A Narrative Review on Biochemical Markers and Emerging Treatments in Prodromal Synucleinopathies
Abstract
:1. Introduction
2. Clinical Markers of Prodromal Synucleinopathy
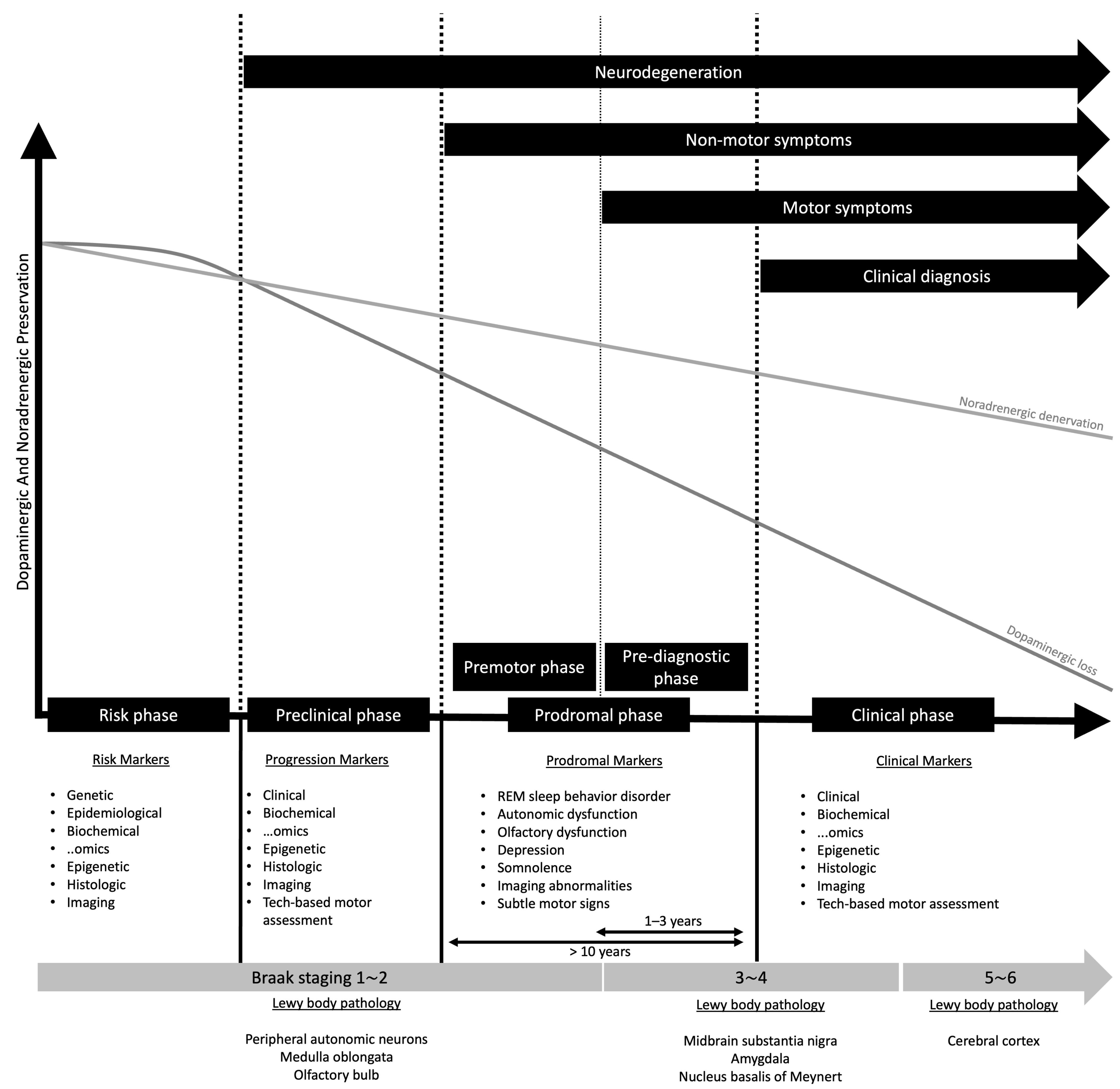
2.1. The Concept of Prodromal State in Parkinson’s Disease
2.2. Isolated Rapid Eye Movement Sleep Behavior Disorder and Synucleinopathy
The Evolving Concept of the Prodrome in REM Sleep Behavior Disorder
2.3. Prodromal Markers of Multiple System Atrophy
2.4. Prodromal Criteria by the Movement Disorder Society
3. Neuroimaging Markers in Prodromal Synucleinopathy
3.1. Imaging Nigral Dopaminergic Change in Prodromal Parkinsonian Syndromes
3.2. Peripheral Autonomic Denervation
3.3. Metabolic Network Activity
3.4. Brain Atrophy
3.5. Amyloid Imaging
4. Biofluid Markers in Prodromal α-Synucleinopathies
4.1. α-Synuclein Assays and Matrices
4.2. Other Biomarkers
4.2.1. Neurofilament Light Chain Concentration
4.2.2. DOPA Decarboxylase
4.2.3. Multiplexed Mass Spectrometry Assay
4.3. Gut Microbiome
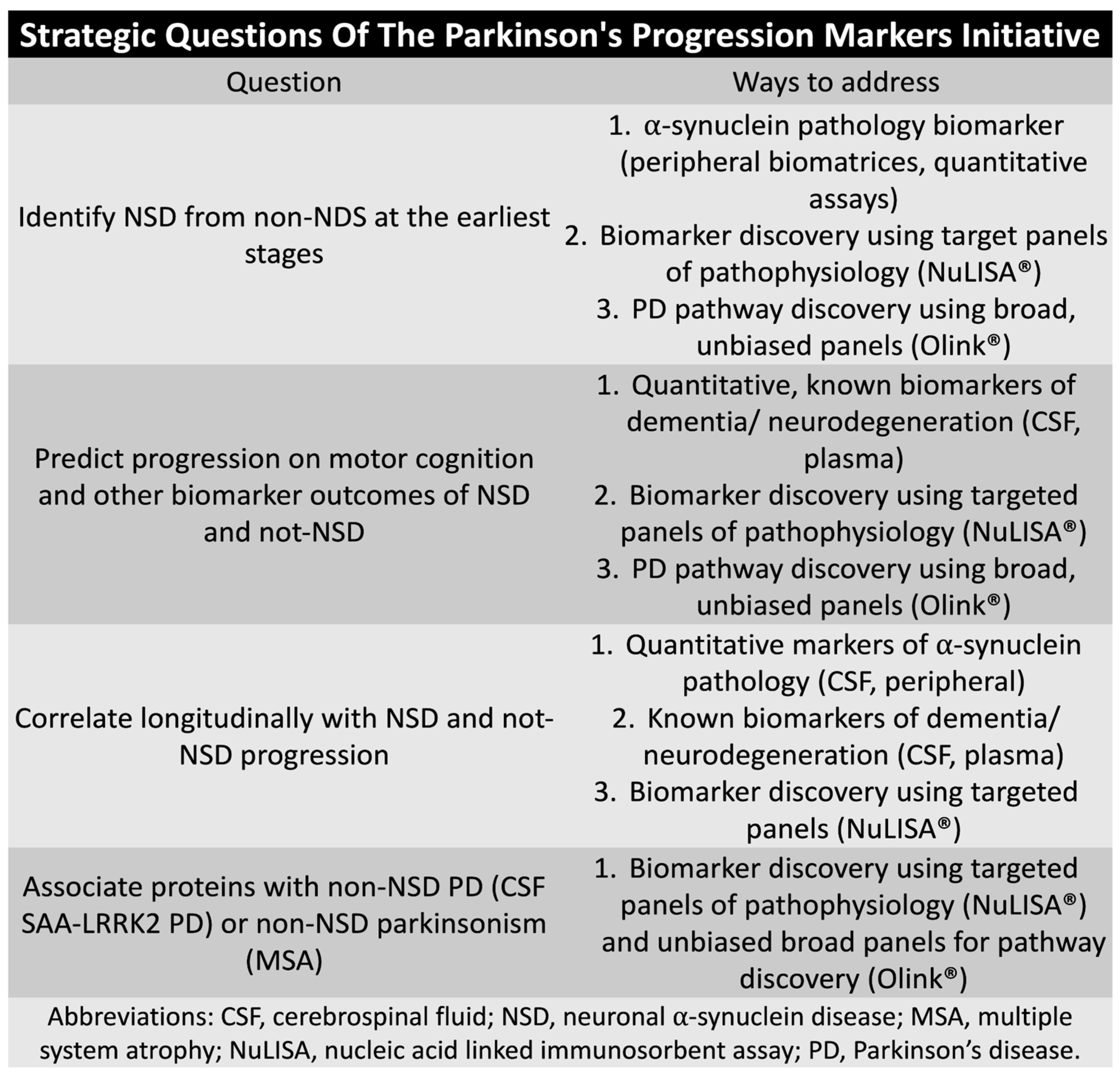
4.4. Parkinson Progression Marker Initiative and Path-to-Prevention Studies
5. Counseling of Prodromal Symptoms in α-Synucleinopathies
5.1. Basic Principles of Early Risk Disclosure
5.1.1. Respect for Autonomy
5.1.2. Beneficence and Non-Maleficence
5.1.3. Risk Disclosure Flow
6. Management of Prodromal Symptoms in α-Synucleinopathies, Evidence-Based Advice to Patients
6.1. Physical Activity
6.2. Diet
6.3. Regarding Individual Food, Food Groups, or Nutritional Supplements
6.4. Smoking
6.5. Sleep and Stress
6.6. Further Work-Up Requirement
7. Clinical Trials
8. Prediction Algorithms
9. Future Studies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mavroeidi, P.; Xilouri, M. Neurons and Glia Interplay in α-Synucleinopathies. Int. J. Mol. Sci. 2021, 22, 4994. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D. Advances in Markers of Prodromal Parkinson Disease. Nat. Rev. Neurol. 2016, 12, 622–634. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Khalil, I.; Sayad, R.; Kedwany, A.M.; Sayed, H.H.; Caprara, A.L.F.; Rissardo, J.P. Cardiovascular Dysautonomia and Cognitive Impairment in Parkinson’s Disease (Review). Med. Int. 2024, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Isaias, I.U.; Marotta, G.; Pezzoli, G.; Sabri, O.; Schwarz, J.; Crenna, P.; Classen, J.; Cavallari, P. Enhanced Catecholamine Transporter Binding in the Locus Coeruleus of Patients with Early Parkinson Disease. BMC Neurol. 2011, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Molsberry, S.A.; Hughes, K.C.; Schwarzschild, M.A.; Ascherio, A. Who to Enroll in Parkinson Disease Prevention Trials? The Case for Composite Prodromal Cohorts. Neurology 2022, 99, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kasten, M.; Hagenah, J.; Graf, J.; Lorwin, A.; Vollstedt, E.-J.; Peters, E.; Katalinic, A.; Raspe, H.; Klein, C. Cohort Profile: A Population-Based Cohort to Study Non-Motor Symptoms in Parkinsonism (EPIPARK). Int. J. Epidemiol. 2013, 42, 128–128k. [Google Scholar] [CrossRef]
- Gaenslen, A.; Wurster, I.; Brockmann, K.; Huber, H.; Godau, J.; Faust, B.; Lerche, S.; Eschweiler, G.W.; Maetzler, W.; Berg, D. Prodromal Features for Parkinson’s Disease--Baseline Data from the TREND Study. Eur. J. Neurol. 2014, 21, 766–772. [Google Scholar] [CrossRef]
- Lerche, S.; Seppi, K.; Behnke, S.; Liepelt-Scarfone, I.; Godau, J.; Mahlknecht, P.; Gaenslen, A.; Brockmann, K.; Srulijes, K.; Huber, H.; et al. Risk Factors and Prodromal Markers and the Development of Parkinson’s Disease. J. Neurol. 2014, 261, 180–187. [Google Scholar] [CrossRef]
- Jennings, D.; Siderowf, A.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K. Conversion to Parkinson Disease in the PARS Hyposmic and Dopamine Transporter-Deficit Prodromal Cohort. JAMA Neurol. 2017, 74, 933–940. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Baker, J.M.; Stephen, C.; Kim, I.Y.; Valeri, L.; Schwarzschild, M.A.; Ascherio, A. Non-Motor Features of Parkinson’s Disease in a Nested Case-Control Study of US Men. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1288–1295. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Gasperi, A.; Djamshidian, A.; Kiechl, S.; Stockner, H.; Willeit, P.; Willeit, J.; Rungger, G.; Poewe, W.; Seppi, K. Performance of the Movement Disorders Society Criteria for Prodromal Parkinson’s Disease: A Population-Based 10-Year Study. Mov. Disord. 2018, 33, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Tanner, C.M.; White, L.R. Pre-Motor Features of Parkinson’s Disease: The Honolulu-Asia Aging Study Experience. Park. Relat. Disord. 2012, 18 (Suppl. S1), S199–S202. [Google Scholar] [CrossRef] [PubMed]
