Time-to-Treatment Delays and Their Prognostic Implications in Pharyngeal Cancer—An Exploratory Analysis in Western Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
Definitions
- Diagnosis to treatment interval (DTI): time from histopathological diagnosis to the start of treatment.
- Treatment package time (TPT): time from the first day with the specialist to the end of curative treatment.
2.2. Diagnosis Process
2.3. Data Collection
- Initial diagnostic of tumor pathology.
- Age at diagnosis.
- Gender.
- Residency environment (rural or urban).
- Tumor localization (nasopharynx, oropharynx, hypopharynx).
- Type of hospitalization (emergency or routine).
- Surgical intervention details.
- Biopsy type and histological diagnosis.
- Details of the patient’s first admission to the ENT ward and oncological treatment.
- Details on adjuvant treatments and anti-neoplastic therapies, along with the prognosis considering the timing of initiation for chemotherapy/radiotherapy and the status of patients (alive or deceased).
2.4. Time Intervals Defined
- First presentation to diagnosis interval: time from the first consultation within the Timisoara Municipal Emergency Clinical Hospital to the final histopathological diagnosis.
- Diagnosis to treatment interval (DTI): time from the final diagnosis to the start of treatment (either the surgery date or the first fraction of chemoradiotherapy).
2.5. Outcome Measurements
2.6. Ethical Statement
2.7. Statistical Analysis
3. Results
3.1. Patient Retrospective Observational Study
3.2. Treatment Intervals
3.3. Oncological Treatment
- (a)
- ChT (chemotherapeutic treatment): This group received combined therapies which included:
- ○
- Single-agent regimens: carboplatin (40 mg/m2 IV weekly for 6–7 wks), docetaxel (75 mg/m2 IV every 3wk), and methotrexate (40 mg/m2 IV weekly (3 wks equals one cycle).
- ○
- Dual-agent combinations: paclitaxel (200 mg/m2 IV every 3 wks) with carboplatin (40 mg/m2 IV weekly for 6–7 wks); cetuximab (400 mg/m2 IV loading dose 1 wk before the start of radiation therapy, then 250 mg/m2 weekly) with carboplatin (40 mg/m2 IV weekly for 6–7 wks); docetaxel (75 mg/m2 IV every 3 wks) with either cisplatin (100 mg/m2 IV on days 1, 22, and 43 or 40 mg/m2 IV weekly for 6–7 wks) or carboplatin (40 mg/m2 IV weekly for 6–7 wks); as well as 5-Fluorouracil (5-FU 800 mg/m2 by continuous IV infusion on days 1–5 given on the days of radiation) with cisplatin (100 mg/m2 IV on days 1, 22, and 43 or 40 mg/m2 IV weekly for 6–7 wks).
- ○
- Triple-agent combinations: paclitaxel (200 mg/m2 IV every 3 wks) combined with carboplatin (40 mg/m2 IV weekly for 6–7 wks) and cetuximab (400 mg/m2 IV loading dose 1 wk before the start of radiation therapy, then 250 mg/m2 weekly) or epirubicin (100 mg/m² IV); and docetaxel (75 mg/m2 IV every 3 wks) combined with carboplatin (40 mg/m2 IV weekly for 6–7 wks) or cisplatin (100 mg/m2 IV on days 1, 22, and 43 or 40 mg/m2 IV weekly for 6–7 wks) and cetuximab (400 mg/m2 IV loading dose 1 wk before the start of radiation therapy, then 250 mg/m2 weekly).
- (b)
- RxT (radiotherapy): Patients were treated with cobalt therapy, often in conjunction with cisplatin, intensity-modulated radiation therapy (IMRT), and three-dimensional conformal radiation therapy (3D CRT). In high-risk patients, it was used: 60–66 Gy (2.0 Gy/fraction), daily Monday–Friday for 6–6.5 weeks; and for those at intermediate risk: 44–50 Gy (2.0 Gy/fraction) to 54–63 Gy (1.6–1.8 Gy/fraction).
