Anterior Hyperfunction Syndrome: Literature Review and Conceptual Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
3. Results
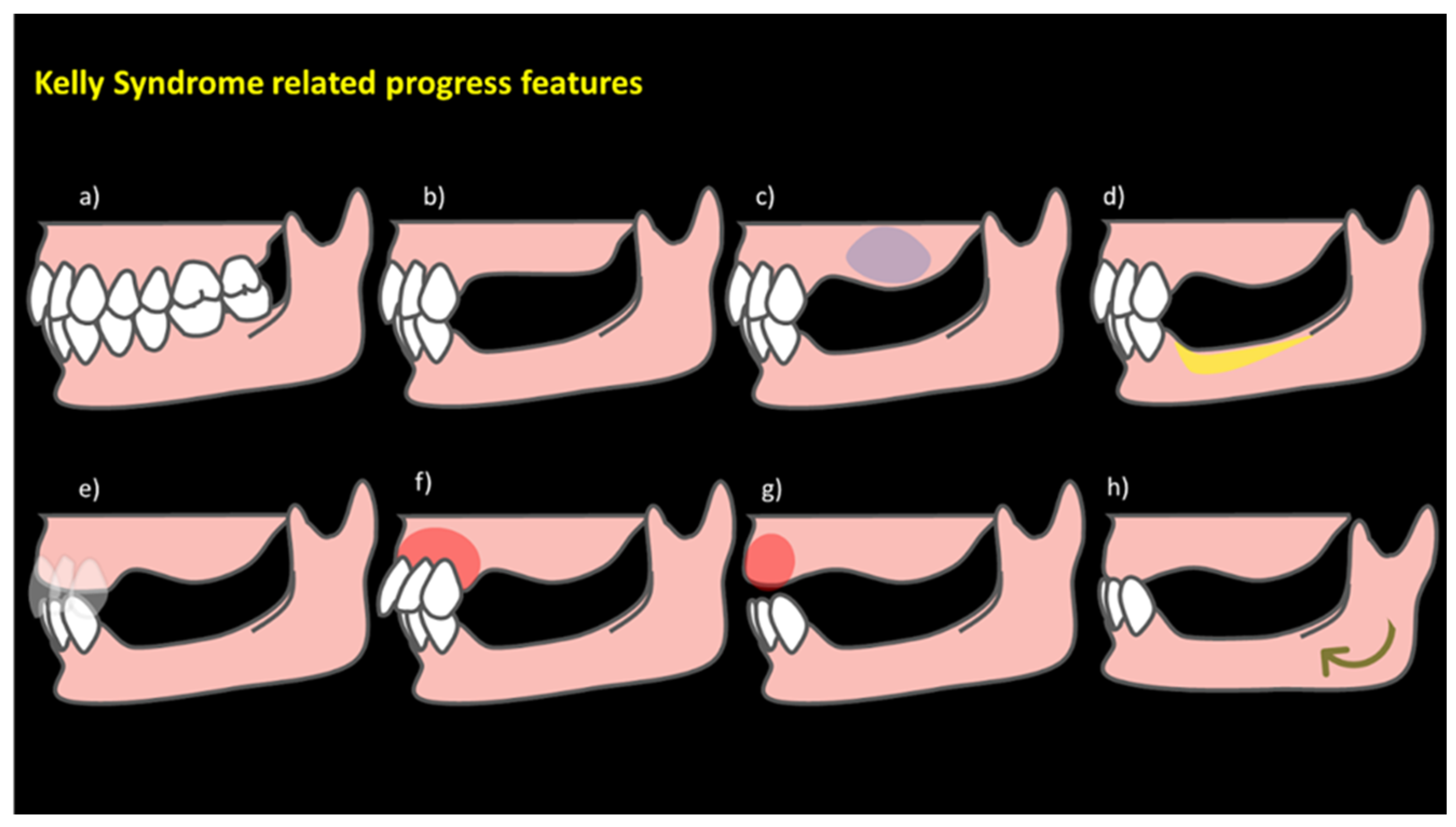
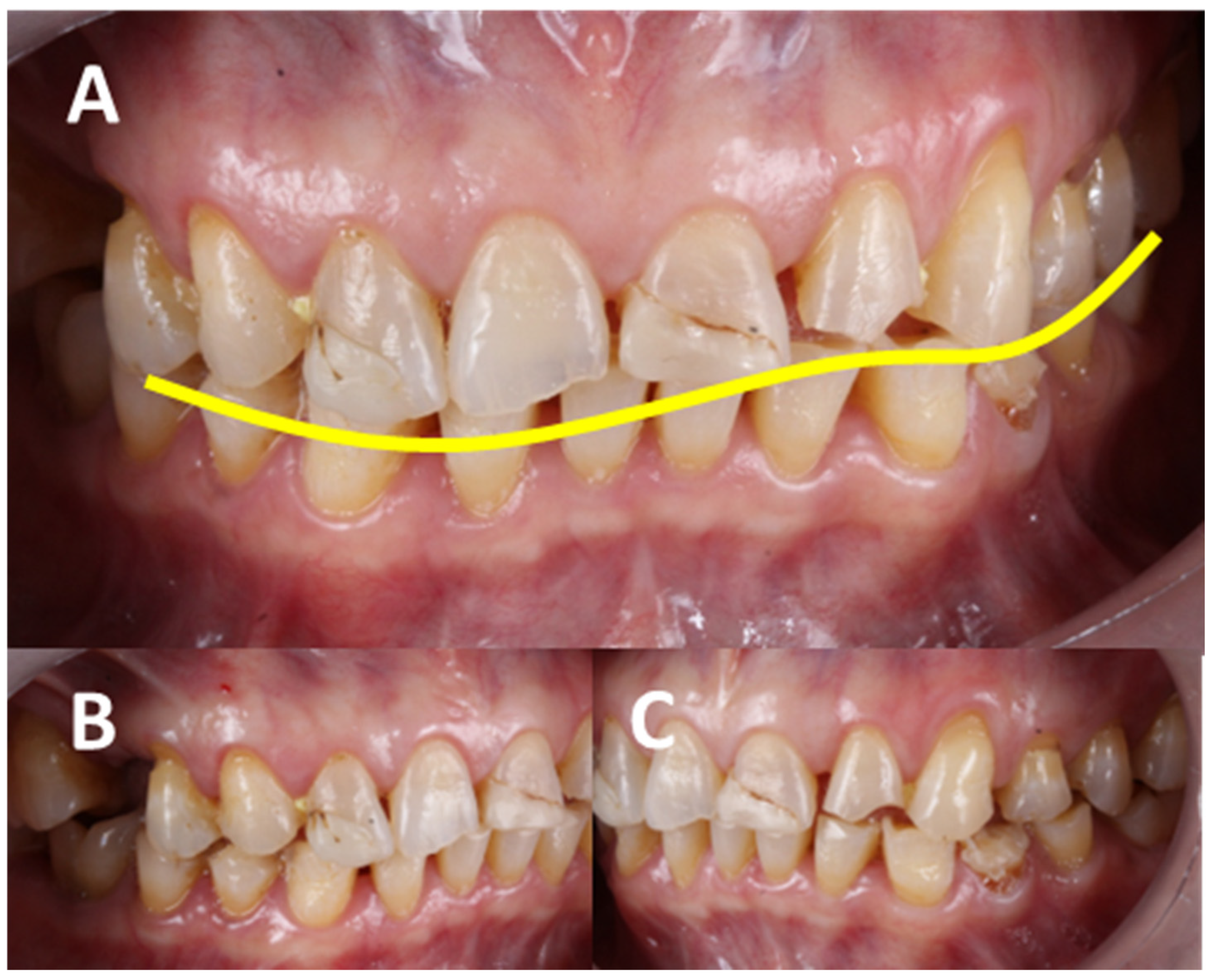
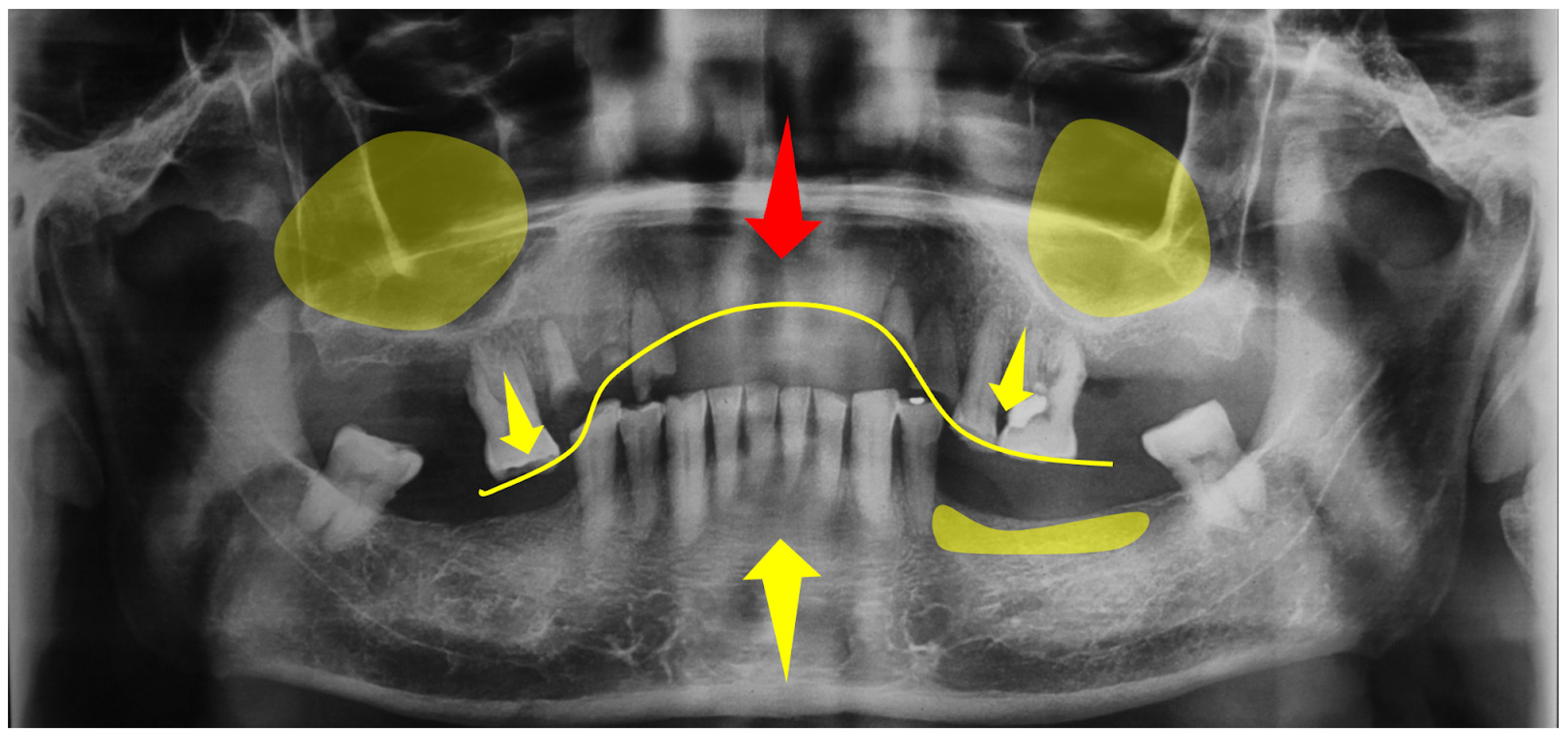
3.1. Assessment and Diagnosis of AHS Risk Factors
3.2. Epidemiology
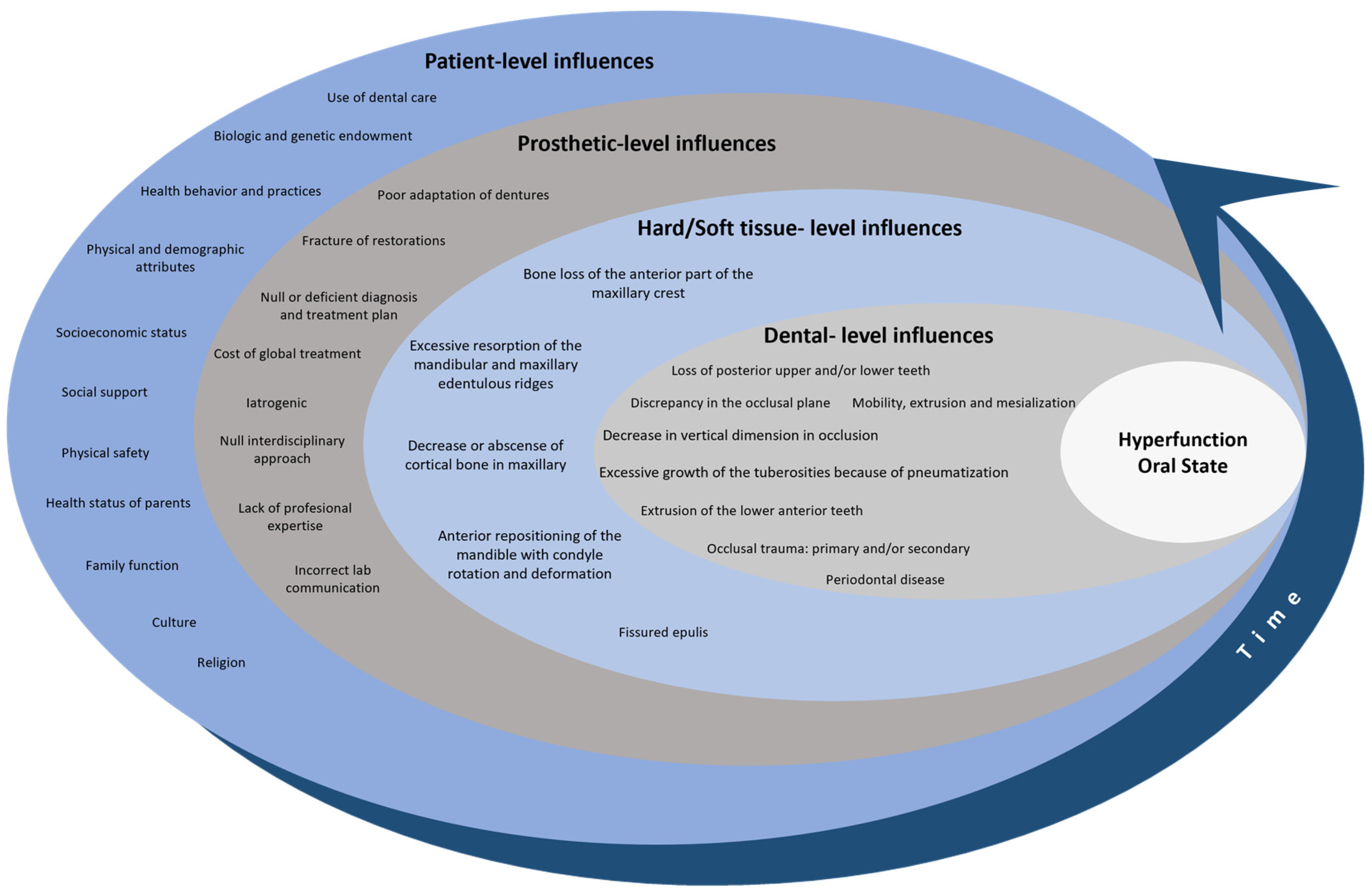
3.3. Hyperfunction Oral State: A Comprehensive Overview for a Conceptual Model
3.3.1. Patient-Level Influences
3.3.2. Prosthetic-Level Influences
3.3.3. Hard/Soft Tissue-Level Influences
3.3.4. Dental-Level Influences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, E. Changes Caused by a Mandibular Removable Partial Denture Opposing a Maxillary Complete Denture. J. Prosthet. Dent. 2003, 90, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Saunders, T.R.; Gillis, R.E.; Desjardins, R.P. The Maxillary Complete Denture Opposing the Mandibular Bilateral Distal-Extension Partial Denture: Treatment Considerations. J. Prosthet. Dent. 1979, 41, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L. Combination Syndrome: Classification and Case Report. J. Oral Implant. 2007, 33, 139–151. [Google Scholar] [CrossRef]
- Driscoll, C.F.; Freilich, M.A.; Guckes, A.D.; Knoernschild, K.L.; Mcgarry, T.J.; Goldstein, G.; Goodacre, C.; Guckes, A.; Mor, S.; Rosenstiel, S.; et al. The Glossary of Prosthodontic Terms. J. Prosthet. Dent. 2017, 117, E1–E105. [Google Scholar] [CrossRef]
- Langer, Y.; Laufer, B.; Cardash, H.S. Modalities of Treatment for the Combination Syndrome. J. Prosthodont. 1995, 4, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L. Management of Biomechanical Complication of Implant-Supported Restoration of a Patient with Combination Syndrome: A Case Report. J. Oral Maxillofac. Surg. 2009, 67, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Gauer, R.L.; Semidey, M.J. Diagnosis and Treatment of Temporomandibular Disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar]
- Patel, M.; Alani, A. Clinical Issues in Occlusion—Part II. Singap. Dent. J. 2015, 36, 2–11. [Google Scholar] [CrossRef]
- Tolstunov, L. Combination Syndrome Symptomatology and Treatment. Compend. Contin. Educ. Dent. 2011, 32, 62–66. [Google Scholar]
- Ogino, Y.; Kihara, M.; Yamada, J.; Toriya, K.; Koyano, K. Implant Treatments for Edentulous Maxilla with Anterior Hyperfunction. J. Oral Implant. 2015, 41, 731–735. [Google Scholar] [CrossRef]
- Kumar, L.; Singla, S. “Kelly’s Syndrome”—Prevention, Using Implant Supported Hybrid Denture: Clinical Considerations and Case Report with 5 Year Follow Up. IOSR J. Dent. Med. Sci. 2017, 16, 21–26. [Google Scholar] [CrossRef]
