Inquiry of the Metabolic Traits in Relationship with Daily Magnesium Intake: Focus on Type 2 Diabetic Population
Abstract
1. Introduction
Objective
2. Methods
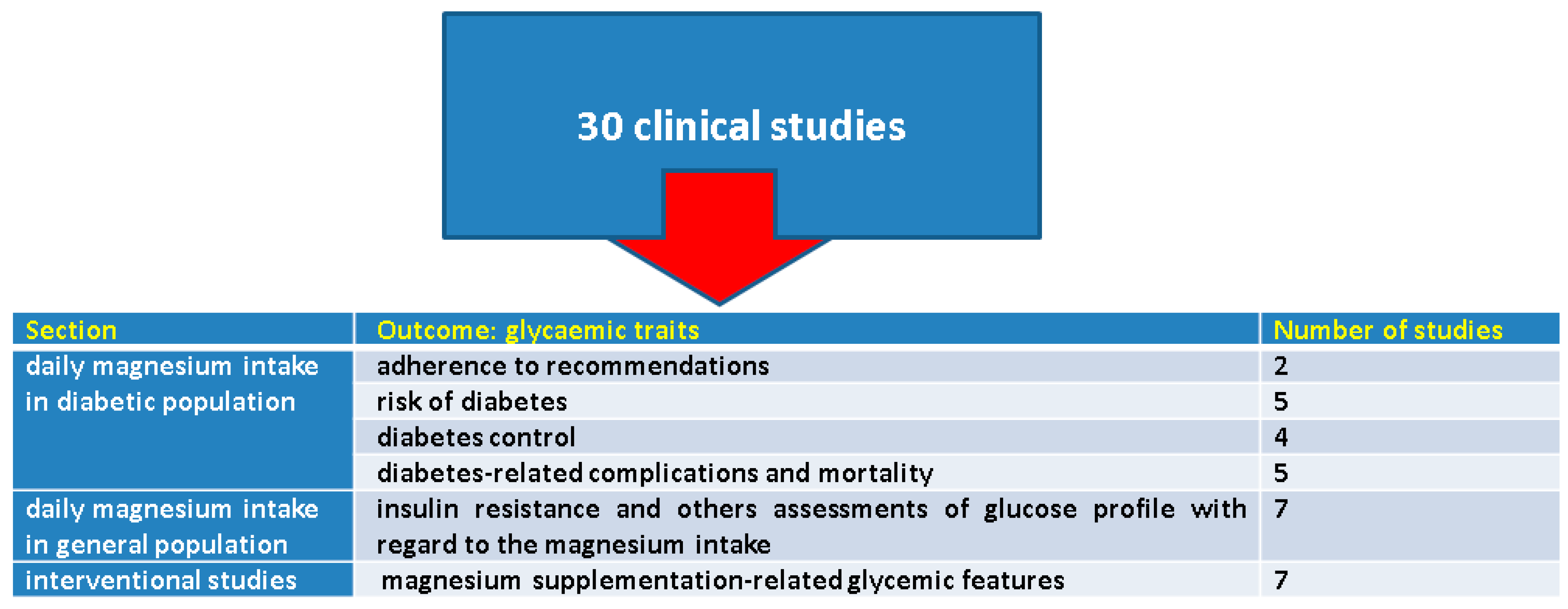
3. Results
3.1. Magnesium Intake in Diabetic Population
3.1.1. Adherence to the Recommendations Regarding Daily Magnesium Intake
3.1.2. Magnesium Intake and the Risk of Diabetes Mellitus
3.1.3. Magnesium Intake and Diabetes Control
3.1.4. Analysing Diabetes-Related Complications and Mortality with Regard to Magnesium Intake
3.2. Insulin Resistance and Others Assessments of Glucose Profile in the General Population with Respect to Magnesium Intake
3.3. The Effects of Magnesium Supplementation on Glycaemic Traits
4. Discussion
4.1. Magnesium Intake and Metabolic Features: Beyond Diabetes Mellitus
4.1.1. Delving into Obesity and Metabolic Syndrome
4.1.2. Cardiovascular Insights
4.2. Other Clinical Elements with Magnesium-Intake-Related Considerations
4.2.1. Interplay between Magnesium Intake and Kidney Status
4.2.2. Dietary Magnesium Amid the Diagnosis of Osteoporosis
4.2.3. Magnesium and Mineral Metabolism: PTH Crossways
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| cAMP | cyclic adenosine monophosphate |
| CI | confidence interval |
| EEFSA | European Food Safety Association |
| HbA1c | glycated haemoglobin A1c |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| NAHSIT | Nutrition and Health Survey in Taiwan |
| NHANES | National Health and Nutrition Examination Surveys (NHANES), |
| NIPAL1 | Nuclear Interaction Partner of Alkaline Phosphatase-like domain containing 1 |
| PTH | parathyroid hormone |
| RDA | recommended dietary allowance |
| QUICKI | quantitative insulin sensitivity check index |
References
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, R.; Davies, A.; Veenhuizen, M.; Campbell, M.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Magnesium Deficiency and Cardiometabolic Disease. Nutrients 2023, 15, 2355. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; López-Garrigós, M.; Flores, E.; Leiva-Salinas, C. Improving diagnosis and treatment of hypomagnesemia. Clin. Chem. Lab. Med. 2023, 62, 234–248. [Google Scholar] [CrossRef]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Schlingmann, K.P.; Gudermann, T. Insights into the molecular nature of magnesium homeostasis. Am. J. Physiol.-Renal Physiol. 2004, 286, F599–F605. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Dietary reference values for nutrients: Summary report. EFSA Support. 2017, 92, e15121. Available online: https://www.efsa.europa.eu/en/supporting/pub/e15121 (accessed on 1 May 2024).