- Hofman, A.; Brusselle, G.G.O.; Darwish Murad, S.; van Duijn, C.M.; Franco, O.H.; Goedegebure, A.; Ikram, M.A.; Klaver, C.C.W.; Nijsten, T.E.C.; Peeters, R.P.; et al. The Rotterdam Study: 2016 Objectives and Design Update. Eur. J. Epidemiol. 2015, 30, 661–708. [Google Scholar] [CrossRef]
- Shrestha, S.; Kamel, F.; Umbach, D.M.; Beane Freeman, L.E.; Koutros, S.; Alavanja, M.; Sandler, D.P.; Chen, H. Nonmotor Symptoms and Parkinson Disease in United States Farmers and Spouses. PLoS ONE 2017, 12, e0185510. [Google Scholar] [CrossRef]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic Presentations of Parkinson’s Disease in Primary Care: A Case-Control Study. Lancet Neurol. 2015, 14, 57–64. [Google Scholar] [CrossRef]
- Searles Nielsen, S.; Warden, M.N.; Camacho-Soto, A.; Willis, A.W.; Wright, B.A.; Racette, B.A. A Predictive Model to Identify Parkinson Disease from Administrative Claims Data. Neurology 2017, 89, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, S.K.L.; Verlinden, V.J.A.; Stricker, B.H.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Trajectories of Prediagnostic Functioning in Parkinson’s Disease. Brain 2017, 140, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Adler, C.H.; Dugger, B.N.; Hentz, J.G.; Shill, H.A.; Driver-Dunckley, E.; Sabbagh, M.N.; Jacobson, S.A.; Belden, C.M.; Sue, L.I.; et al. REM Sleep Behavior Disorder and Neuropathology in Parkinson’s Disease. Mov. Disord. 2015, 30, 1413–1417. [Google Scholar] [CrossRef]
- Diaconu, Ș.; Falup-Pecurariu, O.; Țînț, D.; Falup-Pecurariu, C. REM Sleep Behaviour Disorder in Parkinson’s Disease (Review). Exp. Ther. Med. 2021, 22, 812. [Google Scholar] [CrossRef]
- Bergmann, M.; Högl, B.; Stefani, A. Clinical Neurophysiology of REM Parasomnias: Diagnostic Aspects and Insights into Pathophysiology. Clin. Neurophysiol. Pract. 2024, 9, 53–62. [Google Scholar] [CrossRef]
- Woo, K.A.; Kim, S.; Nam, H.; Kim, Y.K.; Jeon, B.; Lee, J.-Y. Constipation Is Linked to Accelerated Cognitive and Motor Decline in Isolated REM Sleep Behavior Disorder. Park. Relat. Disord. 2024, 119, 105775. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and Predictors of Dementia and Parkinsonism in Idiopathic REM Sleep Behaviour Disorder: A Multicentre Study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J.; Lin, S.C.; Benarroch, E.E.; Schmeichel, A.M.; Ahlskog, J.E.; Caselli, R.J.; Jacobson, S.; Sabbagh, M.; et al. Clinicopathologic Correlations in 172 Cases of Rapid Eye Movement Sleep Behavior Disorder with or without a Coexisting Neurologic Disorder. Sleep Med. 2013, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Gagnon, J.-F.; Bertrand, J.-A.; Génier Marchand, D.; Montplaisir, J.Y. Parkinson Risk in Idiopathic REM Sleep Behavior Disorder: Preparing for Neuroprotective Trials. Neurology 2015, 84, 1104–1113. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Yao, C.; Pelletier, A.; Montplaisir, J.Y.; Gagnon, J.-F.; Postuma, R.B. Evolution of Prodromal Parkinson’s Disease and Dementia with Lewy Bodies: A Prospective Study. Brain 2019, 142, 2051–2067. [Google Scholar] [CrossRef]
- Joza, S.; Hu, M.T.; Jung, K.-Y.; Kunz, D.; Stefani, A.; Dušek, P.; Terzaghi, M.; Arnaldi, D.; Videnovic, A.; Schiess, M.C.; et al. Progression of Clinical Markers in Prodromal Parkinson’s Disease and Dementia with Lewy Bodies: A Multicentre Study. Brain 2023, 146, 3258–3272. [Google Scholar] [CrossRef]
- Joza, S.; Hu, M.T.; Jung, K.-Y.; Kunz, D.; Arnaldi, D.; Lee, J.-Y.; Ferini-Strambi, L.; Antelmi, E.; Sixel-Döring, F.; De Cock, V.C.; et al. Prodromal Dementia with Lewy Bodies in REM Sleep Behavior Disorder: A Multicenter Study. Alzheimers Dement. 2024, 20, 91–102. [Google Scholar] [CrossRef]
- Stefani, A.; Gabelia, D.; Högl, B.; Mitterling, T.; Mahlknecht, P.; Stockner, H.; Poewe, W.; Frauscher, B. Long-Term Follow-up Investigation of Isolated Rapid Eye Movement Sleep Without Atonia Without Rapid Eye Movement Sleep Behavior Disorder: A Pilot Study. J. Clin. Sleep Med. 2015, 11, 1273–1279. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Chau, S.W.H.; Man Yu, M.W.; Chan, N.Y.; Chan, J.W.Y.; Li, S.X.; Huang, B.; Wang, J.; Feng, H.; et al. Evolution of Prodromal REM Sleep Behavior Disorder to Neurodegeneration: A Retrospective Longitudinal Case-Control Study. Neurology 2022, 99, e627–e637. [Google Scholar] [CrossRef]