- (c)
- CRT (chemoradiotherapy): this group received a combined treatment based on chemotherapy and radiotherapy.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guha, N.; Boffetta, P.; Wünsch Filho, V.; Eluf Neto, J.; Shangina, O.; Zaridze, D.; Curado, M.P.; Koifman, S.; Matos, E.; Menezes, A.; et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: Results of two multicentric case-control studies. Am. J. Epidemiol. 2007, 166, 1159–1173. [Google Scholar] [CrossRef]
- Tsang, C.M.; Lui, V.W.Y.; Bruce, J.P.; Pugh, T.J.; Lo, K.W. Translational genomics of nasopharyngeal cancer. Semin. Cancer Biol. 2020, 61, 84–100. [Google Scholar] [CrossRef]
- Murphy, C.T.; Galloway, T.J.; Handorf, E.A.; Egleston, B.L.; Wang, L.S.; Mehra, R.; Flieder, D.B.; Ridge, J.A. Survival Impact of Increasing Time to Treatment Initiation for Patients With Head and Neck Cancer in the United States. J. Clin. Oncol. 2016, 34, 169–178. [Google Scholar] [CrossRef]
- Albahout, K.S.; Lopez, R.A. Anatomy, Head and Neck, Pharynx. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Jafari, A.; Najafi, S.H.; Moradi, F.; Kharazifard, M.; Khami, M. Delay in the diagnosis and treatment of oral cancer. J. Dent. 2013, 14, 146–150. [Google Scholar]
- Kis, A.M.; Watz, C.G.; Motofelea, A.C.; Chiriac, S.; Poenaru, M.; Dehelean, C.A.; Borza, C.; Ionita, I. Challenges of Pharyngeal Cancer Screening in Lower-Income Countries during Economic and Social Transitions: A Population-Based Analysis. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 2226–2237. [Google Scholar] [CrossRef]
- Chen, R.C.; Khorsandi, A.S.; Shatzkes, D.R.; Holliday, R.A. The radiology of referred otalgia. AJNR Am. J. Neuroradiol. 2009, 30, 1817–1823. [Google Scholar] [CrossRef]
- Virk, J.S.; Ingle, M.; Podesta, C.M.; Gujral, D.M.; Awad, Z. Survival outcomes for head and neck cancer patients with N3 cervical nodal metastases. Clin. Otolaryngol. 2020, 45, 342–349. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Center Network (Ed.) NCCN Guidelines for Head and Neck Cancers; National Comprehensive Cancer Center Network: Madrid, Spain, 2018. [Google Scholar]
- Board of the Faculty of Clinical Oncology; The Royal College of Radiologists; The Society and The College of Radiographers; Institute of Physics and Engineering in Medicine. Development and Implementation of Conformal Radiotherapy in the United Kingdom; Royal College of Radiologists: London, UK, 2002. [Google Scholar]