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013, 36, 3821–3842. [Google Scholar] [CrossRef]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon 2020, 6, e05390. [Google Scholar] [CrossRef]
- Pardo, M.R.; Garicano Vilar, E.; San Mauro Martín, I.; Camina Martín, M.A. Bioavailability of magnesium food supplements: A systematic review. Nutrition 2021, 89, 111294. [Google Scholar] [CrossRef]
- Severino, P.; Netti, L.; Mariani, M.V.; Maraone, A.; D’Amato, A.; Scarpati, R.; Infusino, F.; Pucci, M.; Lavalle, C.; Maestrini, V.; et al. Prevention of Cardiovascular Disease: Screening for Magnesium Deficiency. Cardiol. Res. Pract. 2019, 2019, 4874921. [Google Scholar] [CrossRef]
- Zenoaga-Barbăroșie, C.; Berca, L.; Vassu-Dimov, T.; Toma, M.; Nica, M.I.; Alexiu-Toma, O.A.; Ciornei, C.; Albu, A.; Nica, S.; Nistor, C.; et al. The Predisposition for Type 2 Diabetes Mellitus and Metabolic Syndrome. Balkan J. Med. Genet. 2023, 26, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Anghel, D.; Ciobîcă, L.M.; Stanciu, S.M.; Jurcuț, C.V.; Stoicescu, G.D.; Răduță, I.A.; Coca, A. Ankylosing spondylitis and cardiovascular risk—Case report. Rom. J. Mil. Med. 2016, CXIX, 39–42. [Google Scholar]
- Dandurand, K.; Ali, D.S.; Khan, A.A. Primary Hyperparathyroidism: A Narrative Review of Diagnosis and Medical Management. J. Clin. Med. 2021, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Ciobîcă, M.L.; Ionescu, O.P.; Săndulescu, B.A. Osteoporosis and the fracture risk in systemic lupus erythematosus. Rom. J. Mil. Med. 2020, CXXIII, 341–347. [Google Scholar]
- Fisher, S.B.; Perrier, N.D. Primary hyperparathyroidism and hypertension. Gland. Surg. 2020, 9, 142–149. [Google Scholar] [CrossRef]
- Ionescu, O.P.; Stanciu, S.M.; Ciobîcă, M.L. Atherosclerosis in rheumatoid arthritis—The importance of imaging testing. Rom. J. Mil. Med. 2020, CXXIII, 26–31. [Google Scholar]
- Barnett, M.J. Association between Primary Hyperparathyroidism and Secondary Diabetes Mellitus: Findings from a Scoping Review. Cureus 2023, 15, e40743. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Chiou, J.; Lo, K.; Wen, C. Magnesium in joint health and osteoarthritis. Nutr. Res. 2021, 90, 24–35. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Reddy, S.T.; Soman, S.S.; Yee, J. Magnesium Balance and Measurement. Adv. Chronic Kidney Dis. 2018, 25, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, H.; Jing, Z.; Wang, Y.; Cheng, Y.; Wang, W.; Sun, W. Role of Magnesium in Type 2 Diabetes Mellitus. Biol. Trace Elem. Res. 2020, 196, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Massimino, E.; Izzo, A.; Castaldo, C.; Amoroso, A.P.; Rivellese, A.A.; Capaldo, B.; Della Pepa, G. Dietary micronutrient adequacies and adherence to the Mediterranean diet in a population of older adults with type 2 diabetes: A cross-sectional study. Clin. Nutr. ESPEN 2023, 57, 337–345. [Google Scholar] [CrossRef]
- Li, M.C. Associations between Adherence to the Taiwan Dietary Reference Intakes of Micronutrients and the Risk of Type 2 Diabetes. Int. J. Environ. Res. Public Health 2022, 19, 12242. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, Y.; Fang, J.; Sun, M.; Liu, Q.; Ma, Z.; Hu, D.; Gong, X.; Liu, Y.; Jin, L.; et al. Associations between dietary magnesium intake and hypertension, diabetes, and hyperlipidemia. Hypertens. Res. 2024, 47, 331–341. [Google Scholar] [CrossRef]
- Shah, I.U.; Sameen, A.; Manzoor, M.F.; Ahmed, Z.; Gao, J.; Farooq, U.; Siddiqi, S.M.; Siddique, R.; Habib, A.; Sun, C.; et al. Association of dietary calcium, magnesium, and vitamin D with type 2 diabetes among US adults: National health and nutrition examination survey 2007–2014-A cross-sectional study. Food Sci. Nutr. 2021, 9, 1480–1490. [Google Scholar] [CrossRef]
- McClure, S.T.; Schlechter, H.; Oh, S.; White, K.; Wu, B.; Pilla, S.J.; Maruthur, N.M.; Yeh, H.C.; Miller, E.R.; Appel, L.J. Dietary intake of adults with and without diabetes: Results from NHANES 2013–2016. BMJ Open Diabetes Res. Care 2020, 8, e001681. [Google Scholar] [CrossRef]
- Golmohamadi, M.; Hosseinpour-Niazi, S.; Hadaegh, P.; Mirmiran, P.; Azizi, F.; Hadaegh, F. Association between dietary antioxidants intake and the risk of type 2 diabetes mellitus in a prospective cohort study: Tehran Lipid and Glucose Study. Br. J. Nutr. 2024, 131, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ma, X.; Liang, H.; Li, H.; Chen, J.; Fang, L.; Yang, Q.; Zhang, Z. Dietary Magnesium Intake Affects the Association between Serum Vitamin D and Type 2 Diabetes: A Cross-Sectional Study. Front. Nutr. 2021, 8, 763076. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, S.; Wang, X.; Wang, X.; Gao, P. Lower Dietary Magnesium Is Associated with a Higher Hemoglobin Glycation Index in the National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2024, 202, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Datta, S.; Little, B.B.; Kong, M. Magnesium dietary intake and physical activity in Type 2 diabetes by gender in White, African-American and Mexican American: NHANES 2011–2014. Endocrinol. Diabetes Metab. 2020, 4, e00203. [Google Scholar] [CrossRef] [PubMed]
- Ozcaliskan Ilkay, H.; Sahin, H.; Tanriverdi, F.; Samur, G. Association between Magnesium Status, Dietary Magnesium Intake, and Metabolic Control in Patients with Type 2 Diabetes Mellitus. J. Am. Coll. Nutr. 2019, 38, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, E.; Akturk, M.; Koksal, E. Relationships between serum and dietary magnesium, calcium, and metabolic parameters in women with type 2 diabetes mellitus. Clin. Nutr. ESPEN 2023, 54, 304–310. [Google Scholar] [CrossRef]
- Bahrampour, N.; Mirzababaei, A.; Abaj, F.; Hosseininasab, D.; Clark, C.C.T.; Mirzaei, K. The association between dietary micronutrient patterns and odds of diabetic nephropathy: A case-control study. Food Sci. Nutr. 2023, 11, 3255–3265. [Google Scholar] [CrossRef]
- Xu, H.; Dong, X.; Wang, J.; Cheng, X.; Qu, S.; Jia, T.; Liu, J.; Li, Z.; Yao, Y. Association of Calcium, Magnesium, Zinc, and Copper Intakes with Diabetic Retinopathy in Diabetics: National Health and Nutrition Examination Survey, 2007–2018. Curr. Eye Res. 2023, 48, 485–491. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, X.; Yuan, T.; Guo, C.; Zhou, Z.; Wang, L.; Dou, G. Certain Dietary Nutrients Reduce the Risk of Eye Affliction/Retinopathy in Individuals with Diabetes: National Health and Nutrition Examination Survey, 2003–2018. Int. J. Environ. Res. Public Health 2022, 19, 12173. [Google Scholar] [CrossRef]
