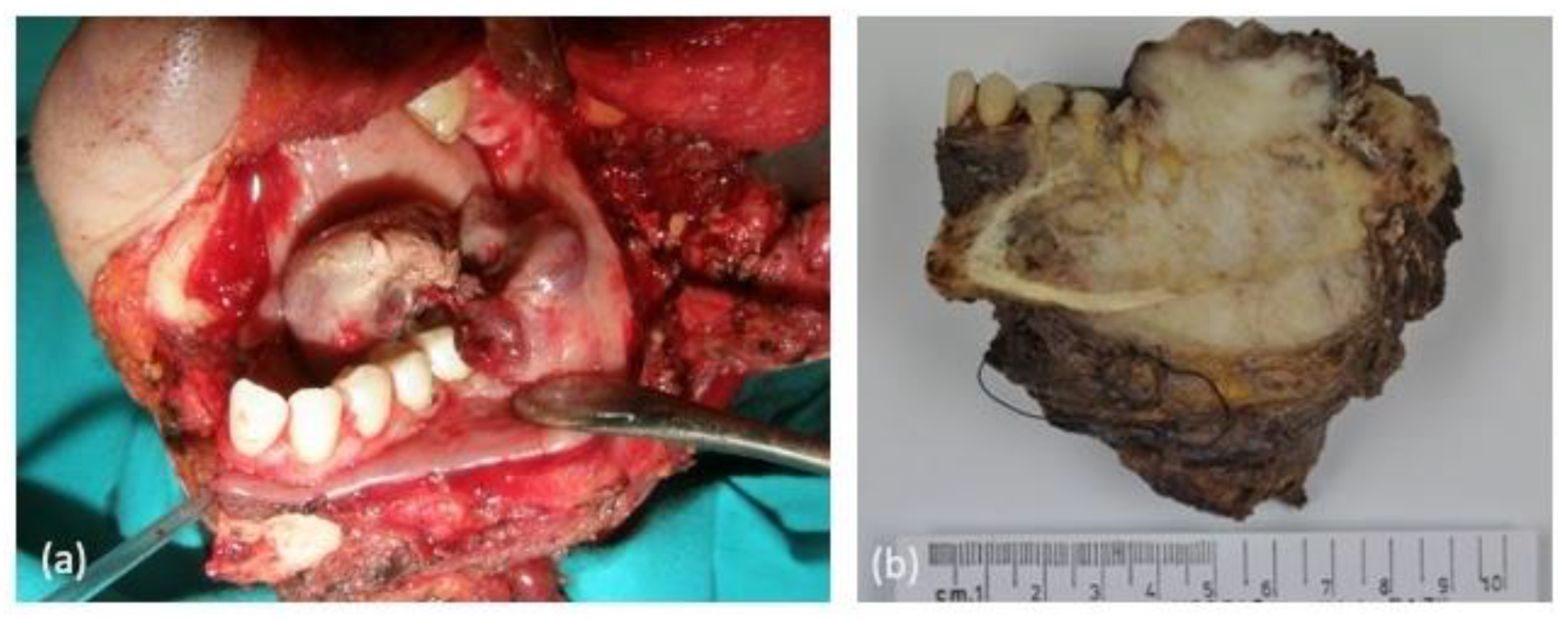

| Case | Age | Sex | Medical History | Signs and Symptoms | Bone | Side |
|---|---|---|---|---|---|---|
| 1 | 51 | M | Pneumothorax at 26 years | -Sensation of nasal obstruction -Progressive proptosis -Diplopia | Maxilla | Left |
| 2 | 35 | F | None | Painful swelling | Maxilla | Left |
| 3 | 56 | F | Cavum cancer (T2N2M0) at 45 years. Treated with CTX and RTX | Painful swelling | Mandible (angle) | Right |
| 4 | 56 | F | None | Asymptomatic mass | Mandible (body and angle) | Right |
| 5 | 49 | F | Uterine myoma. Breast fibroadenoma | -Inferior dental nerve hypoesthesia -Painful swelling -Dental mobility of involved teeth | Mandible (body and angle) | Left |
| 6 | 47 | F | Maxillar cemento-ossifying fibroma at 42 years | Asymptomatic mass | Maxilla | Right |
| 7 | 10 | F | Retinoblastoma at 3 years. Treated with CTX and RTX. Right eye enucleation | Painful swelling | Maxilla | Right |
| 8 | 31 | F | Appendectomy at 21 years | -Painful swelling -Oral cavity bleeding | Mandible (body and angle) | Left |
| Case | Histological Morphology | Grade | Broder’s Classification | Soft-Tissue Involvement | Lymphovascular Invasion |
|---|---|---|---|---|---|
| 1 | Fibroblastic | High | Grade 3–4 | No | No |
| 2 | Chondroblastic | High | Grade 3 | No Yes (after recurrence) | No Yes (after 3rd recurrence) |
| 3 | Epithelioid | High | Grade 3 | Yes | No |
| 4 | Osteoblastic | High | Grade 4 | No | No |
| 5 | Osteoblastic | High | Grade 3 | Yes | No |
| 6 | Chondroblastic | High | Grade 2–3 | Yes | No |
| 7 | Osteoblastic | High | Grade 4 | No | No |
| 8 | Epithelioid | High | Grade 4 | No | Yes |
| Case | Surgical Treatment | Final Surgical Margin | Resection | Neoadjuvant Therapy | Necrosis % Post Neoadjuvant Therapy | Adjuvant Therapy | Recurrences | Metastasis | Survival and Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Partial maxillectomy and eye enucleation | + | R1 | No | N/A | CTX: ifosfamide-cisplatin + RT: tomotherapy 6MV (5 cycles) | No | No | FOD/15 years |
| Epirubicin (3 cycles) | |||||||||