- Withers, H.R.; Taylor, J.M.; Maciejewski, B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988, 27, 131–146. [Google Scholar] [CrossRef]
- Evers, C.; Ostheimer, C.; Sieker, F.; Vordermark, D.; Medenwald, D. Benefit from surgery with additional radiotherapy in N1 head and neck cancer at the time of IMRT: A population-based study on recent developments. PLoS ONE 2020, 15, e0229266. [Google Scholar] [CrossRef]
- Carroll, W.R.; Kohler, C.L.; Carter, V.L.; Hannon, L.; Skipper, J.B.; Rosenthal, E.L. Barriers to early detection and treatment of head and neck squamous cell carcinoma in African American men. Head Neck 2009, 31, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Goy, J.; Hall, S.F.; Feldman-Stewart, D.; Groome, P.A. Diagnostic delay and disease stage in head and neck cancer: A systematic review. Laryngoscope 2009, 119, 889–898. [Google Scholar] [CrossRef]
- Graboyes, E.M.; Gross, J.; Kallogjeri, D.; Piccirillo, J.F.; Al-Gilani, M.; Stadler, M.E.; Nussenbaum, B. Association of compliance with process-related quality metrics and improved survival in oral cavity squamous cell carcinoma. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 430–437. [Google Scholar] [CrossRef]
- Schoonbeek, R.C.; de Vries, J.; Bras, L.; Sidorenkov, G.; Plaat, B.E.C.; Witjes, M.J.H.; van der Laan, B.F.A.M.; van den Hoek, J.G.M.; van Dijk, B.A.C.; Langendijk, J.A.; et al. The effect of treatment delay on quality of life and overall survival in head and neck cancer patients. Eur. J. Cancer Care 2022, 31, e13589. [Google Scholar] [CrossRef]
- Schutte, H.W.; van den Broek, G.B.; Steens, S.C.A.; Hermens, R.P.M.G.; Honings, J.; Marres, H.A.M.; Merkx, M.A.W.; Weijs, W.L.J.; Arens, A.I.J.; van Engen-van Grunsven, A.C.H.; et al. Impact of optimizing diagnostic workup and reducing the time to treatment in head and neck cancer. Cancer 2020, 126, 3982–3990. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bekelman, J.; Lin, A.; Lukens, J.N.; Roman, B.R.; Mitra, N.; Swisher-McClure, S. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with oropharyngeal squamous cell carcinoma. Oral. Oncol. 2016, 56, 17–24. [Google Scholar] [CrossRef]
- Tsai, W.C.; Kung, P.T.; Wang, Y.H.; Huang, K.H.; Liu, S.A. Influence of time interval from diagnosis to treatment on survival for oral cavity cancer: A nationwide cohort study. PLoS ONE 2017, 12, e0175148. [Google Scholar] [CrossRef]