- Wang, H.W.; Huang, Y.T.; Jiang, M.Y. Association of dietary magnesium intake and glycohemoglobin with mortality risk in diabetic patients. PLoS ONE 2022, 17, e0277180. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Cao, S.; Duan, Y.; Xu, C.; Gan, D.; He, W. Dietary Antioxidant Indices in Relation to All-Cause and Cause-Specific Mortality among Adults with Diabetes: A Prospective Cohort Study. Front. Nutr. 2022, 9, 849727. [Google Scholar] [CrossRef]
- Palacios, C.; Pérez, C.M.; González-Sepúlveda, L.; Corsino, L.; Albrecht, S.S.; Siega-Riz, A.M.; Durazo-Arvizu, R.A.; Casagrande, S.; Sotres-Alvarez, D.; Avilés-Santa, M.L. Vitamin D, Calcium, Magnesium, and Potassium Consumption and Markers of Glucose Metabolism in the Hispanic Community Health Study/Study of Latinos. J. Am. Nutr. Assoc. 2022, 41, 20–29. [Google Scholar] [CrossRef]
- Yang, N.; He, L.; Li, Y.; Xu, L.; Ping, F.; Li, W.; Zhang, H. Reduced Insulin Resistance Partly Mediated the Association of High Dietary Magnesium Intake with Less Metabolic Syndrome in a Large Chinese Population. Diabetes Metab. Syndr. Obes. 2020, 13, 2541–2550. [Google Scholar] [CrossRef]
- Bavani, N.G.; Saneei, P.; Hassanzadeh Keshteli, A.; Yazdannik, A.; Falahi, E.; Sadeghi, O.; Esmaillzadeh, A. Magnesium intake, insulin resistance and markers of endothelial function among women. Public Health Nutr. 2021, 24, 5777–5785. [Google Scholar] [CrossRef]
- Mirrafiei, A.; Jabbarzadeh, B.; Hosseini, Y.; Djafarian, K.; Shab-Bidar, S. No association between dietary magnesium intake and body composition among Iranian adults: A cross-sectional study. BMC Nutr. 2022, 8, 39. [Google Scholar] [CrossRef]
- Yan, S.; Li, M.; Ma, X.; Jiang, S.; Sun, M.; Wang, C.; Pan, Y.; Sun, C.; Yao, Y.; Jin, L.; et al. Association of multiple mineral and vitamin B group intake with blood glucose using quantile regression analysis: NHANES 2007–2014. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Xu, M.; Cai, J.; Mo, X.; Liu, Q.; Zhang, J.; Wei, Y.; Liu, S.; Lin, Y.; Huang, S.; Mo, C.; et al. Association of Dietary and Plasma Magnesium with Glycaemic Markers in a Chinese Population. Biol. Trace Elem. Res. 2023, 201, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Bentil, H.J.; Abreu, A.M.; Adu-Afarwuah, S.; Rossi, J.S.; Tovar, A.; Oaks, B.M. Association between Dietary Magnesium Intake and Glycemic Markers in Ghanaian Women of Reproductive Age: A Pilot Cross-Sectional Study. Nutrients 2021, 13, 4141. [Google Scholar] [CrossRef]
- Hamedifard, Z.; Farrokhian, A.; Reiner, Ž.; Bahmani, F.; Asemi, Z.; Ghotbi, M.; Taghizadeh, M. The effects of combined magnesium and zinc supplementation on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Lipids Health Dis. 2020, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.Y.; Shon, Y.H. Natural Magnesium-Enriched Deep-Sea Water Improves Insulin Resistance and the Lipid Profile of Prediabetic Adults: A Randomized, Double-Blinded Crossover Trial. Nutrients 2020, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Afitska, K.; Clavel, J.; Kisters, K.; Vormann, J.; Werner, T. Magnesium citrate supplementation decreased blood pressure and HbA1c in normomagnesemic subjects with metabolic syndrome: A 12-week, placebo-controlled, double-blinded pilot trial. Magnes. Res. 2021, 34, 130–139. [Google Scholar] [CrossRef]
- Drenthen, L.C.A.; de Baaij, J.H.F.; Rodwell, L.; van Herwaarden, A.E.; Tack, C.J.; de Galan, B.E. Oral magnesium supplementation does not affect insulin sensitivity in people with insulin-treated type 2 diabetes and a low serum magnesium: A randomised controlled trial. Diabetologia 2024, 67, 52–61. [Google Scholar] [CrossRef]
- Salehidoost, R.; Taghipour Boroujeni, G.; Feizi, A.; Aminorroaya, A.; Amini, M. Effect of oral magnesium supplement on cardiometabolic markers in people with prediabetes: A double blind randomized controlled clinical trial. Sci. Rep. 2022, 12, 18209. [Google Scholar] [CrossRef]
- Sadeghian, M.; Azadbakht, L.; Khalili, N.; Mortazavi, M.; Esmaillzadeh, A. Oral Magnesium Supplementation Improved Lipid Profile but Increased Insulin Resistance in Patients with Diabetic Nephropathy: A Double-Blind Randomized Controlled Clinical Trial. Biol. Trace Elem. Res. 2020, 193, 23–35. [Google Scholar] [CrossRef]
- Halawa, N.; Elsaid, T.W.; El Wakeel, L.M.; Shawki, M.A. Impact of magnesium supplementation on clinical outcome and disease progression of patients with diabetic nephropathy: A prospective randomized trial. Ther. Adv. Chronic Dis. 2023, 14, 20406223231214641. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Chen, C.; Lu, L.; Bidulescu, A.; Fly, A.D.; Xun, P.; Judd, S.E.; Cushman, M.; Kahe, K. Magnesium intake is inversely associated with the risk of metabolic syndrome in the REasons for geographic and racial differences in stroke (REGARDS) cohort study. Clin. Nutr. 2021, 40, 2337–2342. [Google Scholar] [CrossRef]
- Mirmiran, P.; Moslehi, N.; Hosseinpanah, F.; Sarbazi, N.; Azizi, F. Dietary determinants of unhealthy metabolic phenotype in normal weight and overweight/obese adults: Results of a prospective study. Int. J. Food Sci. Nutr. 2020, 71, 891–901. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Morales-Gurrola, G.; Preza-Rodríguez, L.; Gómez-Barrientos, A.; Olivas-Martínez, A.I.; Simental-Mendía, L.E. Magnesium intake is associated with the metabolically healthy obese phenotype. J. Investig. Med. 2022, 70, 800–804. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between Dietary Magnesium Intake and Metabolic Syndrome. Nutrients 2022, 14, 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; He, Y.; Wu, F.; Zhao, L.; Wu, C.; Lu, Y.; Zang, J.; Wang, Z.; Sun, J.; Huang, J.; et al. The Associations of Dietary Iron, Zinc and Magnesium with Metabolic Syndrome in China’s Mega Cities. Nutrients 2020, 12, 659. [Google Scholar] [CrossRef]