- de Kanter, R.J.A.M.; Battistuzzi, P.G.F.C.M.; Truin, G.-J. Temporomandibular Disorders: “Occlusion” Matters! Pain Res. Manag. 2018, 2018, 8746858. [Google Scholar] [CrossRef] [PubMed]
- Ogino, Y.; Ayukawa, Y. Anterior Hyperfunction by Mandibular Anterior Teeth: A Narrative Review. Healthcare 2023, 11, 2967. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, G.E. Responses of Jawbone to Pressure. Gerodontology 2004, 21, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Buzayan, M.M.; Sivakumar, I.; Choudhary, S.; Tawfiq, O.; Mahdey, H.M.; Binti Mahmood, W.A.A. Prosthodontic Management of Combination Syndrome Case with Metal Reinforced Maxillary Complete Denture and Mandibular Teeth Supported Overdenture. Periodontics Prosthodont. 2018, 4, 105. [Google Scholar] [CrossRef]
- Schmitt, S.M. Combination Syndrome: A Treatment Approach. J. Prosthet. Dent. 1985, 54, 664–671. [Google Scholar] [CrossRef]
- Shen, K.; Gongloff, R.K. Prevalence of the ‘Combination Syndrome’ among Denture Patients. J. Prosthet. Dent. 1989, 62, 642–644. [Google Scholar] [CrossRef]
- Jameson, W.S. The Use of Linear Occlusion to Treat a Patient with Combination Syndrome: A Clinical Report. J. Prosthet. Dent. 2001, 85, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Cabianca, M. Combination Syndrome: Treatment with Dental Implants. Implant. Dent. 2003, 12, 300–305. [Google Scholar] [CrossRef]
- Palmqvist, S.; Carlsson, G.E.; Wall, B. The Combination Syndrome: A Literature Review. J. Prosthet. Dent. 2003, 90, 270–275. [Google Scholar] [CrossRef]
- Madan, N.; Datta, K. Combination Syndrome. J. Indian Prosthodont. Soc. 2006, 6, 10–13. [Google Scholar] [CrossRef]
- Salvador, M.C.G.; do Valle, A.L.; Ribeiro, M.C.M.; Pereira, J.R. Assessment of the Prevalence Index on Signs of Combination Syndrome in Patients Treated at Bauru School of Dentistry, University of Sao Paulo. J. Appl. Oral Sci. 2007, 15, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D. Screwless Fixed Detachable Partial Overdenture Treatment for Atrophic Partial Edentulism of the Anterior Maxilla. J. Oral Implant. 2008, 34, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Daher, T.; Dermendjian, S.; Morgano, S.M. Obtaining Maxillomandibular Records and Definitive Impressions in a Single Visit for a Completely Edentulous Patient with a History of Combination Syndrome. J. Prosthet. Dent. 2008, 99, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.C.; Preoteasa, E. The Anterior Hyperfunction Syndrom—Fem Simulation. Rom. J. Oral Rehabil. 2009, 1, 54–58. [Google Scholar]