- Lee, W.-J.; Baek, S.-H.; Im, H.-J.; Lee, S.-K.; Yoon, J.-E.; Thomas, R.J.; Wing, Y.-K.; Shin, C.; Yun, C.-H. REM Sleep Behavior Disorder and Its Possible Prodromes in General Population: Prevalence, Polysomnography Findings, and Associated Factors. Neurology 2023, 101, e2364–e2375. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.J.; Sandness, D.J.; McCarter, A.R.; Feemster, J.C.; Teigen, L.N.; Timm, P.C.; Boeve, B.F.; Silber, M.H.; St Louis, E.K. REM Sleep Muscle Activity in Idiopathic REM Sleep Behavior Disorder Predicts Phenoconversion. Neurology 2019, 93, e1171–e1179. [Google Scholar] [CrossRef]
- Kunz, D.; Stotz, S.; de Zeeuw, J.; Papakonstantinou, A.; Dümchen, S.; Haberecht, M.; Plotkin, M.; Bes, F. Prognostic Biomarkers in Prodromal α-Synucleinopathies: DAT Binding and REM Sleep without Atonia. J. Neurol. Neurosurg. Psychiatry 2023, 94, 532–540. [Google Scholar] [CrossRef]
- Nepozitek, J.; Varga, Z.; Dostalova, S.; Perinova, P.; Keller, J.; Robinson, S.; Ibarburu, V.; Prihodova, I.; Bezdicek, O.; Ruzicka, E.; et al. Magnetic Susceptibility Changes in the Brainstem Reflect REM Sleep without Atonia Severity in Isolated REM Sleep Behavior Disorder. NPJ Park. Dis. 2023, 9, 112. [Google Scholar] [CrossRef]
- Postuma, R.B.; Gagnon, J.F.; Rompré, S.; Montplaisir, J.Y. Severity of REM Atonia Loss in Idiopathic REM Sleep Behavior Disorder Predicts Parkinson Disease. Neurology 2010, 74, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Berini, S.E.; Sandroni, P.; Fealey, R.D.; Coon, E.A.; Suarez, M.D.; Benarroch, E.E.; Low, P.A. Pure Autonomic Failure: Predictors of Conversion to Clinical CNS Involvement. Neurology 2017, 88, 1129–1136. [Google Scholar] [CrossRef]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.-A.; Biaggioni, I.; Low, P.A.; Singer, W.; Goldstein, D.S.; Peltier, A.C.; Shibao, C.A.; Gibbons, C.H.; et al. Natural History of Pure Autonomic Failure: A United States Prospective Cohort. Ann. Neurol. 2017, 81, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Pelletier, A.; Gagnon, J.-F.; Montplaisir, J. Evolution of Prodromal Multiple System Atrophy from REM Sleep Behavior Disorder: A Descriptive Study. J. Park. Dis. 2022, 12, 983–991. [Google Scholar] [CrossRef]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.-A.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS Research Criteria for Prodromal Parkinson’s Disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Graves, S.M.; Shen, W. Dopaminergic Modulation of Striatal Networks in Health and Parkinson’s Disease. Curr. Opin. Neurobiol. 2014, 29, 109–117. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; Del Rey, N.L.-G.; Hernandez, L.F.; Obeso, J.A. Compensatory Mechanisms in Parkinson’s Disease: Circuits Adaptations and Role in Disease Modification. Exp. Neurol. 2017, 298, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Dreher, J.-C.; Burnod, Y. An Integrative Theory of the Phasic and Tonic Modes of Dopamine Modulation in the Prefrontal Cortex. Neural Netw. 2002, 15, 583–602. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.; Kim, Y.K.; Yoon, E.J.; Nam, H.W.; Jeon, B.; Lee, J.-Y. Brain Metabolic Correlates of Dopaminergic Denervation in Prodromal and Early Parkinson’s Disease. Mov. Disord. 2022, 37, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, G.; Zhang, Y.; Zhang, M.; Chen, Z.; Zhang, L.; Wang, X.; Zhang, M.; Ye, G.; Li, Y.; et al. Increased Free Water in the Substantia Nigra in Idiopathic REM Sleep Behaviour Disorder. Brain 2021, 144, 1488–1497. [Google Scholar] [CrossRef]
- Barber, T.R.; Griffanti, L.; Bradley, K.M.; McGowan, D.R.; Lo, C.; Mackay, C.E.; Hu, M.T.; Klein, J.C. Nigrosome 1 Imaging in REM Sleep Behavior Disorder and Its Association with Dopaminergic Decline. Ann. Clin. Transl. Neurol. 2020, 7, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Pyatigorskaya, N.; Gaurav, R.; Arnaldi, D.; Leu-Semenescu, S.; Yahia-Cherif, L.; Valabregue, R.; Vidailhet, M.; Arnulf, I.; Lehéricy, S. Magnetic Resonance Imaging Biomarkers to Assess Substantia Nigra Damage in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Sleep 2017, 40, zsx149. [Google Scholar] [CrossRef]