- Overgaard, J.; Hansen, H.S.; Specht, L.; Overgaard, M.; Grau, C.; Andersen, E.; Bentzen, J.; Bastholt, L.; Hansen, O.; Johansen, J.; et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomized controlled trial. Lancet 2003, 362, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Kannan, S.; Ghosh-Laskar, S.; Agarwal, J.P. Systematic review and meta-analyses of intensity-modulated radiation therapy versus conventional two-dimensional and/or three-dimensional radiotherapy in curative-intent management of head and neck squamous cell carcinoma. PLoS ONE 2018, 13, e0200137. [Google Scholar] [CrossRef]
- Van Harten, M.C.; Hoebers, F.J.; Kross, K.W.; Van Werkhoven, E.D.; Van Den Brekel, M.W.; Van Dijk, B.A. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015, 51, 272–278. [Google Scholar] [CrossRef]
- Fu, K.K.; Pajak, T.F.; Trotti, A.; Jones, C.U.; Spencer, S.A.; Phillips, T.L.; Garden, A.S.; Ridge, J.A.; Cooper, J.S.; Ang, K.K. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Horiot, J.C.; Bontemps, P.; van den Bogaert, W.; Le Fur, R.; van den Weijngaert, D.; Bolla, M.; Bernier, J.; Lusinchi, A.; Stuschke, M.; Lopez-Torrecilla, J.; et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: Results of the EORTC 22851 randomized trial. Radiother. Oncol. 1997, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Das, S.M.; Roy, N.; Singh, D.; Sardar, P.K.; Das, S. A Comparative Prospective Study Between Conventional Chemo-Radiotherapy and Pure Accelerated Radiotherapy with Concurrent Chemotherapy for the Treatment of Locally Advanced Head and Neck Cancer. Cureus 2023, 15, e42206. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Samlowski, W.E.; Moon, J.; Kuebler, J.P.; Nichols, C.R.; Gandara, D.R.; Ozer, H.; Williamson, S.K.; Atkins, J.N.; Schuller, D.E.; Ensley, J.F. Evaluation of the combination of docetaxel/carboplatin in patients with metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN): A Southwest Oncology Group Phase II study. Cancer Investig. 2007, 25, 182–188. [Google Scholar] [CrossRef]
- Gibson, M.K.; Li, Y.; Murphy, B.; Hussain, M.H.; DeConti, R.C.; Ensley, J.; Forastiere, A.A. Eastern Cooperative Oncology Group. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): An intergroup trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2005, 23, 3562–3567. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, C.D.; Haigentz, M., Jr.; Ow, T.J. Education Committee of the American Head and Neck Society (AHNS). AHNS Series: Do you know your guidelines? Principles of treatment for locally advanced or unresectable head and neck squamous cell carcinoma. Head Neck 2018, 40, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.S.; Harrison, L.B.; Culliney, B.; Sessions, R.B.; Choi, W.H. Cancer of the oropharynx. In Textbook of Radiation Oncology; Leibel, S.A., Phillips, T.L., Eds.; Saunders: Philadelphia, PA, USA, 2004; pp. 601–630. [Google Scholar]