- Gerritsen, A.E.; Allen, P.F.; Witter, D.J.; Bronkhorst, E.M.; Creugers, N.H. Tooth Loss and Oral Health-Related Quality of Life: A Systematic Review and Meta-Analysis. Health Qual. Life Outcomes 2010, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, N.; Shah, N.; Karthik, M.M. Prosthodontic Rehabilitation of Patients with Combination Syndrome. Int. J. Dent. Clin. 2010, 2, 37–44. [Google Scholar]
- Rao, S.; Kumar, G.; Kumar, M.; AD, M. Enigma of Combination Syndrome and Its Prosthodontics Way of Management: A Case Report. J. Int. Oral Health 2011, 3, 51–56. [Google Scholar]
- Kilicarslan, M.A.; Akaltan, F.; Kasko, Y.; Kocabas, Z. Clinical Evaluation of Maxillary Edentulous Patients to Determine the Prevalence and Oral Risk Factors of Combination Syndrome. J. Dent. Sci. 2014, 9, 394–399. [Google Scholar] [CrossRef]
- Peñarrocha, M.; Viña, J.A.; Carrillo, C.; Peñarrocha, D.; Peñarrocha, M. Rehabilitation of Reabsorbed Maxillae with Implants in Buttresses in Patients with Combination Syndrome. J. Oral Maxillofac. Surg. 2012, 70, e322–e330. [Google Scholar] [CrossRef]
- Feng, S.-W.; Liao, P.-B.; Chen, M.-S. Prosthodontic Treatment of a Patient with Combination Syndrome: A Clinical Case Report. J. Prosthodont. Implantol. 2012, 1, 22–25. [Google Scholar]
- Ibrahim, T.O.; Ibrahim, R.O. Evaluation of Two Treatment Modalities for Patients with Combination Syndrome Suffering from Narrow Anterior Maxilla. Life Sci. J. 2013, 10, 2199–2210. [Google Scholar]
- de Resende, C.M.B.M.; Ribeiro, J.A.M.; Dias, K.d.C.; Carreiro, A.d.F.P.; do Rego, M.P.P.; de Queiroz, J.W.N.; Barbosa, G.A.S.; Oliveira, Â.G.R.d.C. Signs of Combination Syndrome and Removable Partial Denture Wearing. Rev. Odontol. UNESP 2014, 43, 390–395. [Google Scholar] [CrossRef]
- Carlino, P.; Pettini, F.; Cantore, S.; Ballini, A.; Grassi, F.R.; Pepe, V. Surgical and Prosthetic Rehabilitation of Combination Syndrome. Case Rep. Dent. 2014, 2014, 186213. [Google Scholar] [CrossRef] [PubMed]
- Ucar Barroeta, A.; Berríos, M. El Síndrome de Combinación En Relación Con Rehabilitaciones Bucales Inadecuadas. MedULA Rev. Fac. Med. 2015, 24, 0798–3166. [Google Scholar]
- De Oliveira Pinto, A.F.; Gonçalves Júnior, N.O.; Teixeira Rodrigues, C.R. Hyperfunction Previous Syndrome: Case Report. Braz. J. Surg. Clin. Res. 2015, 12, 27–32. [Google Scholar]
- Narwal, A.; Chugh, A.; Rohtak, P.; Swami, R. Prosthodontic Management of a Patient with Combination Syndrome: A Clinical Case Report. Int. J. Adv. Res. 2015, 4, 509–513. [Google Scholar]
- Rajendran, S.; Baburajan, F. Combination Syndrome. Int. J. Prosthodont. Restor. Dent. 2012, 2, 156–160. [Google Scholar] [CrossRef]
- Reddy Juturu, R.K.; Mannava, P.; Preet Singh, H. Prevalence of Signs of Combination Syndrome: A Clinical Study. Saudi J. Oral Dent. Res. 2016, 1, 164–166. [Google Scholar]