- Chahine, L.M.; Brumm, M.C.; Caspell-Garcia, C.; Oertel, W.; Mollenhauer, B.; Amara, A.; Fernandez-Arcos, A.; Tolosa, E.; Simonet, C.; Hogl, B.; et al. Dopamine Transporter Imaging Predicts Clinically-Defined α-Synucleinopathy in REM Sleep Behavior Disorder. Ann. Clin. Transl. Neurol. 2021, 8, 201–212. [Google Scholar] [CrossRef]
- Dijkstra, A.A.; Voorn, P.; Berendse, H.W.; Groenewegen, H.J.; Rozemuller, A.J.M.; van de Berg, W.D.J. Stage-Dependent Nigral Neuronal Loss in Incidental Lewy Body and Parkinson’s Disease. Mov. Disord. 2014, 29, 1244–1251. [Google Scholar] [CrossRef]
- Sung, C.; Oh, S.J.; Kim, J.S. Imaging Procedure and Clinical Studies of [18F]FP-CIT PET. Nucl. Med. Mol. Imaging 2024, 58, 185–202. [Google Scholar] [CrossRef]
- Iranzo, A.; Santamaría, J.; Valldeoriola, F.; Serradell, M.; Salamero, M.; Gaig, C.; Niñerola-Baizán, A.; Sánchez-Valle, R.; Lladó, A.; De Marzi, R.; et al. Dopamine Transporter Imaging Deficit Predicts Early Transition to Synucleinopathy in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Ann. Neurol. 2017, 82, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, J.-Y.; Kim, Y.-K.; Shin, S.-A.; Kim, H.; Nam, H.; Jeon, B. Longitudinal Change in Dopamine Transporter Availability in Idiopathic REM Sleep Behavior Disorder. Neurology 2020, 95, e3081–e3092. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.; Siderowf, A.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K. Imaging Prodromal Parkinson Disease: The Parkinson Associated Risk Syndrome Study. Neurology 2014, 83, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, E.; Santin, M.D.; Valabrègue, R.; Mangone, G.; Gaurav, R.; Pyatigorskaya, N.; Hutchison, M.; Yahia-Cherif, L.; Villain, N.; Habert, M.-O.; et al. The Spatiotemporal Changes in Dopamine, Neuromelanin and Iron Characterizing Parkinson’s Disease. Brain 2021, 144, 3114–3125. [Google Scholar] [CrossRef]
- Ehrminger, M.; Latimier, A.; Pyatigorskaya, N.; Garcia-Lorenzo, D.; Leu-Semenescu, S.; Vidailhet, M.; Lehericy, S.; Arnulf, I. The Coeruleus/Subcoeruleus Complex in Idiopathic Rapid Eye Movement Sleep Behaviour Disorder. Brain 2016, 139, 1180–1188. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G. Neurochemical Features of Rem Sleep Behaviour Disorder. J. Pers. Med. 2021, 11, 880. [Google Scholar] [CrossRef]
- Boeve, B.F.; St Louis, E.K.; Kantarci, K. Neuromelanin-Sensitive Imaging in Patients with Idiopathic Rapid Eye Movement Sleep Behaviour Disorder. Brain 2016, 139, 1005–1007. [Google Scholar] [CrossRef]
- Fedorova, T.D.; Knudsen, K.; Andersen, K.B.; Horsager, J.; Skjærbæk, C.; Beier, C.P.; Sommerauer, M.; Svendsen, K.B.; Otto, M.; Borghammer, P. Imaging Progressive Peripheral and Central Dysfunction in Isolated REM Sleep Behaviour Disorder after 3 Years of Follow-Up. Park. Relat. Disord. 2022, 101, 99–104. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Serrano, G.; Sue, L.I.; Walker, D.G.; Dugger, B.N.; Shill, H.A.; Driver-Dunckley, E.; Caviness, J.N.; Intorcia, A.; et al. Prevalence of Submandibular Gland Synucleinopathy in Parkinson’s Disease, Dementia with Lewy Bodies and Other Lewy Body Disorders. J. Park. Dis. 2016, 6, 153–163. [Google Scholar] [CrossRef]
- Ortuño-Lizarán, I.; Beach, T.G.; Serrano, G.E.; Walker, D.G.; Adler, C.H.; Cuenca, N. Phosphorylated α-Synuclein in the Retina Is a Biomarker of Parkinson’s Disease Pathology Severity. Mov. Disord. 2018, 33, 1315–1324. [Google Scholar] [CrossRef]
- Pérez-Acuña, D.; Rhee, K.H.; Shin, S.J.; Ahn, J.; Lee, J.-Y.; Lee, S.-J. Retina-to-Brain Spreading of α-Synuclein after Intravitreal Injection of Preformed Fibrils. Acta Neuropathol. Commun. 2023, 11, 83. [Google Scholar] [CrossRef]
- Hart de Ruyter, F.J.; Morrema, T.H.J.; den Haan, J.; Gase, G.; Twisk, J.W.R.; de Boer, J.F.; Scheltens, P.; Bouwman, F.H.; Verbraak, F.D.; Rozemuller, A.J.M.; et al. α-Synuclein Pathology in Post-Mortem Retina and Optic Nerve Is Specific for α-Synucleinopathies. NPJ Park. Dis. 2023, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Ahn, J.; Oh, S.; Shin, J.Y.; Kim, Y.K.; Nam, H.; Jeon, B. Retina Thickness as a Marker of Neurodegeneration in Prodromal Lewy Body Disease. Mov. Disord. 2020, 35, 349–354. [Google Scholar] [CrossRef]
- Rascunà, C.; Cicero, C.E.; Chisari, C.G.; Russo, A.; Giuliano, L.; Castellino, N.; Terravecchia, C.; Grillo, M.; Longo, A.; Avitabile, T.; et al. Retinal Thickness and Microvascular Pathway in Idiopathic Rapid Eye Movement Sleep Behaviour Disorder and Parkinson’s Disease. Park. Relat. Disord. 2021, 88, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.K.; Romero-Bascones, D.; Cortina-Borja, M.; Williamson, D.J.; Struyven, R.R.; Zhou, Y.; Patel, S.; Weil, R.S.; Antoniades, C.A.; Topol, E.J.; et al. Retinal Optical Coherence Tomography Features Associated with Incident and Prevalent Parkinson Disease. Neurology 2023, 101, e1581–e1593. [Google Scholar] [CrossRef] [PubMed]