- Jun, S.; Cowan, A.E.; Bhadra, A.; Dodd, K.W.; Dwyer, J.T.; Eicher-Miller, H.A.; Gahche, J.J.; Guenther, P.M.; Potischman, N.; Tooze, J.A.; et al. Older adults with obesity have higher risks of some micronutrient inadequacies and lower overall dietary quality compared to peers with a healthy weight, National Health and Nutrition Examination Surveys (NHANES), 2011–2014. Public Health Nutr. 2020, 23, 2268–2279. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Fang, H.Y.; Li, M.C. Relationship between overweight and obesity and insufficient micronutrient intake: A nationwide study in Taiwan. J. Nutr. Sci. 2023, 12, e48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, X.; Li, M.; Yan, S.; Zhao, H.; Pan, Y.; Wang, C.; Yao, Y.; Jin, L.; Li, B. Association between dietary mineral nutrient intake, body mass index, and waist circumference in U.S. adults using quantile regression analysis NHANES 2007–2014. PeerJ 2020, 8, e9127. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, C.; Yang, K.; Zhu, J.; Xun, P.; Shikany, J.M.; He, K. Magnesium intake is inversely associated with risk of obesity in a 30-year prospective follow-up study among American young adults. Eur. J. Nutr. 2020, 59, 3745–3753. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Imoto, S.; Ihara, K.; Nakaji, S. Association between Nutrients and Visceral Fat in Healthy Japanese Adults: A 2-Year Longitudinal Study Brief Title: Micronutrients Associated with Visceral Fat Accumulation. Nutrients 2019, 11, 2698. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.; Chan, T.Y.; Chu, Y.K.; Ling, J.; He, J.; Leung, K.; Ma, R.C.W.; Chan, J.C.N.; Chow, E. Higher dietary magnesium and potassium intake are associated with lower body fat in people with impaired glucose tolerance. Front. Nutr. 2023, 10, 1169705. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, B.; Agraib, L.M.; Al-Dalaeen, A.; Al-Shami, I. Are There Any Correlations between Vitamin D, Calcium, and Magnesium Intake and Coronary and Obesity Indices? J. Am. Nutr. Assoc. 2024, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Izurieta, R.; Nishio, A.; Horita, R.; Yamamoto, M. Nutritional intake and metabolic parameters of Japanese university students with and without obesity: Sex-specific differences. PLoS ONE 2023, 18, e0285088. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Fulda, K.G.; Chen, S.; Tao, M.H. Comparison of Dietary Micronutrient Intakes by Body Weight Status among Mexican-American and Non-Hispanic Black Women Aged 19–39 Years: An Analysis of NHANES 2003–2014. Nutrients 2019, 11, 2846. [Google Scholar] [CrossRef]
- Cheung, M.M.; DeLuccia, R.; Ramadoss, R.K.; Aljahdali, A.; Volpe, S.L.; Shewokis, P.A.; Sukumar, D. Low dietary magnesium intake alters vitamin D-parathyroid hormone relationship in adults who are overweight or obese. Nutr. Res. 2019, 69, 82–93. [Google Scholar] [CrossRef]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Melo, S.R.; Dos Santos, L.R.; da Cunha Soares, T.; Cardoso, B.E.P.; da Silva Dias, T.M.; Morais, J.B.S.; de Paiva Sousa, M.; de Sousa, T.G.V.; da Silva, N.C.; da Silva, L.D.; et al. Participation of Magnesium in the Secretion and Signaling Pathways of Insulin: An Updated Review. Biol. Trace Elem. Res. 2022, 200, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Hodson, D.J.; Mitchell, R.K.; Marselli, L.; Pullen, T.J.; Gimeno Brias, S.; Semplici, F.; Everett, K.L.; Cooper, D.M.; Bugliani, M.; Marchetti, P.; et al. ADCY5 couples glucose to insulin secretion in human islets. Diabetes 2014, 63, 3009–3021. [Google Scholar] [CrossRef]
- Tengholm, A.; Gylfe, E. cAMP signalling in insulin and glucagon secretion. Diabetes Obes. Metab. 2017, 19 (Suppl. S1), 42–53. [Google Scholar] [CrossRef] [PubMed]
- Manialawy, Y.; Khan, S.R.; Bhattacharjee, A.; Wheeler, M.B. The magnesium transporter NIPAL1 is a pancreatic islet-expressed protein that conditionally impacts insulin secretion. J. Biol. Chem. 2020, 295, 9879–9892. [Google Scholar] [CrossRef]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Gommers, L.M.; Hoenderop, J.G.; Bindels, R.J.; de Baaij, J.H. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef]
- Pelczyńska, M.; Moszak, M.; Bogdański, P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients 2022, 14, 1714. [Google Scholar] [CrossRef]
- Ciobîcă, L.M.; Sârbu, I.; Stanciu, S.M.; Coca, A. Behçet disease—Case presentation. Rom. J. Mil. Med. 2016, CXIX, 43–46. [Google Scholar] [CrossRef]
- Jinga, M.; Jurcuţ, C.; Vasilescu, F.; Becheanu, G.; Stancu, S.H.; Ciobaca, L.; Mircescu, G.; Jinga, V. A rare case of digestive hemorrhage in an elderly patient: Diagnosis and treatment difficulties. Rom. J. Morphol. Embryol. 2012, 53 (Suppl. S3), 831–834. [Google Scholar] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Weglicki, W.B. Hypomagnesemia and inflammation: Clinical and basic aspects. Annu. Rev. Nutr. 2012, 32, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Han, S.N. Vitamin D and obesity. Adv. Food Nutr. Res. 2024, 109, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef]
- Cheteu Wabo, T.M.; Wu, X.; Sun, C.; Boah, M.; Ngo Nkondjock, V.R.; Kosgey Cheruiyot, J.; Amporfro Adjei, D.; Shah, I. Association of dietary calcium, magnesium, sodium, and potassium intake and hypertension: A study on an 8-year dietary intake data from the National Health and Nutrition Examination Survey. Nutr. Res. Pract. 2022, 16, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Gea, A.; Ruiz-Estigarribia, L.; Sayón-Orea, C.; Fresán, U.; Barbagallo, M.; Ruiz-Canela, M.; Martínez-González, M.A. Low Dietary Magnesium and Overweight/Obesity in a Mediterranean Population: A Detrimental Synergy for the Development of Hypertension. The SUN Project. Nutrients 2020, 13, 125. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. The ratio potassium-to-magnesium intake and high blood pressure. Eur. J. Clin. Investig. 2019, 49, e13093. [Google Scholar] [CrossRef]
- Wu, Z.; Ruan, Z.; Liang, G.; Wang, X.; Wu, J.; Wang, B. Association between dietary magnesium intake and peripheral arterial disease: Results from NHANES 1999–2004. PLoS ONE 2023, 18, e0289973. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.A.; de Jong, H.B.T.; Blomjous, A.G.A.; Eelderink, C.; Hoekstra, T.; Elders, P.J.M.; de Borst, M.H.; Vervloet, M.G.; van Ballegooijen, A.J.; Beulens, J.W. Magnesium intake and vascular structure and function: The Hoorn Study. Eur. J. Nutr. 2022, 61, 653–664. [Google Scholar] [CrossRef]
- Pickering, R.T.; Bradlee, M.L.; Singer, M.R.; Moore, L.L. Higher Intakes of Potassium and Magnesium, but Not Lower Sodium, Reduce Cardiovascular Risk in the Framingham Offspring Study. Nutrients 2021, 13, 269. [Google Scholar] [CrossRef]
- Cheung, M.M.; Dall, R.D.; Shewokis, P.A.; Altasan, A.; Volpe, S.L.; Amori, R.; Singh, H.; Sukumar, D. The effect of combined magnesium and vitamin D supplementation on vitamin D status, systemic inflammation, and blood pressure: A randomized double-blinded controlled trial. Nutrition 2022, 99–100, 111674. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joosten, M.M.; de Borst, M.H.; Bakker, S.J.L. Magnesium and Blood Pressure: A Physiology-Based Approach. Adv. Chronic Kidney Dis. 2018, 25, 244–250. [Google Scholar] [CrossRef]