- Korunoska-Stevkovska, V.; Guguvcevski, L.; Menceva, Z.; Gigovski, N.; Mijoska, A.N.; Nikolovska, J.; Bajraktarova-Valjakova, E. Prosthodontic Rehabilitation of Patient with Anterior Hyper Function Syndrome. Open Access Maced. J. Med. Sci. 2017, 5, 1000–1004. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, V.P.; Atri, B. Assessment of Signs of Combination Syndrome in Study Population: A clinical study. J. Adv. Med. Dent. Sci. Res. 2018, 6, 28–30. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, M.; Batra, R.; Sharma, C.; Mehta, S. Prosthodontic Management of Patient with Anterior Hyperfunction Syndrome: A Clinical Challenge. Dent. J. Adv. Stud. 2019, 6, 129–133. [Google Scholar] [CrossRef]
- Akhtar, A.; Qadeer, A.; Shakir, S. Frequency of Signs of Kelly’s Syndrome in Patients Presenting to the Prosthodontic Department of Khyber College of Dentistry. J. Khyber Coll. Dent. 2019, 9, 10–15. [Google Scholar]
- Bagga, R.; Robb, N.D.; Fenlon, M.R. An Investigation into the Prevalence of Combination Syndrome. J. Dent. 2019, 82, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Penitente, P.; Freitas da Silva, E.; do Vale Souza, J.; de Freitas Jorge, C.; Bueno Carlini Bittencourt, A.; dos Santos, D.; Lamartine de Moraes Melo Neto, C.; Coelho Goiato, M. Combination Syndrome: A Literature Review of General Aspects and Treatments. J. Stomatol. 2022, 75, 55–59. [Google Scholar] [CrossRef]
- Merriam-Webster. Definition of Hyperfunction. In Merriam-Webster’s Collegiate Dictionary; Merriam-Webster: Springfield, IL, USA, 2021. [Google Scholar]
- Oxford University Press. Definition of Hyperfunction. In Oxford English Dictionary; Editorial Team: Oxford, UK, 2021. [Google Scholar]
- Fisher-Owens, S.A.; Gansky, S.A.; Platt, L.J.; Weintraub, J.A.; Soobader, M.-J.; Bramlett, M.D.; Newacheck, P.W. Influences on Children’s Oral Health: A Conceptual Model. Pediatrics 2007, 120, e510–e520. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.I.; Tellez, M.; Pitts, N.B.; Ekstrand, K.R.; Ricketts, D.; Longbottom, C.; Eggertsson, H.; Deery, C.; Fisher, J.; Young, D.A.; et al. Caries Management Pathways Preserve Dental Tissues and Promote Oral Health. Community Dent. Oral Epidemiol. 2013, 41, e12–e40. [Google Scholar] [CrossRef] [PubMed]
- Reda, S.M.; Krois, J.; Reda, S.F.; Thomson, W.M.; Schwendicke, F. The Impact of Demographic, Health-Related and Social Factors on Dental Services Utilization: Systematic Review and Meta-Analysis. J. Dent. 2018, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E. Factors Contributing to Dentists’ Extraction Decisions in Older Aduits. Spec. Care Dent. 1993, 13, 195–199. [Google Scholar] [CrossRef]