- Holtbernd, F.; Ma, Y.; Peng, S.; Schwartz, F.; Timmermann, L.; Kracht, L.; Fink, G.R.; Tang, C.C.; Eidelberg, D.; Eggers, C. Dopaminergic Correlates of Metabolic Network Activity in Parkinson’s Disease. Hum. Brain Mapp. 2015, 36, 3575–3585. [Google Scholar] [CrossRef]
- Wu, P.; Yu, H.; Peng, S.; Dauvilliers, Y.; Wang, J.; Ge, J.; Zhang, H.; Eidelberg, D.; Ma, Y.; Zuo, C. Consistent Abnormalities in Metabolic Network Activity in Idiopathic Rapid Eye Movement Sleep Behaviour Disorder. Brain 2014, 137, 3122–3128. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.-Y.; Kim, Y.K.; Yoon, E.J.; Kim, H.; Kim, R.; Nam, H.; Jeon, B. Mild Cognitive Impairment and Abnormal Brain Metabolic Expression in Idiopathic REM Sleep Behavior Disorder. Park. Relat. Disord. 2021, 90, 1–7. [Google Scholar] [CrossRef]
- Orso, B.; Mattioli, P.; Yoon, E.-J.; Kim, Y.K.; Kim, H.; Shin, J.H.; Kim, R.; Famà, F.; Brugnolo, A.; Massa, F.; et al. Progression Trajectories from Prodromal to Overt Synucleinopathies: A Longitudinal, Multicentric Brain [18F]FDG-PET Study. NPJ Park. Dis. 2024, 10, 200. [Google Scholar] [CrossRef]
- Mattioli, P.; Orso, B.; Liguori, C.; Famà, F.; Giorgetti, L.; Donniaquio, A.; Massa, F.; Giberti, A.; Vállez García, D.; Meles, S.K.; et al. Derivation and Validation of a Phenoconversion-Related Pattern in Idiopathic Rapid Eye Movement Behavior Disorder. Mov. Disord. 2023, 38, 57–67. [Google Scholar] [CrossRef]
- Orso, B.; Mattioli, P.; Yoon, E.-J.; Kim, Y.K.; Kim, H.; Shin, J.H.; Kim, R.; Liguori, C.; Famà, F.; Donniaquio, A.; et al. Validation of the REM Behaviour Disorder Phenoconversion-Related Pattern in an Independent Cohort. Neurol. Sci. 2023, 44, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Carli, G.; Caminiti, S.P.; Galbiati, A.; Marelli, S.; Casoni, F.; Padovani, A.; Ferini-Strambi, L.; Perani, D. In-Vivo Signatures of Neurodegeneration in Isolated Rapid Eye Movement Sleep Behaviour Disorder. Eur. J. Neurol. 2020, 27, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, P.; Pardini, M.; Famà, F.; Girtler, N.; Brugnolo, A.; Orso, B.; Meli, R.; Filippi, L.; Grisanti, S.; Massa, F.; et al. Cuneus/Precuneus as a Central Hub for Brain Functional Connectivity of Mild Cognitive Impairment in Idiopathic REM Sleep Behavior Patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2834–2845. [Google Scholar] [CrossRef]
- Yoon, E.J.; Lee, J.-Y.; Kim, H.; Yoo, D.; Shin, J.H.; Nam, H.; Jeon, B.; Kim, Y.K. Brain Metabolism Related to Mild Cognitive Impairment and Phenoconversion in Patients with Isolated REM Sleep Behavior Disorder. Neurology 2022, 98, e2413–e2424. [Google Scholar] [CrossRef]
- Carli, G.; Meles, S.K.; Janzen, A.; Sittig, E.; Kogan, R.V.; Perani, D.; Oertel, W.H.; Leenders, K.L. Occipital Hypometabolism Is a Risk Factor for Conversion to Parkinson’s Disease in Isolated REM Sleep Behaviour Disorder. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3290–3301. [Google Scholar] [CrossRef]
- Rahayel, S.; Montplaisir, J.; Monchi, O.; Bedetti, C.; Postuma, R.B.; Brambati, S.; Carrier, J.; Joubert, S.; Latreille, V.; Jubault, T.; et al. Patterns of Cortical Thinning in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2015, 30, 680–687. [Google Scholar] [CrossRef]
- Rahayel, S.; Postuma, R.B.; Montplaisir, J.; Génier Marchand, D.; Escudier, F.; Gaubert, M.; Bourgouin, P.-A.; Carrier, J.; Monchi, O.; Joubert, S.; et al. Cortical and Subcortical Gray Matter Bases of Cognitive Deficits in REM Sleep Behavior Disorder. Neurology 2018, 90, e1759–e1770. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Weintraub, D.; Chahine, L.; Aarsland, D.; Hansson, O.; Westman, E. Cortical Thinning in Patients with REM Sleep Behavior Disorder Is Associated with Clinical Progression. NPJ Park. Dis. 2019, 5, 7. [Google Scholar] [CrossRef]
- Campabadal, A.; Inguanzo, A.; Segura, B.; Serradell, M.; Abos, A.; Uribe, C.; Gaig, C.; Santamaria, J.; Compta, Y.; Bargallo, N.; et al. Cortical Gray Matter Progression in Idiopathic REM Sleep Behavior Disorder and Its Relation to Cognitive Decline. Neuroimage Clin. 2020, 28, 102421. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, H.; Kim, Y.K.; Yoon, E.J.; Nam, H.; Jeon, B.; Lee, J.-Y. Longitudinal Evolution of Cortical Thickness Signature Reflecting Lewy Body Dementia in Isolated REM Sleep Behavior Disorder: A Prospective Cohort Study. Transl. Neurodegener. 2023, 12, 27. [Google Scholar] [CrossRef]