- Dános, K.; Horváth, A.; Halász, J.; Tamás, L.; Polony, G. Patient delay and its clinical significance among head and neck cancer patients in Hungary. Pathol. Oncol. Res. 2023, 29, 1611206. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ellis, M.; Garas, G.; Hardman, J.; Khan, M.; Mehanna, H.; Smith, M.E.; Tikka, T.; Ubayasiri, K.; Williams, R.; Swords, C. Post-treatment head and neck cancer care: National audit and analysis of current practice in the United Kingdom. Clin. Otolaryngol. 2021, 46, 284–294. [Google Scholar]
- Deng, Z.; Hasegawa, M.; Matayoshi, S.; Kiyuna, A.; Yamashita, Y.; Maeda, H.; Suzuki, M. Prevalence and clinical features of human papillomavirus in head and neck squamous cell carcinoma in Okinawa, southern Japan. Eur. Arch. Otorhinolaryngol. 2011, 268, 1625–1631. [Google Scholar] [CrossRef]
- Abdelhakam, D.A.; Huenerberg, K.A.; Nassar, A. Utility of p16 and HPV testing in oropharyngeal squamous cell carcinoma: An institutional review. Diagn. Cytopathol. 2021, 49, 54–59. [Google Scholar] [CrossRef]
| ChT N = 23 | RxT N = 121 | CRT N = 36 | Total N = 180 | p-Value | |
|---|---|---|---|---|---|
| Age | 0.204 | ||||
| Mean (SD) | 57.6 (9.5) | 59.9 (9.2) | 57.1 (8.6) | 59.0 (9.2) | |
| Range | 42.0–78.0 | 31.0–76.0 | 40.0–81.0 | 31.0–81.0 | |
| Gender | 0.615 | ||||
| M | 21.0 (91.3%) | 114.0 (94.2%) | 35.0 (97.2%) | 170.0 (94.4%) | |
| F | 2.0 (8.7%) | 7.0 (5.8%) | 1.0 (2.8%) | 10.0 (5.6%) | |
| Histopathologic Diagnosis | 0.848 | ||||
| SCC | 15.0 (65.2%) | 82.0 (67.8%) | 24.0 (66.7%) | 121.0 (67.2%) | |
| KSCC | 5.0 (21.7%) | 17.0 (14.0%) | 7.0 (19.4%) | 29.0 (16.1%) | |
| SCC IN SITU | 1.0 (4.3%) | 4.0 (3.3%) | 2.0 (5.6%) | 7.0 (4.1%) | |
| UCNT | 2.0 (8.7%) | 12.0 (9.9%) | 3.0 (8.4%) | 17.0 (8.4%) | |
| OTHER CANCERS | 0.0 (0.0%) | 6.0 (5.2%) | 0.0 (0.0%) | 7.0 (4.1%) | |
| Patient delay | 0.375 | ||||
| Unspecified | 15.0 (65.2%) | 56.0 (46.3%) | 14.0 (38.9%) | 85.0 (47.2%) | |
| ˂3 months | 4.0 (17.4%) | 39.0 (32.2%) | 13.0 (36.1%) | 56.0 (31.1%) | |
| ˃3 months | 4.0 (17.4%) | 26.0 (21.5%) | 9.0 (25.0%) | 39.0 (21.7%) | |
| N category | 0.329 | ||||