| 2 | Partial maxillectomy | 1st surgery: − 2nd surgery: − 3rd surgery: n/c 4th surgery: + 5th surgery: n/c | 1st Surgery: R0 2nd Surgery: R0 3rd Surgery: n/c 4th Surgery: R1 5th Surgery: n/c | CTX: cisplatin-adriamycin (1 cycle) | N/D | After 3rd surgery (pregnant in 2nd recurrence): CTX: cisplatin-adriamycin/ Methotrexate (5 cycles) | 3 local recurrences | N/D | N/D |
| 3 | Hemimandibulectomy | − | R0 | No | N/A | In recurrence (no surgery decided) CTX: ifosfamide | 1 local recurrence | No | Death/7 months |
| 4 | Hemimandibulectomy | − | R0 | No | N/A | No | No | No | FOD/15 years |
| 5 | Hemimandibulectomy | − (<1 mm) | R0 | No | N/A | CTX: cisplatin-adriamycin (6 cycles) | No | No | FOD/9 years |
| 6 | Partial maxillectomy | − | R0 | No | N/A | CTX: cisplatin-adriamycin (5 cycles) | No | No | FOD/7 years |
| 7 | Partial maxillectomy | − | R0 | CTX: methotrexate-adriamycin-cisplatin | 95% | CTX: adriamycin (1 cycle) | No | No | FOD/4 years |
| 8 | Hemimandibulectomy | + | R1 | CTX: cisplatin-adriamycin (1 cycle) | 15% | CTX: ifosfamide-etoposide (1 cycle) | 1 local recurrence | Yes | Death/6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Molinero, J.; Pozo-Kreilinger, J.J.; Ruiz-Roca, J.A.; Lopez-Sanchez, A.F.; Cebrian-Carretero, J.L. Clinical and Pathological Features of Osteosarcomas of the Jaws: A Retrospective Study. Clin. Pract. 2024, 14, 965-979. https://doi.org/10.3390/clinpract14030077
Rodriguez-Molinero J, Pozo-Kreilinger JJ, Ruiz-Roca JA, Lopez-Sanchez AF, Cebrian-Carretero JL. Clinical and Pathological Features of Osteosarcomas of the Jaws: A Retrospective Study. Clinics and Practice. 2024; 14(3):965-979. https://doi.org/10.3390/clinpract14030077
Chicago/Turabian StyleRodriguez-Molinero, Jesus, Jose Juan Pozo-Kreilinger, Juan Antonio Ruiz-Roca, Antonio Francisco Lopez-Sanchez, and Jose Luis Cebrian-Carretero. 2024. "Clinical and Pathological Features of Osteosarcomas of the Jaws: A Retrospective Study" Clinics and Practice 14, no. 3: 965-979. https://doi.org/10.3390/clinpract14030077
APA StyleRodriguez-Molinero, J., Pozo-Kreilinger, J. J., Ruiz-Roca, J. A., Lopez-Sanchez, A. F., & Cebrian-Carretero, J. L. (2024). Clinical and Pathological Features of Osteosarcomas of the Jaws: A Retrospective Study. Clinics and Practice, 14(3), 965-979. https://doi.org/10.3390/clinpract14030077