- Rahayel, S.; Postuma, R.; Montplaisir, J.; Misic, B.; Tremblay, C.; Vo, A.; Lewis, S.; Matar, E.; Martens, K.E.; Blanc, F. A Brain Signature of Prodromal Lewy Body Dementia in Isolated REM Sleep Behavior Disorder. J. Sleep Res. 2020, 29, 18–19. [Google Scholar]
- Woo, K.A.; Kim, H.; Yoon, E.J.; Shin, J.H.; Nam, H.; Jeon, B.; Kim, Y.K.; Lee, J.-Y. Brain Olfactory-Related Atrophy in Isolated Rapid Eye Movement Sleep Behavior Disorder. Ann. Clin. Transl. Neurol. 2023, 10, 2192–2207. [Google Scholar] [CrossRef] [PubMed]
- Okkels, N.; Horsager, J.; Labrador-Espinosa, M.; Kjeldsen, P.L.; Damholdt, M.F.; Mortensen, J.; Vestergård, K.; Knudsen, K.; Andersen, K.B.; Fedorova, T.D.; et al. Severe Cholinergic Terminal Loss in Newly Diagnosed Dementia with Lewy Bodies. Brain 2023, 146, 3690–3704. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Galvan, P.; Przybelski, S.A.; Lesnick, T.G.; Schwarz, C.G.; Senjem, M.L.; Gunter, J.L.; Jack, C.R.; Min, H.-K.P.; Jain, M.; Miyagawa, T.; et al. β-Amyloid Load on PET Along the Continuum of Dementia with Lewy Bodies. Neurology 2023, 101, e178–e188. [Google Scholar] [CrossRef]
- Woo, K.A.; Yoon, E.J.; Kim, S.Y.; Jin, B.R.; Lee, S.M.; Nam, H.W.; Park, H.W.; Kim, Y.K.; Lee, J.Y. Association of β-Amyloid Load on PET with Cognitive Profiles Across the Lewy Body Diseases Continuum. Mov. Disord. 2024, 39, 124. [Google Scholar]
- Noyce, A.J.; Lees, A.J.; Schrag, A.-E. The Prediagnostic Phase of Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 871–878. [Google Scholar] [CrossRef]
- Simuni, T.; Chahine, L.M.; Poston, K.; Brumm, M.; Buracchio, T.; Campbell, M.; Chowdhury, S.; Coffey, C.; Concha-Marambio, L.; Dam, T.; et al. A Biological Definition of Neuronal α-Synuclein Disease: Towards an Integrated Staging System for Research. Lancet Neurol. 2024, 23, 178–190. [Google Scholar] [CrossRef]
- Kang, U.J.; Boehme, A.K.; Fairfoul, G.; Shahnawaz, M.; Ma, T.C.; Hutten, S.J.; Green, A.; Soto, C. Comparative Study of Cerebrospinal Fluid α-Synuclein Seeding Aggregation Assays for Diagnosis of Parkinson’s Disease. Mov. Disord. 2019, 34, 536–544. [Google Scholar] [CrossRef]
- Bellomo, G.; De Luca, C.M.G.; Paoletti, F.P.; Gaetani, L.; Moda, F.; Parnetti, L. α-Synuclein Seed Amplification Assays for Diagnosing Synucleinopathies: The Way Forward. Neurology 2022, 99, 195–205. [Google Scholar] [CrossRef]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-Synuclein RT-QuIC in the CSF of Patients with Alpha-Synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC Assay with High Sensitivity and Specificity for Lewy Body-Associated Synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Iranzo, A.; Fairfoul, G.; Ayudhaya, A.C.N.; Serradell, M.; Gelpi, E.; Vilaseca, I.; Sanchez-Valle, R.; Gaig, C.; Santamaria, J.; Tolosa, E.; et al. Detection of α-Synuclein in CSF by RT-QuIC in Patients with Isolated Rapid-Eye-Movement Sleep Behaviour Disorder: A Longitudinal Observational Study. Lancet Neurol. 2021, 20, 203–212. [Google Scholar] [CrossRef]
- Stefani, A.; Iranzo, A.; Holzknecht, E.; Perra, D.; Bongianni, M.; Gaig, C.; Heim, B.; Serradell, M.; Sacchetto, L.; Garrido, A.; et al. Alpha-Synuclein Seeds in Olfactory Mucosa of Patients with Isolated REM Sleep Behaviour Disorder. Brain 2021, 144, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Poggiolini, I.; Gupta, V.; Lawton, M.; Lee, S.; El-Turabi, A.; Querejeta-Coma, A.; Trenkwalder, C.; Sixel-Döring, F.; Foubert-Samier, A.; Pavy-Le Traon, A.; et al. Diagnostic Value of Cerebrospinal Fluid Alpha-Synuclein Seed Quantification in Synucleinopathies. Brain 2022, 145, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Concha-Marambio, L.; Weber, S.; Farris, C.M.; Dakna, M.; Lang, E.; Wicke, T.; Ma, Y.; Starke, M.; Ebentheuer, J.; Sixel-Döring, F.; et al. Accurate Detection of α-Synuclein Seeds in Cerebrospinal Fluid from Isolated Rapid Eye Movement Sleep Behavior Disorder and Patients with Parkinson’s Disease in the DeNovo Parkinson (DeNoPa) Cohort. Mov. Disord. 2023, 38, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Mammana, A.; Muñoz-Lopetegi, A.; Dellavalle, S.; Mayà, G.; Rossi, M.; Serradell, M.; Baiardi, S.; Arqueros, A.; Quadalti, C.; et al. Misfolded α-Synuclein Assessment in the Skin and CSF by RT-QuIC in Isolated REM Sleep Behavior Disorder. Neurology 2023, 100, e1944–e1954. [Google Scholar] [CrossRef]