- Patni, N.; Fatima, M.; Lamis, A.; Siddiqui, S.W.; Ashok, T.; Muhammad, A. Magnesium and Hypertension: Decoding Novel Anti-hypertensives. Cureus 2022, 14, e25839. [Google Scholar] [CrossRef]
- Atarashi, K.; Matsuoka, H.; Takagi, M.; Sugimoto, T. Magnesium ion: A possible physiological regulator of aldosterone production. Life Sci. 1989, 44, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, K.; Dabrowski, W.; Dobija, J.; Wrońskal, J.; Rzecki, Z.; Biernacka, J. The effect of preoperative magnesium supplementation on blood catecholamine concentrations in patients undergoing CABG. Magnes. Res. 2006, 19, 113–122. [Google Scholar]
- Banjanin, N.; Belojevic, G. Relationship of dietary magnesium intake and serum magnesium with hypertension: A review. Magnes. Res. 2021, 34, 166–171. [Google Scholar] [CrossRef]
- Cunha, A.R.; Umbelino, B.; Correia, M.L.; Neves, M.F. Magnesium and vascular changes in hypertension. Int. J. Hypertens. 2012, 2012, 754250. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Plesset, M.R. Oral magnesium supplements decrease high blood pressure (SBP>155 mmHg) in hypertensive subjects on anti-hypertensive medications: A targeted meta-analysis. Magnes. Res. 2013, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.O.; Nicolson, D.J.; Campbell, F.; Cook, J.V.; Beyer, F.R.; Ford, G.A.; Mason, J. Magnesium supplementation for the management of essential hypertension in adults. Cochrane Database Syst. Rev. 2006, 3, CD004640. [Google Scholar] [CrossRef]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Maraj, M.; Hetwer, P.; Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Kuźniewski, M.; Ceranowicz, P. α1-Acid Glycoprotein and Dietary Intake in End-Stage Renal Disease Patients. Nutrients 2021, 13, 3671. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Di Stasio, E.; Viola, A.; Cenerelli, S.; Leo, A.; Santarelli, S.; Monteburini, T. Dietary Daily Sodium Intake Lower than 1500 mg Is Associated with Inadequately Low Intake of Calorie, Protein, Iron, Zinc and Vitamin B1 in Patients on Chronic Hemodialysis. Nutrients 2020, 12, 260. [Google Scholar] [CrossRef]
- Song, A.Y.; Crews, D.C.; Ephraim, P.L.; Han, D.; Greer, R.C.; Boyér, L.L.; Ameling, J.; Gayles, D.J.; Sneed, V.; Carson, K.A.; et al. Sociodemographic and Kidney Disease Correlates of Nutrient Intakes among Urban African Americans with Uncontrolled Hypertension. J. Ren. Nutr. 2019, 29, 399–406. [Google Scholar] [CrossRef]
- Massy, Z.A.; Drüeke, T.B. Magnesium and outcomes in patients with chronic kidney disease: Focus on vascular calcification, atherosclerosis and survival. Clin. Kidney J. 2012, 5 (Suppl. S1), i52–i61. [Google Scholar] [CrossRef] [PubMed]
- Kircelli, F.; Peter, M.E.; Sevinc Ok, E.; Celenk, F.G.; Yilmaz, M.; Steppan, S.; Asci, G.; Ok, E.; Passlick-Deetjen, J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol. Dial. Transplant. 2012, 27, 514–521. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Type 2 Diabetes Mellitus, Obesity, and Metabolic Syndrome. Nutrients 2022, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- van de Wal-Visscher, E.R.; Kooman, J.P.; van der Sande, F.M. Magnesium in Chronic Kidney Disease: Should We Care? Blood Purif. 2018, 45, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; García-Pérez, J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin. Dial. 2009, 22, 37–44. [Google Scholar] [CrossRef]
- Mamilla, M.; Yartha, S.G.R.; Tuli, R.; Konipineni, S.; Rayaprolu, D.T.; Borgharkar, G.; Kalluru, P.K.R.; Thugu, T.R. Role of Magnesium in Diabetic Nephropathy for Better Outcomes. Cureus 2023, 15, e43076. [Google Scholar] [CrossRef]
- Shringi, S.; Raker, C.A.; Tang, J. Dietary Magnesium Intake and Kidney Stone: The National Health and Nutrition Examination Survey 2011–2018. Rhode Isl. Med. J. 2023, 106, 20–25. [Google Scholar] [CrossRef]
- Betz, M.V.; Penniston, K.L. Primary Contributors to Dietary Acid Load in Patients with Urolithiasis. J. Ren. Nutr. 2023, 33, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Israr, B.; Frazier, R.A.; Gordon, M.H. Effects of phytate and minerals on the bioavailability of oxalate from food. Food Chem. 2013, 141, 1690–1693. [Google Scholar] [CrossRef]
- Zimmermann, D.J.; Voss, S.; von Unruh, G.E.; Hesse, A. Importance of magnesium in absorption and excretion of oxalate. Urol. Int. 2005, 74, 262–267. [Google Scholar] [CrossRef]
- Riley, J.M.; Kim, H.; Averch, T.D.; Kim, H.J. Effect of magnesium on calcium and oxalate ion binding. J. Endourol. 2013, 27, 1487–1492. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef]
- Creedon, A.; Flynn, A.; Cashman, K. The effect of moderately and severely restricted dietary magnesium intakes on bone composition and bone metabolism in the rat. Br. J. Nutr. 1999, 82, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Xu, H.; Wang, C.; Qin, H.; An, Z. Magnesium enhances the chondrogenic differentiation of mesenchymal stem cells by inhibiting activated macrophage-induced inflammation. Sci. Rep. 2018, 8, 3406. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Skinner, J.; Hickson, M. Dietary Magnesium May Be Protective for Aging of Bone and Skeletal Muscle in Middle and Younger Older Age Men and Women: Cross-Sectional Findings from the UK Biobank Cohort. Nutrients 2017, 9, 1189. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas 2020, 133, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xing, F.; Sheng, N.; Xiang, Z. Associations of the Dietary Magnesium Intake and Magnesium Depletion Score with Osteoporosis among American Adults: Data from the National Health and Nutrition Examination Survey. Front. Nutr. 2022, 9, 883264. [Google Scholar] [CrossRef]
- Motooka, N.; Matsuo, H. The Affect of Lifestyle on Bone Mineral Density and Bone Turnover in Young Women. Kobe J. Med. Sci. 2020, 65, E124–E131. [Google Scholar]
- Wright, H.H.; Kruger, M.C.; Schutte, W.D.; Wentzel-Viljoen, E.; Kruger, I.M.; Kruger, H.S. Magnesium Intake Predicts Bone Turnover in Postmenopausal Black South African Women. Nutrients 2019, 11, 2519. [Google Scholar] [CrossRef]
- Cui, Y.; Cai, H.; Zheng, W.; Shu, X.O. Associations of Dietary Intakes of Calcium, Magnesium, and Soy Isoflavones with Bone Fracture Risk in Men: A Prospective Study. JBMR Plus 2021, 6, e10563. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, M.; Zhang, Y.; Wu, J.; Gao, M.; Lei, T.; Huang, F.; Chen, H.; Wu, M. Risk factors for the comorbidity of osteoporosis/osteopenia and kidney stones: A cross-sectional study. Arch. Osteoporos. 2023, 18, 128. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef]