| N0 | 12.0 (52.2%) | 70.0 (57.9%) | 15.0 (41.7%) | 97.0 (53.9%) | |
| N1 | 1.0 (4.3%) | 19.0 (15.7%) | 7.0 (19.4%) | 27.0 (15.0%) | |
| N2 | 3.0 (13.0%) | 8.0 (6.6%) | 5.0 (13.9%) | 16.0 (8.9%) | |
| N3 | 7.0 (30.4%) | 24.0 (19.8%) | 9.0 (25.0%) | 40.0 (22.2%) | |
| T Stage | 0.071 | ||||
| T1 | 8.0 (34.7%) | 39.0 (32.2%) | 13.0 (36.1%) | 60.0 (33.4%) | |
| T2 | 9.0 (39.1%) | 54.0 (44.6%) | 10.0 (27.8%) | 73.0 (40.6%) | |
| T3 | 5.0 (21.7%) | 25.0 (20.7%) | 9.0 (25.0%) | 39.0 (21.7%) | |
| T4 | 0.0 (0.0%) | 3.0 (2.5%) | 2.0 (5.6%) | 5.0 (2.8%) | |
| This | 1.0 (4.3%) | 0.0 (0.0%) | 2.0 (5.6%) | 3.0 (1.7%) | |
| Surgery | 0.137 | ||||
| Minor surgery (biopsy) | 20.0 (80.9%) | 108.0 (89.3%) | 32.0 (88.9%) | 160.0 (89.0%) | |
| Major surgery (tracheotomy, cervicotomy, laryngectomy) | 5.0 (21.7%) | 11.0 (9.1%) | 4.0 (11.2%) | 15.0 (11.1%) |
| Mean (SD) | Median | 95% CI for Median | ||
|---|---|---|---|---|
| The first presentation to diagnosis | 15.47 (40.08) | 9 | 8–9 | 180 patients |
| Diagnosis to treatment (DTI) | 68.21 (134.35) | 33 | 29.05–37 | 175 patients |
| Radiation interval | 112.78 (184.06) | 50 | 48–51.6 | 180 patients |
| Treatment package time (TPT) | 114.06 (186) | 50 | 48–52 | 176 patients |
| The first presentation to diagnosis | 15.47 (40.08) | 9 | 8–9 | 180 patients |
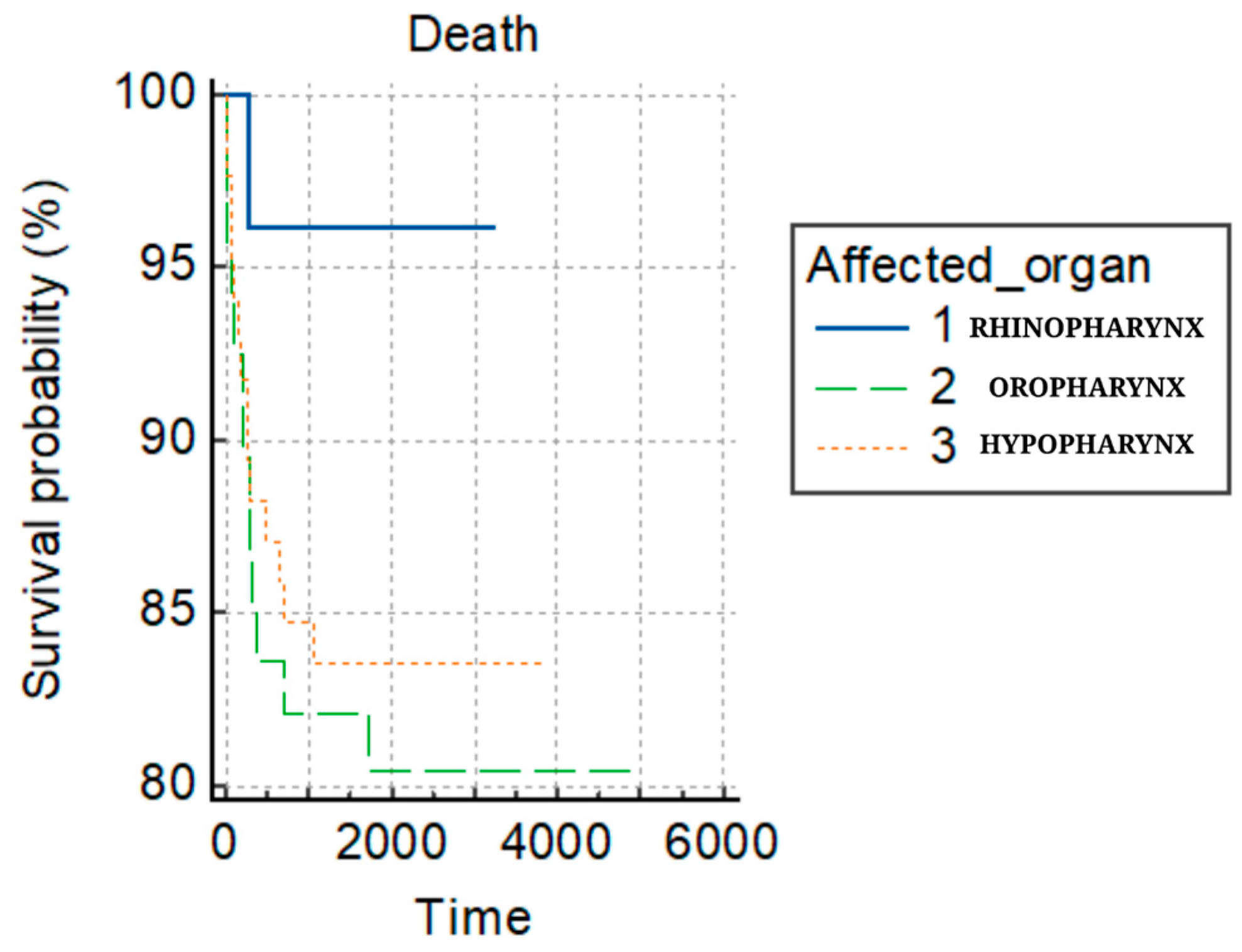
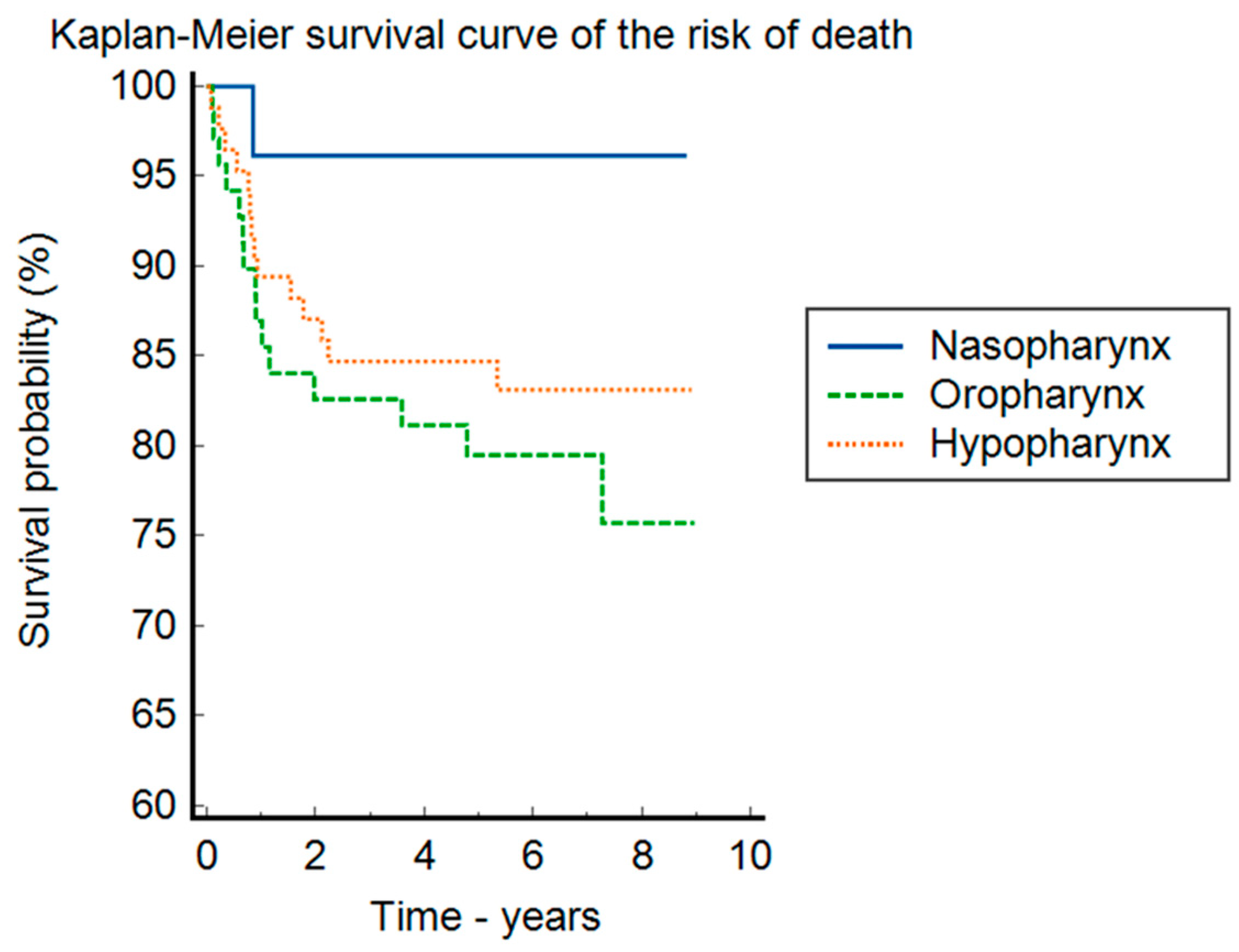
| Parameter | Nasopharynx N = 26 (14.4%) | Oropharynx N = 69 (38.3%) | Hypopharynx N = 85 (47.2%) | Total N = 180 | p-Value |
|---|---|---|---|---|---|
| Days diagnostic–end of treatment M + SD | 211 (423.46) | 162.36 (177.19) | 178.01 (272.15) | 0.232 | |
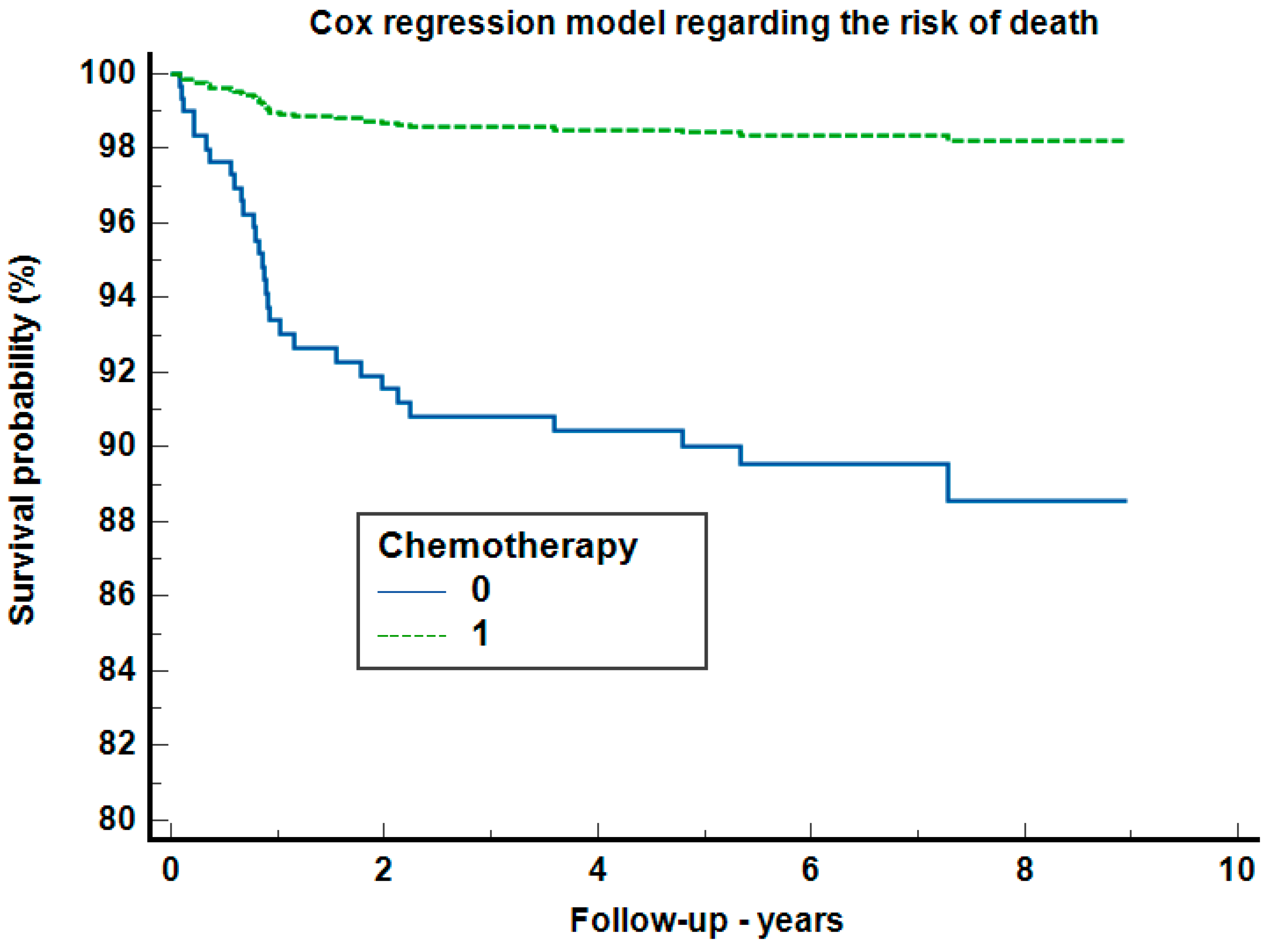
| Chemotherapy | 7 (26.9%) | 21 (30.4%) | 31 (36.5%) | 59 (32.8%) | 0.576 |
| Radiotherapy | 23 (88.5%) | 62 (89.9%) | 73 (85.9%) | 158 (87.8%) | |
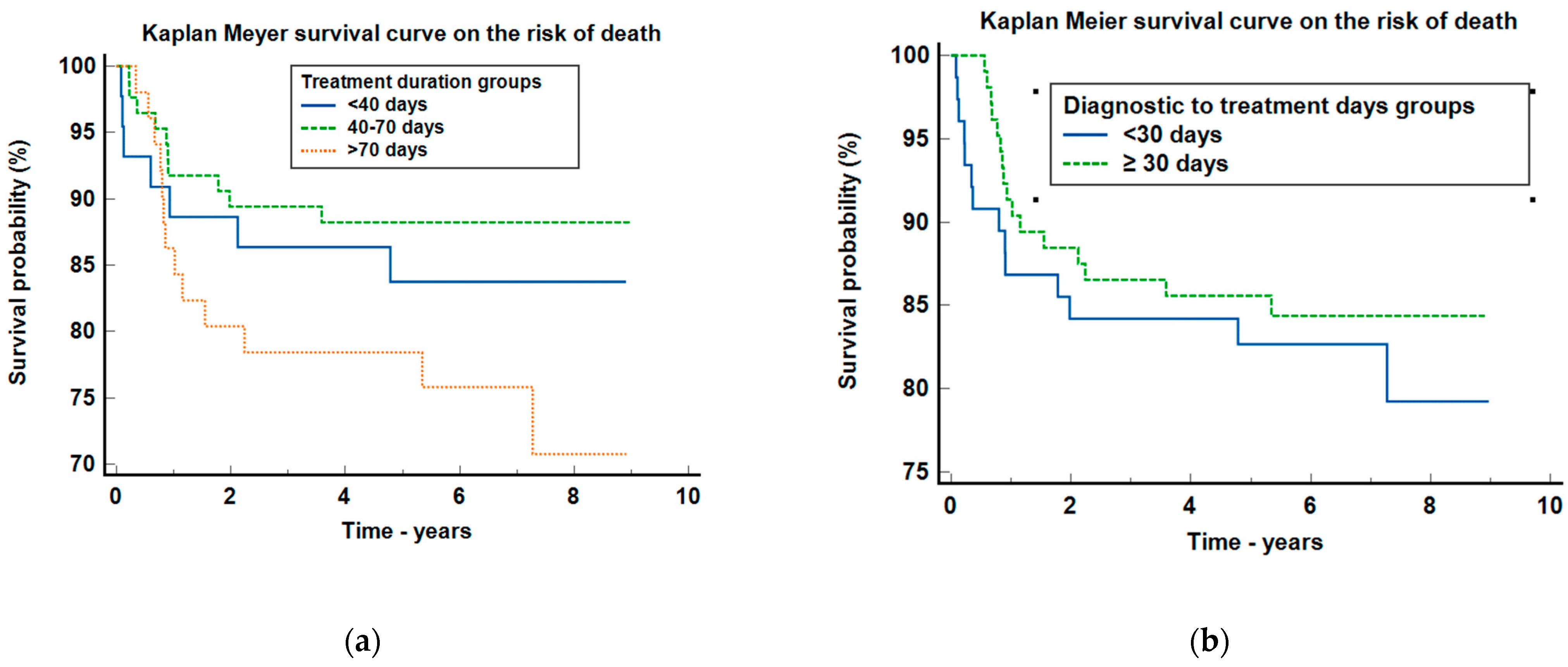
| Treatment duration group (Rxt; CRT, ChT) | |||||
| <40 days | 8 (30.8%) | 11 (15.9%) | 25 (29.4%) | 44 (24.4%) | |
| 40–70 days | 10 (38.4%) | 41 (59.4%) | 34 (40%) | 85 (47.2%) | 0.1162 |
| >70 days | 8 (30.8%) | 17 (24.6%) | 26 (30.6%) | 51 (28.3%) | |
| Diagnosis to treatment group (DTI) | |||||
| <30 days | 14 (53.8%) | 31 (44.9%) | 31 (36.5%) | 76 (42.2%) | |
| ≥30 days | 12 (46.2%) | 38 (55.1%) | 54 (63.5%) | 104 (57.8%) | |
| No Relapse (N = 155) | Relapse (N = 25) | Total (N = 180) | p-Value | |
|---|---|---|---|---|
| Gender | ||||
| M | 146.0 (94.2%) | 24.0 (96.0%) | 170.0 (94.4%) | 0.714 * |