- Liguori, R.; Donadio, V.; Wang, Z.; Incensi, A.; Rizzo, G.; Antelmi, E.; Biscarini, F.; Pizza, F.; Zou, W.; Plazzi, G. A Comparative Blind Study between Skin Biopsy and Seed Amplification Assay to Disclose Pathological α-Synuclein in RBD. NPJ Park. Dis. 2023, 9, 34. [Google Scholar] [CrossRef]
- Okuzumi, A.; Hatano, T.; Matsumoto, G.; Nojiri, S.; Ueno, S.-I.; Imamichi-Tatano, Y.; Kimura, H.; Kakuta, S.; Kondo, A.; Fukuhara, T.; et al. Propagative α-Synuclein Seeds as Serum Biomarkers for Synucleinopathies. Nat. Med. 2023, 29, 1448–1455. [Google Scholar] [CrossRef]
- Siderowf, A.; Concha-Marambio, L.; Lafontant, D.-E.; Farris, C.M.; Ma, Y.; Urenia, P.A.; Nguyen, H.; Alcalay, R.N.; Chahine, L.M.; Foroud, T.; et al. Assessment of Heterogeneity among Participants in the Parkinson’s Progression Markers Initiative Cohort Using α-Synuclein Seed Amplification: A Cross-Sectional Study. Lancet Neurol. 2023, 22, 407–417. [Google Scholar] [CrossRef]
- Dam, T.; Pagano, G.; Brumm, M.C.; Gochanour, C.; Poston, K.L.; Weintraub, D.; Chahine, L.M.; Coffey, C.; Tanner, C.M.; Kopil, C.M.; et al. Neuronal Alpha-Synuclein Disease Integrated Staging System Performance in PPMI, PASADENA, and SPARK Baseline Cohorts. NPJ Park. Dis. 2024, 10, 178. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-Synuclein Strains in Parkinson’s Disease and Multiple System Atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Ma, W.; Ma, Z.; Shi, Y.; Pang, X.; Yimingjiang, M.; Dang, Z.; Cui, W.; Lin, R.; Zhang, W. Comparison of Clinicopathological Features between Cerebral Cystic and Alveolar Echinococcosis: Analysis of 27 Cerebral Echinococcosis Cases in Xinjiang, China. Diagn. Pathol. 2024, 19, 90. [Google Scholar] [CrossRef]
- Tolosa, E.; Vilas, D. Peripheral Synuclein Tissue Markers: A Step Closer to Parkinson’s Disease Diagnosis. Brain 2015, 138, 2120–2122. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Jiang, C.; Janzen, A.; Barber, T.R.; Seger, A.; Sommerauer, M.; Davis, J.J.; Marek, K.; Hu, M.T.; Oertel, W.H.; et al. Neuronally Derived Extracellular Vesicle α-Synuclein as a Serum Biomarker for Individuals at Risk of Developing Parkinson Disease. JAMA Neurol. 2024, 81, 59–68. [Google Scholar] [CrossRef]
- Niu, M.; Li, Y.; Li, G.; Zhou, L.; Luo, N.; Yao, M.; Kang, W.; Liu, J. A Longitudinal Study on α-Synuclein in Plasma Neuronal Exosomes as a Biomarker for Parkinson’s Disease Development and Progression. Eur. J. Neurol. 2020, 27, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Hopfner, F.; Katsikoudi, A.; Hein, R.; Catli, C.; Evetts, S.; Huang, Y.; Wang, H.; Ryder, J.W.; Kuhlenbaeumer, G.; et al. Serum Neuronal Exosomes Predict and Differentiate Parkinson’s Disease from Atypical Parkinsonism. J. Neurol. Neurosurg. Psychiatry 2020, 91, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-Q.; Pu, J.-L.; Zheng, R.; Fang, Y.; Gu, L.-Y.; Guo, T.; Si, X.-L.; Zhou, C.; Chen, Y.; Liu, Y.; et al. Different Patterns of Exosomal α-Synuclein between Parkinson’s Disease and Probable Rapid Eye Movement Sleep Behavior Disorder. Eur. J. Neurol. 2022, 29, 3590–3599. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Yan, S.; Jiang, C.; Tofaris, G.K.; Davis, J.J. Alternating Magnetic Field-Promoted Nanoparticle Mixing: The On-Chip Immunocapture of Serum Neuronal Exosomes for Parkinson’s Disease Diagnostics. Anal. Chem. 2023, 95, 7906–7913. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Dakna, M.; Kruse, N.; Galasko, D.; Foroud, T.; Zetterberg, H.; Schade, S.; Gera, R.G.; Wang, W.; Gao, F.; et al. Validation of Serum Neurofilament Light Chain as a Biomarker of Parkinson’s Disease Progression. Mov. Disord. 2020, 35, 1999–2008. [Google Scholar] [CrossRef]
- Rutledge, J.; Lehallier, B.; Zarifkar, P.; Losada, P.M.; Shahid-Besanti, M.; Western, D.; Gorijala, P.; Ryman, S.; Yutsis, M.; Deutsch, G.K.; et al. Comprehensive Proteomics of CSF, Plasma, and Urine Identify DDC and Other Biomarkers of Early Parkinson’s Disease. Acta Neuropathol. 2024, 147, 52. [Google Scholar] [CrossRef]
