Characteristics, Management, and Outcomes of Acute Life-Threatening Asthma in Adult Intensive Care
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Presentation
3.3. Patient Outcomes
3.4. Outcome Predictors
3.5. Management
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Public Health Profiles—OHID. Available online: https://fingertips.phe.org.uk/search/asthma (accessed on 8 July 2024).
- Pendergraft, T.B.; Stanford, R.H.; Beasley, R.; Stempel, D.A.; Roberts, C.; McLaughlin, T. Rates and characteristics of intensive care unit admissions and intubations among asthma-related hospitalizations. Ann. Allergy Asthma Immunol. 2004, 93, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Stow, P.J.; Pilcher, D.; Wilson, J.; George, C.; Bailey, M.; Higlett, T.; Bellomo, R.; Hart, G.K.; the Australian; New Zealand Intensive Care Society Adult Patient Database Management Committee. Improved outcomes from acute severe asthma in Australian intensive care units (1996 2003). Thorax 2007, 62, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Talbot, T.; Roe, T.; Dushianthan, A. Management of Acute Life-Threatening Asthma Exacerbations in the Intensive Care Unit. Appl. Sci. 2024, 14, 693. [Google Scholar] [CrossRef]
- Gibbison, B.; Griggs, K.; Mukherjee, M.; Sheikh, A. Ten years of asthma admissions to adult critical care units in England and Wales. BMJ Open 2013, 3, e003420. [Google Scholar] [CrossRef]
- Gupta, D.; Keogh, B.; Chung, K.F.; Ayres, J.G.; Harrison, D.A.; Goldfrad, C.; Brady, A.R.; Rowan, K. Characteristics and outcome for admissions to adult, general critical care units with acute severe asthma: A secondary analysis of the ICNARC Case Mix Programme Database. Crit. Care 2004, 8, R112–R121. [Google Scholar] [CrossRef]
- Secombe, P.; Stewart, P.; Singh, S.; Campbell, L.; Stephens, D.; Tran, K.; White, H.; Sheehy, R.; Gibson, J.; Cooke, R.; et al. Clinical management practices of life-threatening asthma: An audit of practices in intensive care. Crit. Care Resusc. 2019, 21, 53–62. [Google Scholar] [CrossRef]
- Younan, R.; Augy, J.L.; Hermann, B.; Guidet, B.; Aegerter, P.; Guerot, E.; Novara, A.; Hauw-Berlemont, C.; Hamdan, A.; Bailleul, C.; et al. Severe asthma exacerbation: Changes in patient characteristics, management, and outcomes from 1997 to 2016 in 40 ICUs in the greater Paris area. J. Intensive Med. 2024, 4, 209–215. [Google Scholar] [CrossRef]
- Althoff, M.D.; Holguin, F.; Yang, F.; Grunwald, G.K.; Moss, M.; Vandivier, R.W.; Ho, P.M.; Kiser, T.H.; Burnham, E.L. Noninvasive Ventilation Use in Critically Ill Patients with Acute Asthma Exacerbations. Am. J. Respir. Crit. Care Med. 2020, 202, 1520–1530. [Google Scholar] [CrossRef]
- Metwally, M.M.; Elshinnawy, O.; Abdelaleem, N.; Mokhtar, W. Assessment of non-invasive ventilation in acute severe asthma patients. ERJ Open Res. 2020, 6, 28. [Google Scholar] [CrossRef]
- Asthma. British Thoracic Society. Better Lung Health for All. Available online: https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (accessed on 8 July 2024).
- 2022 GINA Main Report. Global Initiative for Asthma—GINA. Available online: https://ginasthma.org/gina-reports/ (accessed on 8 July 2024).