- Appukuttan, D. Strategies to Manage Patients with Dental Anxiety and Dental Phobia: Literature Review. Clin. Cosmet. Investig. Dent. 2016, 10, 35–50. [Google Scholar] [CrossRef]
- Al-Nasser, L.; Lamster, I.B. Prevention and Management of Periodontal Diseases and Dental Caries in the Older Adults. Periodontol. 2000 2020, 84, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Harish, P.; Joseph, S.A.; Sirajuddin, S.; Gundapaneni, V.; Chungkham, S.A. Iatrogenic Damage to the Periodontium Caused by Fixed Prosthodontic Treatment Procedures. Open Dent. J. 2015, 9, 190–196. [Google Scholar] [CrossRef]
- Brantley, C.F.; Bader, J.D.; Shugars, D.A.; Nesbit, S.P. Does The Cycle of Rerestoration Lead To Larger Restorations? JADA 1995, 126, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.Q.; Gonda, T.; Maeda, Y.; Ikebe, K. Average Rate of Ridge Resorption in Denture Treatment: A Systematic Review. J. Prosthodont. Res. 2021, 65, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, F.; Zafeiriadis, A.A.; Hans, M.G. Growth of the Craniofacial Complex. In Pediatric Dentistry; Kotsanos, N., Sarnat, H., Park, K., Eds.; Textbooks in Contemporary Dentistry; Springer: Cham, Switzerland, 2022; pp. 155–175. [Google Scholar] [CrossRef]
- Nassani, M.Z. Aspects of Malpractice in Prosthodontics. J. Prosthodont. 2017, 26, 672–681. [Google Scholar] [CrossRef]
- Markovics, D.; Szendi, R.; Vicko, K.; Rajnics, Z.; Marada, G.; Radnai, M. Incidence of Combination Syndrome Based on the Orthopantomograms Made between 2009 És 2014 at the Department of Prosthodontics, University of Pécs, Hungary. Fogorvosi Szle. 2016, 109, 23–27. [Google Scholar] [CrossRef]



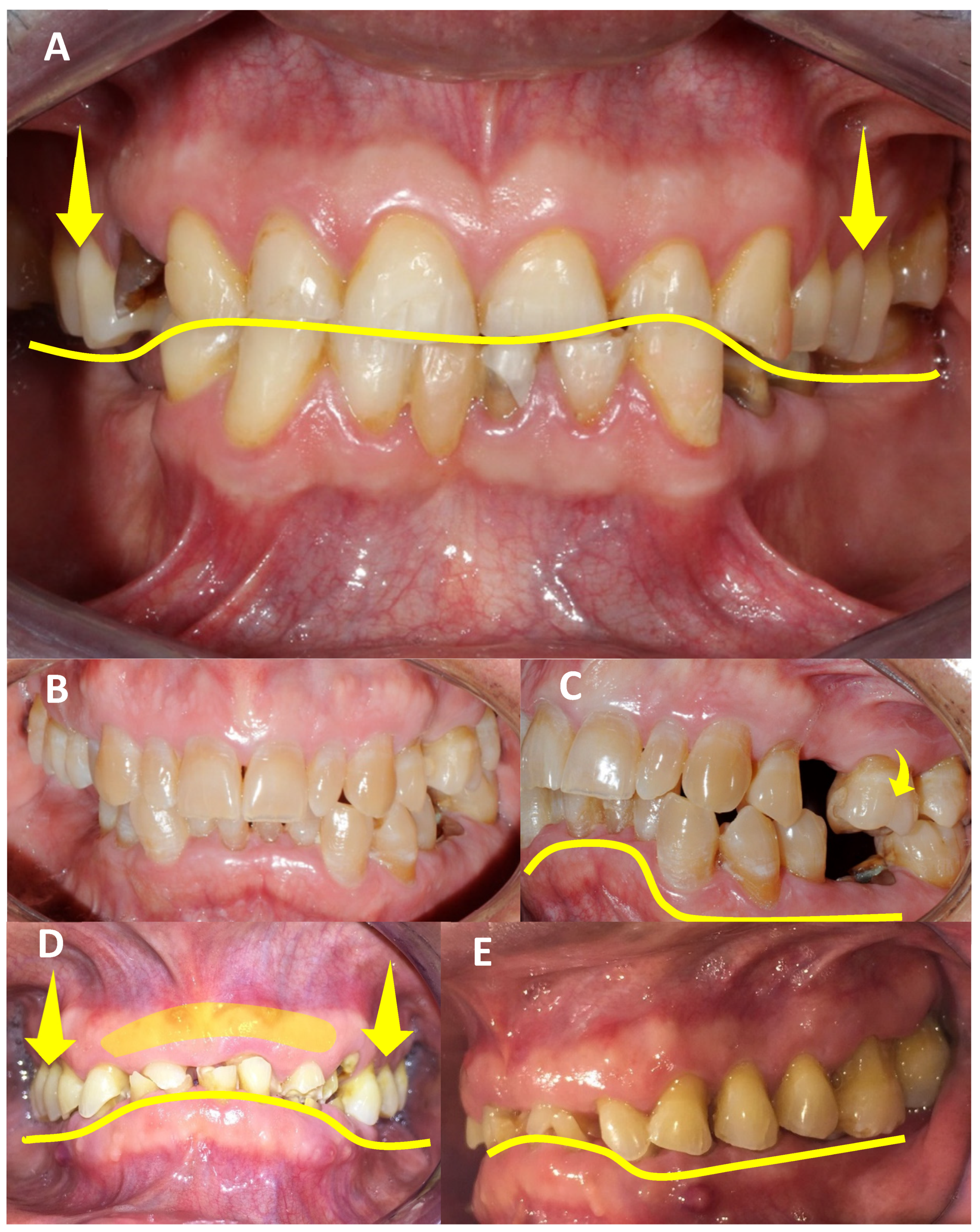
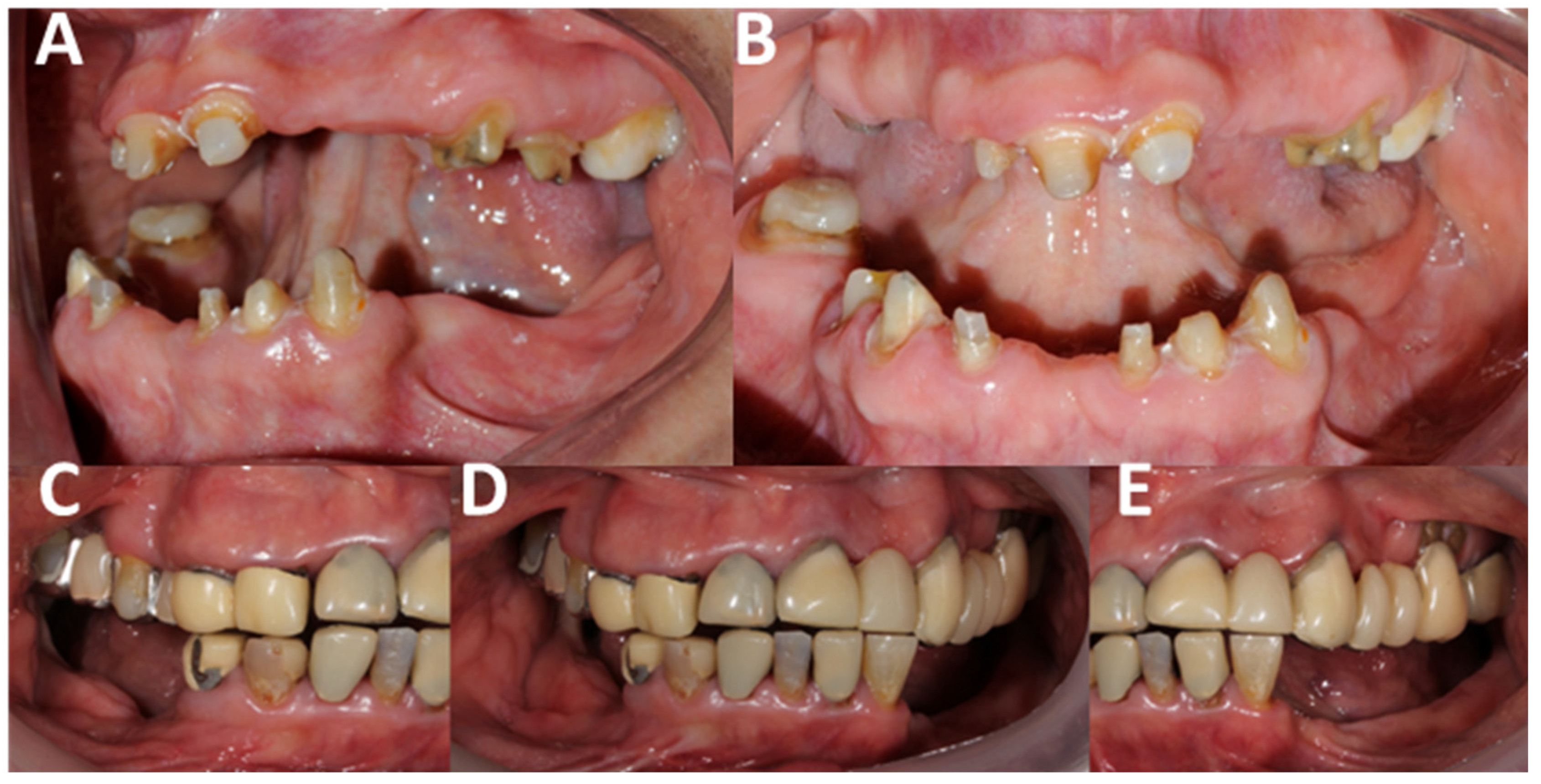
| Authors | AHS Risk Factors |
|---|---|
| Kelly, 1972 [1] | Bone loss of the anterior part of the maxillary crest; excessive growth of tuberosities; papillary hyperplasia in the hard palate; extrusion of the lower anterior teeth; excessive resorption of the mandibular edentulous ridges; excessive force; shearing forces; inadequate denture base coverage; underlying systemic causes |
| Saunders et al., 1979 [2] | Loss of vertical dimension in occlusion; discrepancy in the occlusal plane; anterior repositioning of the mandible; poor adaptation of prostheses; epulis fissuratum; periodontal disease; systemic disease; caries; oral hygiene |
| Schmitt et al., 1983 [16] | Occlusal stress |
| Shen et al., 1989 [17] | Periodontal disease |
| Langer et al., 1995 [5] | Poorly designed mandibular RPD |
| Jameson et al., 2001 [18] | Excessive anterior force |
| Cabianca et al., 2003 [19] | Posterior tooth loss; unstable occlusal plane |