- de Baaij, J.H.F. Magnesium reabsorption in the kidney. Am. J. Physiol. Renal Physiol. 2023, 324, F227–F244. [Google Scholar] [CrossRef]
- Tinawi, M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus 2021, 13, e12420. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, A.J.; Levine, B.S.; Rodriguez, M. Pathophysiology of Calcium, Phosphorus, and Magnesium Dysregulation in Chronic Kidney Disease. Semin. Dial. 2015, 28, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Suksridechacin, N.; Thongon, N. Fibroblast growth factor-23 and parathyroid hormone suppress small intestinal magnesium absorption. Physiol. Rep. 2022, 10, e15247. [Google Scholar] [CrossRef]
- Khan, N.B.; Nawaz, M.A.; Ijaz, A.; Memon, A.A.; Asif, N.; Khadim, M.T.; Sawal, H.A. Biochemical spectrum of parathyroid disorders diagnosed at a tertiary care setting. J. Pak. Med. Assoc. 2020, 70, 243–247. [Google Scholar] [CrossRef]
- Na, D.; Tao, G.; Shu-Ying, L.; Qin-Yi, W.; Xiao-Li, Q.; Yong-Fang, L.; Yang-Na, O.; Zhi-Feng, S.; Yan-Yi, Y. Association between hypomagnesemia and severity of primary hyperparathyroidism: A retrospective study. BMC Endocr. Disord. 2021, 21, 170. [Google Scholar] [CrossRef]
- Düğer, H.; Uçan, B.; Çalışkan, M.; Bostan, H.; Demirci, T.; Gül, Ü.; Çakal, E.; Kızılgül, M. Hypomagnesemia may be associated with symptomatic disease in patients with primary hyperparathyroidism. Endocrine 2024, 83, 466–472. [Google Scholar] [CrossRef]
- Saponaro, F.; Marcocci, C.; Apicella, M.; Mazoni, L.; Borsari, S.; Pardi, E.; Di Giulio, M.; Carlucci, F.; Scalese, M.; Bilezikian, J.P.; et al. Hypomagnesuria is Associated with Nephrolithiasis in Patients with Asymptomatic Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2020, 105, dgaa233. [Google Scholar] [CrossRef]

| First Author Year of Publication Reference Number | Study Design | Studied Population | Main Findings |
|---|---|---|---|
| Massimino 2023 [26] | Cross-sectional | N = 138 patients with T2DM (65.95% men + 34.06% women) | Mean Mg intake (mg/day) Men: 160 ± 55 Women: 160 ± 49 Adherence to Mg intake recommendations:
Women: 36.2%
Women: 2.1% |
| Li 2022 [27] | Retrospective | N = 2685 patients (48.2% men + 51.8% women) included: N1 = 173 patients with T2DM (57.23% men + 42.77% women): N2 = 2512 patients without T2DM (47.53% men + 52.47% women) | N1: No association between the adherence to DRI (Mg) and newly diagnosed T2DM: Men: OR (95% CI) = 0.70 (0.44–1.11) Women: OR (95% CI) = 0.89 (0.52–1.52) |
| First Author Year of Publication Reference Number | Study Design | Studied Population | Daily Mg Intake | Main Findings |
|---|---|---|---|---|
| Han 2024 [28] | Cross-sectional | N = 9950 patients with DM | Five quintiles based on daily Mg intake (Q1→Q5) Median (min, max) mg/day Q1: 156.5 (9.5–191) Q5: 455.25 (382–1721) | Adjusted models: OR (95% CI) between daily Mg intake and DM: Q2: 0.70 (0.53–0.92) Q3: 0.71 (0.53–0.97) Q4: 0.62 (0.45–0.87) Q5: 0.56 (0.39–0.81) p < 0.001 |
| Golmohamadi 2023 [31] | Prospective | N = 2188 patients with DM | Five quintiles based on daily Mg intake (Q1→Q5) Median (IQR) = 422 (335–524) mg | HR (95% CI) daily Mg intake and T2DM: Q2: 1.09 (0.81–1.49) Q3: 0.76 (0.53–1.3) p = 0.051 HR (95% CI) Mg intake ≥422 mg/day and T2DM according to BMI: normal weight: 0.33 (0.14–0.84) overweight: 1.02 (0.67–1.53) obese: 1.09 (0.76–1.57) p = 0.030 |
| Huang 2021 [32] | Cross-sectional | N = 10,249 (47.7% men + 52.3% women) included: N1 = 5094 patients with Mg intake < 267 mg/day (26% men + 64% women) N2 = 5155 patients with Mg intake >267 mg/day (57.7% men + 42.3% women) | Mean daily Mg intake (mg/day): N: 309.6 ± 3.1 N1: 191.7 ± 12 N2: 411.5 ± 2.5 | OR (95% CI) vitamin D and DM: N1: 0.925 (0.883–0.970), p = 0.002 N2: 0.968 (0.919–1.020), p = 0.225 p for interaction = 0.001 |
| Shah 2021 [29] | Cross-sectional | N = 20,480 patients included: N1 = 3432 patients with T2DM (51.6% me + 48.4% women) N2 = 17,048 patients without T2DM (47.4% men + 52.6% women) | Median Mg mg/day according to quintiles: Q1: 152 Q2: 215 Q3: 265 Q4: 328 Q5: 445 | In the overall population: OR (95% CI) daily Mg intake and T2DM: Adjustment for race, sex, age: Q2: 0.8 (0.7, 0.9) Q3: 0.8 (0.6, 0.9) Q4: 0.7 (0.5, 0.8) Q5: 0.5 (0.4, 0.6) p = 0.000 No statistically significant difference after further multivariable adjustments (p = 0.699 and p = 0.906 for model 2 and 3) Men: OR (95% CI) daily Mg intake and T2DM: Adjustment for race, sex, age: Q2: 0.9 (0.6, 1.2) Q3: 0.8 (0.6, 1.1) Q4: 0.7 (0.5, 0.9) Q5: 0.6 (0.4, 0.8) p = 0.01 No statistically significant difference after further multivariable adjustments (p = 0.566 and p = 0.787 for model 2 and 3) Women: OR (95% CI) daily Mg intake and T2DM: Adjustment for race, sex, age: Q2: 0.7 (0.6, 0.9) Q3: 0.7 (0.6, 0.9) Q4: 0.6 (0.5, 0.8) Q5: 0.4 (0.3, 0.6) p = 0.00 No statistically significant difference after further multivariable adjustments (p = 0.936 and p = 0.972 for model 2 and 3) OR (95% CI) Ca:Mg ratio and T2DM: Q2: 1.0 (0.8, 1.1) Q3: 1.0 (0.8, 1.2) Q4: 1.1 (0.9, 1.3) Q5: 1.2 (1.0, 1.5) p = 0.010 |
| McClure 2020 [30] | Cross-sectional | N = 4299 patients included: N1 = 947 patients with DM (51.7% men + 48.3% women) N2 = 3352 patients without DM (48.1% men + 51.9% women) | Mean (95% CI) Mg mg/2000 kcal: N1: 296 (285, 308) N2: 294 (286, 302) p = 0.739 | Daily Mg intake (mean; 95% CI) in mg/2000 kcal N1 (HbA1c > 9%): 272 (246, 299) N1 (HbA1c < 6.5%): 283 (268, 298) p = 0.007 |
| First Author Reference Number Year of Publication | Study Design | Studied Population | Daily Mg Intake | Main Findings |
|---|---|---|---|---|
| Chen 2024 [33] | Cross-sectional | N = 4249 patients (51.5% males + 48.7% women) included: N1 = 11.53% of the N (patients with DM) | Mg intake and haemoglobin glycation index: beta (95% CI) = −0.00016 (−0.0003, −0.00003), p = 0.019 N1: linear dose–response relationship, at ≥427 mg/day (for non-linearity p = 0.156) Non-N1: L-shaped dose–response relationship, with plateau at ≥495 mg/day (for non-linearity p = 0.565) | Mg intake mg/day: Median (IQR): overall: 248 (176–345) men: 284 (200–389.5) women: 212 (156–292) p < 0.001 |
| Kocyigit 2023 [36] | Case–control | N = 80 females included: N1 = 40 patients with T2DM N2 = 40 controls | Mean ± SD mg/day N1: 376.4 ± 103.62 N2: 402.2 ± 117.97 p = 0.303 % of dietary reference index N1: 67.5% N2: 75% p = 0.459 | N1: no correlation between daily Mg intake and: HbA1c: r = −0.273, p = 0.089 Insulin: r = −0.220, p = 0.172 |