- Holguin, F.; Cardet, J.C.; Chung, K.F.; Diver, S.; Ferreira, D.S.; Fitzpatrick, A.; Gaga, M.; Kellermeyer, L.; Khurana, S.; Knight, S.; et al. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2020, 55, 1900588. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Moorhouse, P.; Rockwood, K. Frailty and its quantitative clinical evaluation. J. R. Coll. Physicians Edinb. 2012, 42, 333–340. [Google Scholar] [CrossRef]
- Fowler, S.J.; O’Byrne, P.M.; Buhl, R.; Shaw, D. Two pathways, one patient; UK asthma guidelines. Thorax 2018, 73, 797–798. [Google Scholar] [CrossRef]
- Manyeruke, F.; Calligaro, G.L.; Raine, R.; van Zyl-Smit, R.N. Asthma in the intensive care unit: A review of patient characteristics and outcomes. Afr. J. Thorac. Crit. Care Med. 2023, 29, 48–51. [Google Scholar] [CrossRef]
- Wunsch, H.; Angus, D.C.; Harrison, D.A.; Linde-Zwirble, W.T.; Rowan, K.M. Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am. J. Respir. Crit. Care Med. 2011, 183, 1666–1673. [Google Scholar] [CrossRef]
- Brenner, B.; Corbridge, T.; Kazzi, A. Intubation and Mechanical Ventilation of the Asthmatic Patient in Respiratory Failure. Proc. Am. Thorac. Soc. 2009, 6, 371–379. [Google Scholar] [CrossRef]
- Leatherman, J. Mechanical Ventilation for Severe Asthma. Chest 2015, 147, 1671–1680. [Google Scholar] [CrossRef]
- BTS National Audit Reports (Full List). British Thoracic Society. Better Lung Health for All. Available online: https://www.brit-thoracic.org.uk/quality-improvement/clinical-audit/bts-national-audit-reports-full-list/ (accessed on 8 July 2024).
- Patel, S.; Shah, N.M.; Malhotra, A.M.; Lockie, C.; Camporota, L.; Barrett, N.; Kent, B.D.; Jackson, D.J. Inflammatory and microbiological associations with near-fatal asthma requiring extracorporeal membrane oxygenation. ERJ Open Res. 2020, 6, 00267-2019. [Google Scholar] [CrossRef] [PubMed]
- Rabah, H.; Itani, A.; Chalhoub, M. Leukocytes in Critical Patients With Asthma Exacerbation. Cureus 2021, 13, e20520. [Google Scholar] [CrossRef] [PubMed]
- Travers, A.H.; Milan, S.J.; Jones, A.P.; Camargo, C.A.; Rowe, B.H. Addition of intravenous beta(2)-agonists to inhaled beta(2)-agonists for acute asthma. Cochrane Database Syst. Rev. 2012, 12, CD010179. [Google Scholar]