| Palmqvist et al., 2003 [20] | Supraerupted maxillary molars; artificial denture teeth; |
| Carlsson et al., 2004 [14] | Anatomy; psychosocial aspects, mechanical devices; gender; age; facial morphology; duration of edentulousness; denture wearing habits; number of dentures worn; oral hygiene; parafunctions; occlusal loading; denture quality; nutrition; general health; medication; systemic; diseases; osteoporosis; corticosteroid treatment for asthma |
| Madan et al., 2006 [21] | Combination of complete maxillary dentures opposing class I mandibular RPD; retaining weak posterior teeth as abutments; conventional lower denture |
| Gonzalves et al., 2007 [22] | Lack of prosthesis adaptation |
| Tolstunov, 2007 [3] | Type of edentulism |
| Flanagan et al., 2008 [23] | Inappropriate treatment |
| Daher et al., 2008 [24] | Lack of professional expertise |
| Magureanu et al., 2009 [25] | Excessive pression in frontal region |
| Tolstunov, 2009; 2011 [6,9] | Abnormal traumatic occlusal forces |
| Gerritsen et al., 2010 [26] | Low quality of life |
| Jyoti et al., 2010 [27] | Null or deficient diagnosis and treatment plan |
| Rao et al., 2011 [28] | Deficient mandibular RPD |
| Kilicarslan et al., 2012 [29] | Edentulous maxilla |
| Peñarrocha et al., 2012 [30] | Marginal bone loss related to maxillary atrophy class |
| Feng et al., 2012 [31] | Edentulous maxilla opposed to natural mandibular anterior teeth; distal-extension RPD |
| Ibrahim et al., 2013 [32] | Low quality of bone in edentulous maxilla; deficient diagnosis; time |
| Resende et al., 2014 [33] | Mandibular RPD with inadequate technical quality; RPD absence |
| Carlino et al., 2014 [34] | Complete maxillary denture opposing complete denture attached to implants by bars or ball attachments; biomechanical stress to anterior maxilla of implants supported by prosthesis; lack of pre-prosthetic surgical intervention; no consideration of occlusion, vertical dimension, or occlusal plane; lack of follow-up |
| Barroeta et al., 2015 [35] | Deficient diagnosis; inadequate oral rehabilitation, lack of professional expertise; absence of lower RPD, inadequate lower RPD; lack of preventive treatment; decreased vertical dimension; maladaptation of the upper prosthesis; inverted prosthetic plane |
| Oliveira et al., 2015 [36] | Lack of diagnosis of the patient’s characteristics before treatment; combination of an upper tissue-supported prosthesis with lower RPD; inadequate occlusal schemes in prostheses; failure to eliminate the contact between the anterior teeth and the lower teeth |
| Narwal et al., 2015 [37] | Increasing pressure on the premaxillary alveolar ridge; loss of adequate posterior occlusal |