| Wu 2020 [34] | Retrospective | N = 2831 patients with T2DM on oral medication included Non-Hispanic White: N1 = 1572 (46.7% men + 53.3% women) Non-Hispanic Black: N2 = 814 (44.2% men + 55.8% women) Mexican American: N3 = 445 (51.5% men + 48.5% women) | N1: Males: mean = 326.1 ± 168.7 mg/day N1: Females: mean = 265 ± 125 mg/day p < 0.001 N2: Males: mean = 288.7 ± 159.5 mg/day N2: Females: mean = 247.4 ± 128.9 mg/day p < 0.001 N3: Males: mean = 367.1 ± 196.7 mg/day N3: Females: mean = 293.4 ± 131.8 mg/day p < 0.001 | Path coefficients: Physical Activity > Mg: 0.205 Physical Activity > Mg > HbA1c: −0.026 Education > Mg: 0.152 Education > Mg > HbA1c: −0.019 Gender > Mg: 0.373 Gender > Mg > HbA1c: −0.047 |
| Ozcaliskan 2019 [35] | Cross-sectional | N = 119 with T2DM (21.85% men + 78.15% women) | <67% of the recommended daily allowance in 23.5% of subjects | Correlation between daily Mg intake and the following: Lean body mass: r = 0.268, p = 0.003 Waist-to-hip ratio: r = 0.213, p = 0.020 HbA1c: r = −0.107, p = 0.249 Fasting plasma glucose: r = −0.056, p = 0.550 Postprandial plasma glucose: r = −0.136, p = 0.186 BMI: r = −0.055, p = 0.550 Waist circumference: r = 0.156, p = 0.091 Body fat percentage: r = −0.149, p = 0.107 |
| First Author Reference Number Year of Publication Study Design | Studied Population | Daily Mg Intake | Main Findings |
|---|---|---|---|
| Diabetes-related complications | |||
| Bahrampour 2023 Case–control study [37] | N = 210 females with T2DM included: N1 = 105 patients with diabetic nephropathy N2 = 105 controls | High minerals group: 327.35 ± 33.26 mg Low minerals group: 291.66 ± 48.31 mg Principal factor loading of Mg for the mineral pattern = 0.794 | OR (95% CI) high mineral pattern and diabetic nephropathy: Crude model: 0.56 (0.32–0.97), p = 0.03 Adjusted model: 0.51 (0.28–0.95), p = 0.03 |
| Xu 2023 Retrospective study [38] | N = 3794 patients with DM: N1 = 791 patients with diabetic retinopathy (52.1% men + 47.9% women) N2 = 3003 patients without retinopathy (52.26% men + 47.44% women) | Median (IQR) N1: 250 (190–340) mg/day N2: 270 (200–360) mg/day p = 0.009 Q1: <200 mg/day Q2: 200–270 mg/day Q3: 270–360 mg/day Q4: ≥360 mg/day | OR (95% CI) Mg intake and diabetic retinopathy, adjusted for multiple variables: Q2: 0.73 (0.51–1.06), p = 0.093 Q3: 0.62 (0.43–0.92), p = 0.016 Q4: 0.48 (0.32–0.73), p = 0.001 U-shaped association between Mg intake and diabetic retinopathy: Stable risk when Mg reaches 380 mg/day (OR = 0.76, 95% CI: 0.60–0.76) Association not statistically significant for Mg over 480 mg/day (OR = 0.72, 95% CI: 0.52–1.00) |
| Zhang 2022 Cross-sectional study [39] | N = 4595 patients with DM (49.49% men + 50.51% women) | NA | Univariate regression: OR (95% CI) Mg intake and diabetic retinopathy Q2: 0.95 (0.77–1.17) Q3: 0.91 (0.73–1.13) Q4: 0.77 (0.61–0.96) Q5: 0.79 (0.64–0.99) Multivariate regression: OR (95% CI) Mg intake and diabetic retinopathy adjusted for sex and age: Q2: 0.94 (0.76–1.17) Q3: 0.90 (0.72–1.12) Q4: 0.76 (0.60–0.95) Q5: 0.78 (0.62–0.98) |
| Diabetes-related mortality | |||
| Wang 2022 Retrospective study [40] | N = 2045 patients with DM (49.1% men + 50.9% women) | ≤250 mg =983 (45.62%) >250 mg =1062 (54.38%) | HR (95% CI) Mg intake ≤250 mg/day and all-cause mortality: 1.56 (1.13–2.16) p < 0.01 other-cause mortality: 1.68 (1.09–2.60) p < 0.05 |
| Wang 2022 Prospective cohort [41] | N = 4699 patients with DM According to dietary antioxidant index (three tertiles) | Mean = 265.70 ± 1.71 mg/day | HR (96% CI) daily Mg intake and: all-cause mortality: tertile 2: 0.75 (0.61–0.91) tertile 3: 0.65 (0.51–0.81) p < 0.001 cardiovascular mortality: tertile 2: 0.71 (0.52–0.98) tertile 3: 0.69 (0.41–1.09) p = 0.093 cancer mortality: tertile 2: 0.59 (0.36–0.96) tertile 3: 0.8 (0.45–1.4) p = 0.460 |
| First Author Reference Number Year of Publication Study Design | Studied Population | Daily Mg Intake | Main Findings |
|---|---|---|---|
| Insulin resistance in general population | |||
| Kocyigit ** 2023 Case–control study [36] | N = 80 females included: N1 = 40 patients with T2DM N2 = 40 controls | Mean ± SD mg/day N1: 376.4 ± 103.62 N2: 402.2 ± 117.97 p = 0.303 | N1:correlation between daily Mg intake and HOMA-IR: r = −0.393, p = 0.012 |
| Mirrafiei 2022 Cross-sectional study [45] | N = 778 individuals (232 males + 546 females) | Mean ± SD mg/day Males: 294 ± 140 mg/day Females: 262 ± 112 mg/day p < 0.001 | No statistically significant association between daily Mg intake and: TyG: men β ± SE = 0.000 ± 0.000, p = 0.61 women β ± SE = −0.000 ± 0.000, p = 0.55 |
| Palacios 2022 Case–control study [42] | N = 10,609 individuals included: Prediabetes N1 = 3169 (48.8% men + 51.2% women) Normoglycemia N2 = 7740 (48.4% men + 51.6% women) | N1 ***: Q1 < 124.72 mg/day Q2: between 124.72 and 155.68 mg/day Q3: between 155.69 and 193.80 mg/day Q4 > 193.80 mg/day N2 ***: Q1 < 117.21 mg/day Q2: between 117.21 and 145.18 mg/day Q3 between 145.19 and 182.47 mg/day Q4 > 182.47 mg/day | Geometric mean (SE) HOMA-IR based on daily Mg intake quartiles: N1: Q1: 1.14 (0.03) Q2: 1.10 (0.03) Q3: 1.02 (0.05) Q4: 0.90 (0.03) p < 0.0001 N2: Q1: 0.78 (0.03) Q2: 0.79 (0.02) Q3: 0.71 (0.02) Q4: 0.61 (0.03) p < 0.0001 |
| Bavani 2021 Cross-sectional study [44] | N = 345 females | Mg intake (mg/kg) according to quintiles: Mean, SE (mg/day) Q1: 205, 7 Q2: 221, 8 Q3: 254, 7 Q4: 355, 9 | Mean (SE) QUICKI based on daily Mg intake quartiles: Multivariable adjustment: Q1: 0.34 (0.02) Q2: 0.36 (0.01) Q3: 0.40 (0.01) Q4: 0.39 (0.02) p = 0.02 After adjustment for endothelial dysfunction parameters, p = 0.09 No statistically significant association between daily Mg intake and fasting plasma glucose (p = 0.93), insulin (p = 0.08), HOMA-IR (p = 0.11) |
| Yang 2020 Cross-sectional study [43] | N = 8120 individuals | Mean Mg intake (mg/kg) according to quintiles: Q1: 2.91 ± 0.52 Q2: 4.09 ± 0.28 Q3: 5.14 ± 0.36 Q4: 7.66 ± 2.06 Mean (mg/day) Q1: 190.61 ± 46.16 Q2: 254.04 ± 45.52 Q3: 301.93 ± 52.99 Q4: 426.05 ± 124.43 | PR (95% CI) daily Mg intake per kg body weight and insulin resistance (adjustment for multiple variables) Q2: 0.589 (0.511, 0.678) Q3: 0.421 (0.360, 0.493) Q4: 0.250 (0.204, 0.306) p < 0.001 |
| Glucose profile in general population | |||