- Howton, J.C.; Rose, J.; Duffy, S.; Zoltanski, T.; Levitt, M.A. Randomized, double-blind, placebo-controlled trial of intravenous ketamine in acute asthma. Ann. Emerg. Med. 1996, 27, 170–175. [Google Scholar] [CrossRef] [PubMed]


| All Patients (n = 100) | Invasive Mechanical Ventilation (n = 30) | Non-Invasive Respiratory Support (n = 38) | No Respiratory Support (n = 32) | p | |
|---|---|---|---|---|---|
| Patient Characteristics | |||||
| Age (years) | 42.5 (32.0–53.3) | 43.0 (32.0–50.0) | 46.0 (32.5–55.8) | 41.0 (30.3–50.0) | 0.573 |
| Female sex | 67 (67) | 20 (70) | 26 (68) | 20 (63) | 0.869 |
| White ethnicity | 88 (88) | 27 (90) | 34 (89) | 27 (84) | 0.745 |
| Body Mass Index (kg/m2) | 27.7 (24.0–34.6) | 27.6 (23.9–37.1) | 29.1 (24.1–34.6) | 27.2 (23.7–33.3) | 0.755 |
| Charlson CI | 0 (0–2) | 0 (0–3) | 2 (0–3) | 0 (0–1) | 0.064 |
| Clinical Frailty Scale | 2 (1–3) | 2 (1–4) | 2 (1–4) | 1 (1–3) | 0.199 |
| Current smoker | 22 (22) | 7 (23) | 9 (24) | 6 (19) | 0.865 |
| Former smoker | 26 (26) | 8 (27) | 9 (24) | 8 (25) | 0.961 |
| COPD | 9 (9) | 0 (0) | 8 (21) | 1 (3) | <0.001 |
| Asthma Characteristics | |||||
| Previous ICU admission | 32 (32) | 13 (43) | 5 (13) | 14 (44) | 0.007 |
| Long-term oral steroids | 15 (15) | 5 (17) | 4 (11) | 6 (19) | 0.314 |
| Biologic therapy | 13 (13) | 3 (10) | 2 (5) | 7 (22) | 0.030 |
| Respiratory Data | |||||
| Respiratory rate (per min) | 28 (20–34) | 29 (17–37) | 30 (25–35) | 24 (19–32) | 0.099 |
| Heart rate (per min) | 112 (100–126) | 117 (102–130) | 114 (106–126) | 108 (94–123) | 0.274 |
| Clinically exhausted | 70 (70) | 26 (87) | 28 (74) | 16 (50) | 0.001 |
| Altered mental status | 29 (29) | 20 (67) | 8 (21) | 1 (3) | <0.001 |
| pH | 7.37 (7.30–7.41) | 7.28 (7.21–7.36) | 7.38 (3.35–7.42) | 7.39 (7.36–7.43) | <0.001 |
| PaO2/FiO2 ratio (mmHg) | 236 (155–313) | 209 (129–419) | 241 (158–314) | 245 (208–274) | 0.823 |
| PaCO2 (mmHg) | 39 (33–47) | 53 (43–69) | 39 (35–45) | 33 (30–38) | <0.001 |
| Bicarbonate (mmol/L) | 22.9 (19.9–25.9) | 24.7 (22.7–27.2) | 23.0 (19.9–26.6) | 20.5 (18.1–22.9) | <0.001 |
| Base excess (mmol/L) | −2.7 (−4.7–−0.1) | −3.2 (−4.5–−0.6) | −1.8 (−4.9–0.3) | −3.5 (−5.1–−1.9) | 0.284 |