| Patel et al., 2015 [8] | Incorrect and inappropriate occlusal diagnosis for treatment planning |
| Rajendran et al., 2015 [38] | Lack of evaluation of dental history and the condition of the remaining mandibular anterior teeth; stress on the maxillary ridge, angle class III jaw relationships, parafunctional habits, and prolonged function with mandibular anterior teeth; degenerative changes in edentulous regions; inadequate treatment planning; failure to maintain oral tissue health; insufficient diagnosis, planning, and treatment implementation |
| Ogino et al., 2015 [10] | Patients failing to attend follow-up appointments; inadequate relationship between implant position and optimal artificial tooth positions; low quality and quantity of bone; absence of keratinized tissue; non-personalized treatments |
| Reddy et al., 2016 [39] | Lack of maxillary denture adaptation; need for replacement of maxillary denture; lack of mandibular denture adaptation; sex |
| Kumar et al., 2017 [11] | Inter-arch distance and relationship; lack of or null analysis of the anatomy of the maxilla using all tools available, including diagnostic models, X-ray images (radiographs, CBCT); incorrect impression technique; financial limitations for additional implants; lack of bone to support an adequate number of implants; loss of supporting structures for the lips and surrounding tissue; avoidance of bone grafting; no use of a tissue implant-supported hybrid denture as a less expensive and simpler option, within certain guidelines |
| Stevkovska et al., 2017 [40] | Lack of interdisciplinary therapy approach; delayed diagnosis; deficient treatment plan |
| Sharma et al., 2018 [41] | Lack of maxillary denture adaptation; not replacing the maxillary denture |
| Buzayan et al., 2018 [15] | Presence of a large torus palatinus and enlarged tuberosities; partially dentate mandibular arch with remaining anterior teeth; compromised sulcus depth: lack of pre-prosthetic surgical procedures; economic factors affecting treatment options; patient’s desires influencing the treatment plan; bone availability for dental implants; patient’s general health considerations; potential for progressive destructive changes in oral tissue without proper management |
| Verma et al., 2018 [42] | Reduced posterior occlusal contact; lack of use of implant-retained prostheses in the mandibular posterior area; extraction of upper posterior teeth; imbalanced occlusion |