| F | 9.0 (5.8%) | 1.0 (4.0%) | 10.0 (5.6%) | |
| Age | ||||
| Mean (SD) | 58.9 (9.2) | 59.6 (9.3) | 59.0 (9.2) | 0.728 ** |
| Range | 31.0–76.0 | 42.0–81.0 | 31.0–81.0 | |
| Patient delay | 0.132 * | |||
| Unspecified | 73.0 (47.1%) | 12.0 (48.0%) | 85.0 (47.2%) | |
| ˂3 Months | 45.0 (29.0%) | 11.0 (44.0%) | 56.0 (31.1%) | |
| ˃3 Months | 37.0 (23.9%) | 2.0 (8.0%) | 39.0 (21.7%) | |
| Histopathologic diagnosis | 0.093 * | |||
| SCC | 109.0 (70.3%) | 13.0 (52.0%) | 122.0 (67.8%) | |
| KSCC | 25.0 (16.1%) | 4.0 (16.0%) | 29.0 (16.1%) | |
| SCC In Situ | 3.0 (1.9%) | 2.0 (8.0%) | 5.0 (2.8%) | |
| UCNT | 13.0 (8.4%) | 3.0 (12.0%) | 16.0 (8.9%) | |
| Others | 5.0 (3.2%) | 3.0 (12.0%) | 8.0 (4.4%) | |
| GRADING | 0.055 * | |||
| In situ | 3.0 (1.9%) | 3.0 (12.0%) | 6.0 (3.3%) | |
| G1 | 24.0 (15.4%) | 0.0 (0.0%) | 27.0 (15.0%) | |
| G2 | 111.0 (71.6%) | 14.0 (56.0%) | 125.0 (69.4%) | |
| G3 | 17.0 (11.0%) | 5.0 (20.0%) | 22.0 (12.2%) | |
| Therapy | 0.027 * | |||
| Chemotherapy | 18.0 (11.6%) | 5.0 (20.0%) | 23.0 (12.8%) | |
| Radiotherapy | 110.0 (71.0%) | 11.0 (44.0%) | 121.0 (67.2%) | |
| Chemoradiotherapy | 27.0 (17.4%) | 9.0 (36.0%) | 36.0 (20.0%) | |
| Emergency | 0.804 * | |||
| N-Miss | 135.0 | 21.0 | 156.0 | |
| Acute Respiratory failure | 19.0 (95.0%) | 4.0 (100.0%) | 23.0 (95.9%) | |
| Chronic Respiratory failure | 1.0 (5.0%) | 0.0 (0.0%) | 1.0 (4.2%) | |
| Stage | 0.321 * | |||
| N-Miss | 2.0 | 1.0 | 3.0 | |
| Stage 1 | 56.0 (36.6%) | 10.0 (41.7%) | 66.0 (37.3%) | |
| Stage 2 | 28.0 (18.3%) | 7.0 (29.2%) | 35.0 (19.8%) | |
| Stage 3 | 35.0 (22.9%) | 2.0 (8.3%) | 37.0 (20.9%) | |
| Stage 4 | 34.0 (22.2%) | 5.0 (20.8%) | 39.0 (22.0%) | |
| N Stage | 0.722 * | |||
| N0 | 83.0 (53.5%) | 14.0 (56.0%) | 97.0 (53.9%) | |
| N1 | 25.0 (16.1%) | 2.0 (8.0%) | 27.0 (15.0%) | |
| N2 | 13.0 (8.4%) | 3.0 (12.0%) | 16.0 (8.9%) | |
| N3 | 34.0 (21.9%) | 6.0 (24.0%) | 40.0 (22.2%) | |
| T Stage | 0.171 * | |||
| T1 | 52.0 (33.5%) | 8.0 (32.0%) | 60.0 (33.4%) | |
| T2 | 63.0 (40.6%) | 10.0 (40.0%) | 73.0 (40.6%) | |
| T3 | 35.0 (22.6%) | 4.0 (16.0%) | 39.0 (21.7%) | |
| T4 | 4.0 (2.6%) | 1.0 (4.0%) | 5.0 (2.8%) | |
| Tis | 1.0 (0.6%) | 2.0 (8.0%) | 3.0 (1.7%) | |
| Environment | ||||
| R | 71.0 (45.8%) | 9.0 (36.0%) | 80.0 (44.4%) | 0.360 * |
| U | 84.0 (54.2%) | 16.0 (64.0%) | 100.0 (55.6%) | |
| Year | ||||
| 2014 | 29.0 (18.7%) | 7.0 (28.0%) | 36.0 (20.0%) | 0.153 * |
| 2015 | 24.0 (15.5%) | 7.0 (28.0%) | 31.0 (17.2%) | |