| Lactate | 2.2 (0.9–4.4) | 1.2 (0.7–2.3) | 1.9 (0.9–3.8) | 4.2 (2.2–5.5) | 0.001 |
| Microbiology Data | |||||
| White cell count (×109/L) | 13.6 (10.4–18.6) | 17.3 (12.6–22.8) | 14.5 (10.9–17.0) | 11.5 (10.1–13.8) | 0.003 |
| Neutrophil count (×109/L) | 11.7 (8.8–15.7) | 14.4 (10.8–19.0) | 12.0 (8.8–14.9) | 10.0 (8.7–12.1) | 0.022 |
| Lymphocyte count (×109/L) | 0.8 (0.6–1.3) | 1.0 (0.6–1.4) | 0.8 (0.6–1.1) | 0.7 (0.6–1.2) | 0.636 |
| NLR | 14.0 (7.8–23.4) | 13.8 (7.3–31.6) | 14.8 (9.0–23.5) | 12.9 (7.3–17.9) | 0.593 |
| CRP (mg/L) | 22 (8–77) | 25 (11–82) | 41 (9–96) | 11 (5–27) | 0.028 |
| Proven viral infection | 18 (18) | 6 (20) | 9 (24) | 3 (9) | 0.112 |
| Proven bacterial infection | 8 (8) | 6 (20) | 2 (5) | 0 (0) | 0.003 |
| Patient Outcomes | |||||
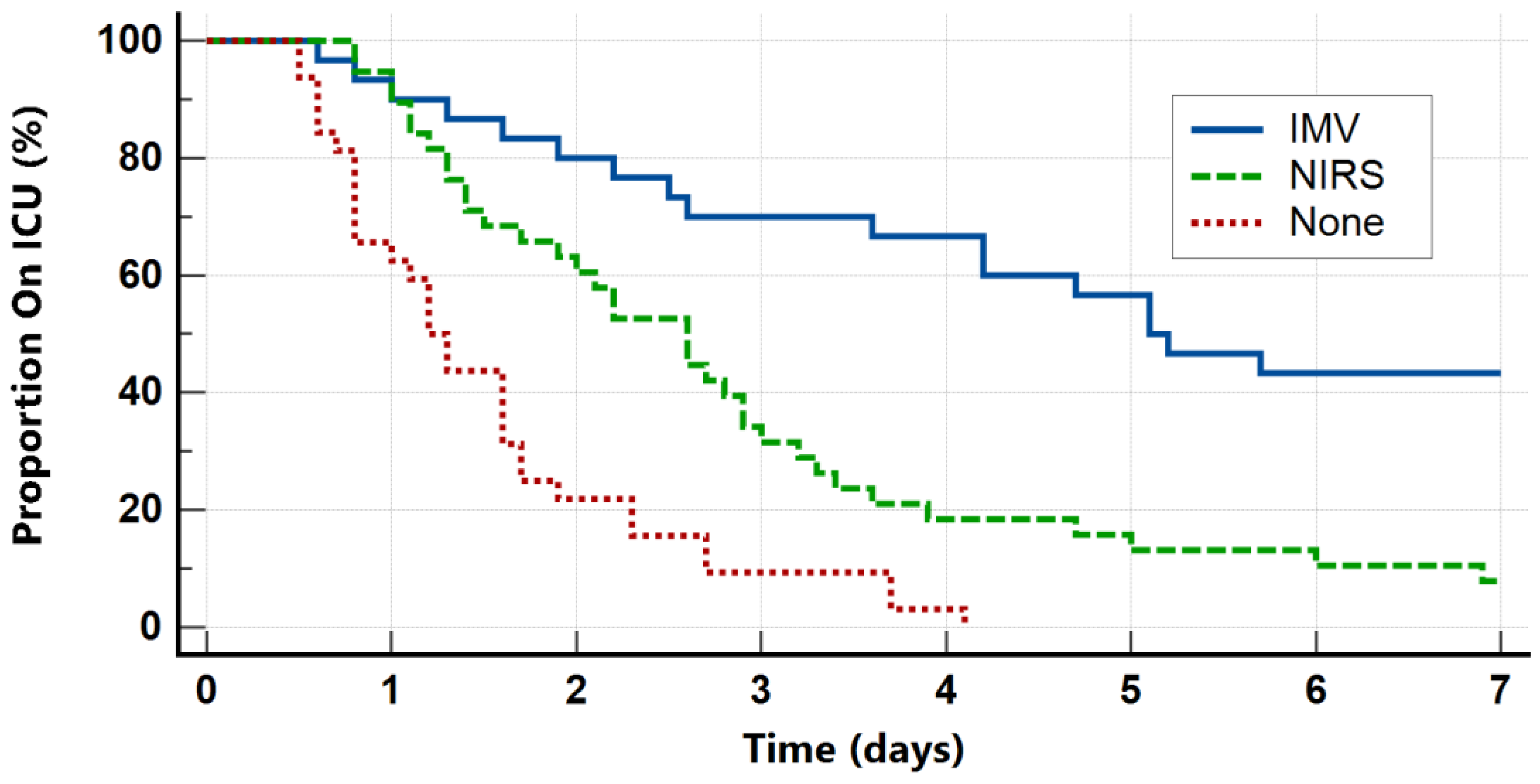
| ICU length of stay (days) | 2.3 (1.3–4.4) | 5.1 (2.5–9.4) | 2.6 (1.4–3.4) | 1.3 (0.8–1.8) | <0.001 |
| ECMO/ECCO2R | 2 (2) | 2 (6) | 0 (0) | 0 (0) | 0.029 |
| In-ICU mortality | 1 (1) | 1 (3) | 0 (0) | 0 (0) | 0.125 |
| Twenty-eight-day mortality | 1 (1) | 1 (3) | 0 (0) | 0 (0) | 0.125 |
| Predictor Variable | Univariate OR (95% CI, p) | AUC (95% CI, p) |
|---|---|---|
| Respiratory rate | 1.01 (0.97–1.06, 0.637) | 0.513 (0.370–0.657, 0.855) |
| Heart rate | 1.01 (0.99–1.03, 0.595) | 0.539 (0.406–0.67, 0.569) |
| Exhaustion | 3.84 (1.21–12.24, 0.023) | 0.619 (0.505–0.733, 0.041) |
| Altered mental status | 13.56 (4.83–38.06, <0.001) | 0.769 (0.658–0.880, <0.001) |
| pH (per 0.1) | 0.33 (0.19–0.56, <0.001) | 0.772 (0.676–0.851, <0.001) |
| PaCO2 (mmHg) | 1.08 (1.04–1.13, <0.001) | 0.809 (0.705–0.914, <0.001) |
| PaO2/FiO2 ratio | 1.00 (0.99–1.00, 0.186) | 0.460 (0.316–0.604, 0.584) |
| Lactate (mmol/L) | 0.71 (0.55–0.92, 0.011) | 0.676 (0.574–0.768, 0.002) |
| WCC (×109/L) | 1.14 (1.05–1.23, 0.001) | 0.689 (0.569–0.810, 0.002) |
| CRP (mg/L) | 1.00 (0.99–1.01, 0.254) | 0.558 (0.430–0.686, 0.375) |
| Viral infection | 1.21 (0.41–3.59, 0.734) | 0.514 (0.389–0.639, 0.823) |
| Bacterial infection | 8.50 (1.61–45.00, 0.012) | 0.586 (0.457–0.714, 0.192) |
| All Patients (n = 100) | Invasive Mechanical Ventilation (n = 30) | Non-Invasive Respiratory Support (n = 38) | No Respiratory Support (n = 32) | p | |
|---|---|---|---|---|---|