| Akhtar et al., 2019 [43] | Inadequate surgical and prosthodontic treatment and lack of follow-up; tooth extrusion associated with RPD wearing; unsatisfactory lower dentures; non-simultaneous rehabilitation of residual arches; presence of preexisting signs before the provision of removable dentures; alveolar bone resorption as a natural phenomenon post-tooth loss; lack of scheduled follow-up sessions and proper guidance on denture care; poor preservation of posterior occlusion; inadequate treatment modalities, including poor-quality RPDs; failure to address papillary hyperplasia; lack of surgical procedures and proper impression techniques for flaccid tissue; poor fit, hygiene, and occlusion maintenance; insufficient use of implants to convert mandibular Kennedy Class 1 and 2 to Class 3, which could improve the masticatory efficiency, stability, and aesthetics; lack of implant-supported RPDs to reduce bone resorption |
| Bagga et al., 2019 [44] | Unsatisfactory dentures; periodontitis; maxillary complete dentures opposing mandibular anterior teeth |
| Penitente et al., 2022 [45] | Excessive bone resorption in the maxilla; occlusal architecture rearrangement; discrepancies between dental arches; divergent bone quality between maxilla and mandible; faster bone loss post-tooth extraction; greater bone loss with complete dentures; insufficient implant use; inadequate prosthetic and surgical planning; imbalanced occlusion; inadequate prosthesis material; insufficient posterior stabilization of the mandible |
| Ogino et al., 2023 [13] | Shift in mastication to anterior regions; excessive anterior occlusal function; occlusal trauma; lack of timely implant treatment; traumatic occlusion by preserved anterior teeth; excessive bone resorption in maxilla; lack of posterior occlusion; insufficient follow-up care; inadequate prosthetic treatment; loss of posterior occlusion support; improper implant placement; lack of cross-arch stabilization; inadequate bone quality and quantity; poor oral hygiene control; absence of keratinized tissue; inadequate prosthetic design; lack of individualized treatment approach |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda-Herrera, B.; Cruz, T.R.A.-d.l.; Jurado, C.A.; Garcia-Contreras, R. Anterior Hyperfunction Syndrome: Literature Review and Conceptual Model. Clin. Pract. 2024, 14, 1584-1600. https://doi.org/10.3390/clinpract14040128
Aranda-Herrera B, Cruz TRA-dl, Jurado CA, Garcia-Contreras R. Anterior Hyperfunction Syndrome: Literature Review and Conceptual Model. Clinics and Practice. 2024; 14(4):1584-1600. https://doi.org/10.3390/clinpract14040128
Chicago/Turabian StyleAranda-Herrera, Benjamin, Tania Rubi Agudo-de la Cruz, Carlos Alberto Jurado, and Rene Garcia-Contreras. 2024. "Anterior Hyperfunction Syndrome: Literature Review and Conceptual Model" Clinics and Practice 14, no. 4: 1584-1600. https://doi.org/10.3390/clinpract14040128