| 2016 | 40.0 (25.8%) | 7.0 (28.0%) | 47.0 (26.1%) | |
| 2017 | 32.0 (20.6%) | 3.0 (12.0%) | 35.0 (19.4%) | |
| 2018 | 30.0 (19.4%) | 1.0 (4.0%) | 31.0 (17.2%) | |
| Tumor site | ||||
| Nasopharynx | 23.0 (14.8%) | 3.0 (12.0%) | 26.0 (14.4%) | 0.808 * |
| Hypopharynx | 58.0 (37.4%) | 11.0 (44.0%) | 69.0 (38.3%) | |
| Oropharynx | 74.0 (47.7%) | 11.0 (44.0%) | 85.0 (47.2%) | |
| Docetaxel | 0.143 * | |||
| 0 | 135.0 (87.1%) | 19.0 (76.0%) | 154.0 (85.6%) | |
| 1 | 20.0 (12.9%) | 6.0 (24.0%) | 26.0 (14.4%) | |
| Paclitaxel | 0.130 * | |||
| 0 | 148.0 (95.5%) | 22.0 (88.0%) | 170.0 (94.4%) | |
| 1 | 7.0 (4.5%) | 3.0 (12.0%) | 10.0 (5.6%) | |
| Carboplatin | 0.057 * | |||
| 0 | 130.0 (83.9%) | 17.0 (68.0%) | 147.0 (81.7%) | |
| 1 | 25.0 (16.1%) | 8.0 (32.0%) | 33.0 (18.3%) | |
| Cisplatin | 0.104 * | |||
| 0 | 89.0 (57.4%) | 10.0 (40.0%) | 99.0 (55.0%) | |
| 1 | 66.0 (42.6%) | 15.0 (60.0%) | 81.0 (45.0%) | |
| Deceased | 0.509 * | |||
| 0 | 132.0 (85.2%) | 20.0 (80.0%) | 152.0 (84.4%) | |
| 1 | 23.0 (14.8%) | 5.0 (20.0%) | 28.0 (15.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiș, A.M.; Buzatu, R.; Chisavu, L.; Poenaru, M.; Borza, C.; Iftode, A.; Sarau, O.S.; Dehelean, C.A.; Ardelean, S. Time-to-Treatment Delays and Their Prognostic Implications in Pharyngeal Cancer—An Exploratory Analysis in Western Romania. Clin. Pract. 2024, 14, 1270-1284. https://doi.org/10.3390/clinpract14040103
Kiș AM, Buzatu R, Chisavu L, Poenaru M, Borza C, Iftode A, Sarau OS, Dehelean CA, Ardelean S. Time-to-Treatment Delays and Their Prognostic Implications in Pharyngeal Cancer—An Exploratory Analysis in Western Romania. Clinics and Practice. 2024; 14(4):1270-1284. https://doi.org/10.3390/clinpract14040103
Chicago/Turabian StyleKiș, Andreea Mihaela, Roxana Buzatu, Lazar Chisavu, Marioara Poenaru, Claudia Borza, Andrada Iftode, Oana Silvana Sarau, Cristina Adriana Dehelean, and Simona Ardelean. 2024. "Time-to-Treatment Delays and Their Prognostic Implications in Pharyngeal Cancer—An Exploratory Analysis in Western Romania" Clinics and Practice 14, no. 4: 1270-1284. https://doi.org/10.3390/clinpract14040103
APA StyleKiș, A. M., Buzatu, R., Chisavu, L., Poenaru, M., Borza, C., Iftode, A., Sarau, O. S., Dehelean, C. A., & Ardelean, S. (2024). Time-to-Treatment Delays and Their Prognostic Implications in Pharyngeal Cancer—An Exploratory Analysis in Western Romania. Clinics and Practice, 14(4), 1270-1284. https://doi.org/10.3390/clinpract14040103