| Pharmacological Treatment | |||||
| Nebulised Bronchodilators | 100 (100) | 100 (100) | 100 (100) | 100 (100) | 1.000 |
| Corticosteroids | 95 (95) | 29 (97) | 36 (95) | 30 (94) | 0.593 |
| Antibiotics | 64 (64) | 23 (77) | 26 (68) | 14 (44) | 0.005 |
| Mucolytics | 27 (27) | 16 (53) | 8 (21) | 3 (9) | <0.001 |
| IV Magnesium | 96 (96) | 30 (100) | 35 (92) | 30 (94) | 0.125 |
| IV Aminophylline | 62 (62) | 24 (80) | 23 (61) | 15 (49) | 0.007 |
| IV Salbutamol | 28 (28) | 13 (43) | 6 (16) | 9 (28) | 0.012 |
| IV Adrenaline | 21 (21) | 17 (57) | 2 (5) | 2 (6) | <0.001 |
| IV Ketamine | 20 (20) | 17 (57) | 1 (3) | 2 (6) | <0.001 |
| Inhaled Isoflurane | 4 (4) | 4 (13) | 0 (0) | 0 (0) | 0.002 |
| First Respiratory Support | |||||
| HFNO | 23 (23) | 6 (20) | 17 (45) | 0 (0) | <0.001 |
| CPAP | 4 (4) | 1 (3) | 3 (8) | 0 (0) | 0.090 |
| NIV | 24 (24) | 6 (20) | 18 (47) | 0 (0) | <0.001 |
| IMV | 17 (17) | 17 (57) | 0 (0) | 0 (0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, A.J.R.; Roe, T.; Arscott, O.; Thomas, C.; Ward, J.; Beecham, R.; Browning, D.; Saeed, K.; Dushianthan, A. Characteristics, Management, and Outcomes of Acute Life-Threatening Asthma in Adult Intensive Care. Clin. Pract. 2024, 14, 1886-1897. https://doi.org/10.3390/clinpract14050149
Watson AJR, Roe T, Arscott O, Thomas C, Ward J, Beecham R, Browning D, Saeed K, Dushianthan A. Characteristics, Management, and Outcomes of Acute Life-Threatening Asthma in Adult Intensive Care. Clinics and Practice. 2024; 14(5):1886-1897. https://doi.org/10.3390/clinpract14050149
Chicago/Turabian StyleWatson, Adam J. R., Thomas Roe, Oliver Arscott, Charlotte Thomas, James Ward, Ryan Beecham, David Browning, Kordo Saeed, and Ahilanandan Dushianthan. 2024. "Characteristics, Management, and Outcomes of Acute Life-Threatening Asthma in Adult Intensive Care" Clinics and Practice 14, no. 5: 1886-1897. https://doi.org/10.3390/clinpract14050149
APA StyleWatson, A. J. R., Roe, T., Arscott, O., Thomas, C., Ward, J., Beecham, R., Browning, D., Saeed, K., & Dushianthan, A. (2024). Characteristics, Management, and Outcomes of Acute Life-Threatening Asthma in Adult Intensive Care. Clinics and Practice, 14(5), 1886-1897. https://doi.org/10.3390/clinpract14050149