| Xu 2023 Cross-sectional study [47] | N = 2373 individuals (37.5% men + 62.5% women) | Median (IQR): 205.94 (144–249) mg/day | Adjusted models for relationship between the following: Daily Mg intake and HbA1c: beta (96% CI): overall: 0.072 (−0.019, 0.405), p = 0.074 men: 0.132 (−0.007, 0.771), p = 0.054 women: 0.033 (−0.165, 0.330), p = 0.512 Daily Mg intake and fasting plasma glucose: beta (96% CI): overall: −0.087 (−0.512, −0.034), p = 0.025 men: −0.061 (−0.610, 0.221), p = 0.358 women: −0.098 (−0.597, −0.014), p = 0.040 |
| Bentil 2021 Cross-sectional, pilot study [48] | N = 63 women Mean age = 29.5 ± 8.5 years BMI: underweight 1.6%, normal weight 54%, overweight 30.2%, obese 14.3% | Mean = 200 ± 116 mg 15.9% met RDA | Association between daily Mg intake and fasting blood glucose: Beta coefficient [95% CI]: unadjusted model: 0.31 (0.07–0.55), p = 0.01 adjusted for age and BMI: 0.22 (−0.03–0.46), p = 0.08 Association between daily Mg intake and HbA1c: Beta coefficient [95% CI]: unadjusted model: 0.26 (0.01–0.51), p = 0.04 adjusted for age and BMI: 0.15 (−0.08–0.39), p = 0.2 |
| Yan 2019 Cross-sectional study [46] | N = 8322 individuals (50.1% men + 49.9% women) | Mean (95% CI) Males: 372.71 (362.48, 382.94) mg/day Females: 305.34 (294.20, 316.48) mg/day | Regression coefficients for daily Mg intake and fasting plasma glucose quantiles: Men: 0.1: −4.165 (−5.612, −0.411) 0.2: −4.926 (−8.654, −1.038) 0.3: −5.093 (−8.407, −1.436) 0.4: −4.670 (−7.303, −0.611) 0.5: −3.387 (−6.739, −0.480) 0.6: −1.803 (−7.486, −0.182) 0.7: −3.473 (−7.141, −1.137) 0.8: −4.036 (−6.633, −2.231) 0.9: −5.208 (−11.844, −2.033) p < 0.05 Women: 0.3: −4.300 (−7.034, −0.739) 0.4: −3.244 (−6.630, −0.913) 0.6: −2.141 (−5.627, −0.214) 0.7: −3.983 (−8.388, −0.616) 0.8: −7.295 (−11.42, −1.695) 0.9: −9.674 (−16.319, −0.700) p < 0.05 |
| First Author Reference Number Year of Publication Study Design | Studied Population | Mg Supplementation Dose Per Day | Outcome |
|---|---|---|---|
| Drenthen 2024 Randomized controlled trial [52] | N = 14 patients (50% women) with insulin-treated T2DM and hypomagnesaemia (serum Mg ≤0.79 mmol/L) Mean age = 67 ± 6 years Mean BMI = 31 ± 5 kg/m2 | Supplementation with 150 mL Mg gluconate (=360 mg Mg) | Mg versus placebo: insulin dose p = 0.869 HbA1c p = 0.851 |
| Salehidoost 2022 Randomized controlled trial [53] | N = 86 patients with prediabetes (21.1% men + 78.9% women) Magnesium supplementation: N1 = 37 (24.33% men + 75.67% women) Mean age = 56.7 ± 5.9 years Placebo: N2 = 34 (17.65% men + 82.35% women) Mean age = 54.8 ± 4.9 years | Supplementation with 250 mg Mg oxide | Mg versus placebo: HOMA-IR p = 0.17 HbA1c p = 0.63 fasting plasma glucose p = 0.57 |
| Ham 2020 Randomized double-blind and crossover trial [50] | N = 37 patients with prediabetes | Supplementation with 350 mg Mg | Balanced deep-sea water with 350 mg Mg/440 mL versus placebo: change in HOMA-IR: −0.27 ± 1.01 versus 0.17 ± 0.70, p = 0.049 change in fasting plasma glucose: 0.73 ± 4.88 versus 0.46 ± 6.91, p = 0.837 |
| Hamedifard 2020 Randomized double-blind controlled trial [49] | N = 55 females with T2DM and coronary heart disease Mg and zinc: N1 = 27 Mean age = 61.7 ± 9.4 years Placebo: N2 = 28 Mean age = 62.6 ± 10.8 years | Supplementation with 250 mg Mg oxide + 150 mg Zn sulphate | Supplementation with 250 mg Mg oxide and 150 mg Zn sulphate versus placebo β (95% CI): fasting plasma glucose: −9.44 (−18.30, −0.57), p = 0.03 insulin: −1.37 (−2.57, −0.18), p = 0.02 HOMA-IR: −0.36 (− 0.75, 0.02), p = 0.06 QUICKI: 0.006 (−0.002, 0.01), p = 0.12 |
| Afitska 2021 Randomized controlled trial [51] | N = 24 patients with metabolic syndrome and normal serum Mg (41.7% men + 58.3% women) N1 = 13 (39% men + 61% women) Mean age = 61.8 ± 10.7 years Placebo: N2 = 11 (45% men + 55% women) Mean age = 71.9 ± 7.8 years | Supplementation with 400 mg Mg citrate | Change in HbA1c in Mg group: –0.28 ± 0.27, p = 0.0036 Mg versus placebo: p = 0.02 |
| Specific outcomes in diabetic nephropathy | |||
| Halawa 2023 Randomized controlled trial [55] | N = 54 patients with T2DM and nephropathy (59% men + 41% women) (Mg) N1 = 26 (61.5% men + 38.5% women) Mean age = 61.4 ± 7.5 years (Control) N2 = 28 (57.1% men + 42.9% women) Mean age = 63 ± 7.2 years | Supplementation with 2.25 g Mg citrate (=360 mg Mg) | Mg supplementation versus placebo → percent change in: urinary-albumin-to-creatinine ratio: −6.87 (−9.17, −4.84) versus −0.9 (−1.797, −1.58), p = 0.001 eGFR: 21.74 (12.14, 37.41) versus 0 (−4.01, − 0), p = 0.001 |
| Sadeghian 2020 Randomized double-blind, controlled trial [54] | N = 80 patients with T2DM and early-stage diabetic nephropathy (Mg) N1 = 40 (65.9% women, 34.1% men) Mean age = 41.2 ± 8.8 years (Placebo) N2 = 40 (67.5% women, 32.5% men) Mean age = 42.8 ± 8.4 years | Mg supplementation group: mean dietary intake = 195.5 ± 77.7 mg/day Placebo group: mean Mg dietary intake = 210.5 ± 160.3 mg/day | Mg supplementation with 250 mg Mg oxide versus placebo → change in: microalbuminuria: −14 ± 9.9 versus −3.1 ± 2.2, p = 0.09 HbA1c: 0.09 ± 1.3 versus 0.29 ± 0.1, p = 0.36 fasting plasma glucose: −1.4 ± 58.5 versus −7.9 ± 39.2, p = 0.47 HOMA-IR: 1.9 ± 4 versus 0.2 ± 2.2, p = 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, A.-M.; Ciobica, M.-L.; Nistor, C.; Gurzun, M.-M.; Sandulescu, B.-A.; Stanciu, M.; Popa, F.L.; Carsote, M. Inquiry of the Metabolic Traits in Relationship with Daily Magnesium Intake: Focus on Type 2 Diabetic Population. Clin. Pract. 2024, 14, 1319-1347. https://doi.org/10.3390/clinpract14040107
Gheorghe A-M, Ciobica M-L, Nistor C, Gurzun M-M, Sandulescu B-A, Stanciu M, Popa FL, Carsote M. Inquiry of the Metabolic Traits in Relationship with Daily Magnesium Intake: Focus on Type 2 Diabetic Population. Clinics and Practice. 2024; 14(4):1319-1347. https://doi.org/10.3390/clinpract14040107
Chicago/Turabian StyleGheorghe, Ana-Maria, Mihai-Lucian Ciobica, Claudiu Nistor, Maria-Magdalena Gurzun, Bianca-Andreea Sandulescu, Mihaela Stanciu, Florina Ligia Popa, and Mara Carsote. 2024. "Inquiry of the Metabolic Traits in Relationship with Daily Magnesium Intake: Focus on Type 2 Diabetic Population" Clinics and Practice 14, no. 4: 1319-1347. https://doi.org/10.3390/clinpract14040107
APA StyleGheorghe, A.-M., Ciobica, M.-L., Nistor, C., Gurzun, M.-M., Sandulescu, B.-A., Stanciu, M., Popa, F. L., & Carsote, M. (2024). Inquiry of the Metabolic Traits in Relationship with Daily Magnesium Intake: Focus on Type 2 Diabetic Population. Clinics and Practice, 14(4), 1319-1347. https://doi.org/10.3390/clinpract14040107