- Appleton, E.; Khosousi, S.; Ta, M.; Nalls, M.; Singleton, A.B.; Sturchio, A.; Markaki, I.; Paslawski, W.; Iwaki, H.; Svenningsson, P. DOPA-Decarboxylase Is Elevated in CSF, but Not Plasma, in Prodromal and de Novo Parkinson’s Disease. Transl. Neurodegener. 2024, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Hällqvist, J.; Bartl, M.; Dakna, M.; Schade, S.; Garagnani, P.; Bacalini, M.-G.; Pirazzini, C.; Bhatia, K.; Schreglmann, S.; Xylaki, M.; et al. Plasma Proteomics Identify Biomarkers Predicting Parkinson’s Disease up to 7 Years before Symptom Onset. Nat. Commun. 2024, 15, 4759. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic Bacterial Composition in Parkinson’s Disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The Nasal and Gut Microbiome in Parkinson’s Disease and Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef]
- Sampson, T. Microbial Amyloids in Neurodegenerative Amyloid Diseases. FEBS J. 2023. [Google Scholar] [CrossRef]
- Schmit, K.J.; Garcia, P.; Sciortino, A.; Aho, V.T.E.; Pardo Rodriguez, B.; Thomas, M.H.; Gérardy, J.-J.; Bastero Acha, I.; Halder, R.; Cialini, C.; et al. Fiber Deprivation and Microbiome-Borne Curli Shift Gut Bacterial Populations and Accelerate Disease in a Mouse Model of Parkinson’s Disease. Cell Rep. 2023, 42, 113071. [Google Scholar] [CrossRef]
- Schaeffer, E.; Kluge, A.; Schulte, C.; Deuschle, C.; Bunk, J.; Welzel, J.; Maetzler, W.; Berg, D. Association of Misfolded α-Synuclein Derived from Neuronal Exosomes in Blood with Parkinson’s Disease Diagnosis and Duration. J. Park. Dis. 2024, 14, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, M.A.; Sharp, R.R.; Sandness, D.J.; Feemster, J.C.; Junna, M.; Kotagal, S.; Lipford, M.C.; Tippmann-Peikert, M.; Boeve, B.F.; Silber, M.H.; et al. Physician and Patient Determinants of Prognostic Counseling in Idiopathic REM Sleep-Behavior Disorder. Sleep Med. 2019, 62, 80–85. [Google Scholar] [CrossRef]
- Pérez-Carbonell, L.; Simonet, C.; Chohan, H.; Gill, A.; Leschziner, G.; Schrag, A.; Noyce, A.J. The Views of Patients with Isolated Rapid Eye Movement Sleep Behavior Disorder on Risk Disclosure. Mov. Disord. 2023, 38, 1089–1093. [Google Scholar] [CrossRef]
- Teigen, L.N.; Sharp, R.R.; Hirsch, J.R.; Campbell, E.; Timm, P.C.; Sandness, D.J.; Feemster, J.C.; Gossard, T.R.; Faber, S.M.; Steele, T.A.; et al. Specialist Approaches to Prognostic Counseling in Isolated REM Sleep Behavior Disorder. Sleep Med. 2021, 79, 107–112. [Google Scholar] [CrossRef]
- Gossard, T.R.; Teigen, L.N.; Yoo, S.; Timm, P.C.; Jagielski, J.; Bibi, N.; Feemster, J.C.; Steele, T.; Carvalho, D.Z.; Junna, M.R.; et al. Patient Values and Preferences Regarding Prognostic Counseling in Isolated REM Sleep Behavior Disorder. Sleep 2023, 46, zsac244. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, A.; Bogucki, A.; Kroemeke, A.; Gajos, A. To Know or Not to Know? Opinions of Patients with Parkinson’s Disease on Disclosing Risk of Phenoconversion in RBD. Neurol. Neurochir. Pol. 2023, 57, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Rogge, A.; Nieding, K.; Helmker, V.; Letsch, C.; Hauptmann, B.; Berg, D. Patients’ Views on the Ethical Challenges of Early Parkinson Disease Detection. Neurology 2020, 94, e2037–e2044. [Google Scholar] [CrossRef]
- Yilmaz, R.; Dilek, G.R.; Kayis, G.; Abali, T.; Yalçın-Çakmaklı, G.; Arda, B.; Elibol, B.; Akbostancı, M.C. Disclosing the News of Future Risk of Parkinson’s Disease: What Do Patients Think? Park. Relat. Disord. 2023, 116, 105895. [Google Scholar] [CrossRef] [PubMed]
- Dommershuijsen, L.J.; Darweesh, S.K.L.; Luik, A.I.; Kieboom, B.C.T.; Koudstaal, P.J.; Boon, A.J.W.; Ikram, M.A.; Ikram, M.K.; Bunnik, E.M. Ethical Considerations in Screening for Rapid Eye Movement Sleep Behavior Disorder in the General Population. Mov. Disord. 2020, 35, 1939–1944. [Google Scholar] [CrossRef]
- Verbrugge, J.; Cook, L.; Miller, M.; Rumbaugh, M.; Schulze, J.; Heathers, L.; Wetherill, L.; Foroud, T. Outcomes of Genetic Test Disclosure and Genetic Counseling in a Large Parkinson’s Disease Research Study. J. Genet. Couns. 2021, 30, 755–765. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Maruff, P.; Getter, C.; Snyder, P.J. Disclosure of Positron Emission Tomography Amyloid Imaging Results: A Preliminary Study of Safety and Tolerability. Alzheimers Dement. 2016, 12, 454–458. [Google Scholar] [CrossRef]
- Burns, J.M.; Johnson, D.K.; Liebmann, E.P.; Bothwell, R.J.; Morris, J.K.; Vidoni, E.D. Safety of Disclosing Amyloid Status in Cognitively Normal Older Adults. Alzheimers Dement. 2017, 13, 1024–1030. [Google Scholar] [CrossRef]
- Taswell, C.; Donohue, C.; Mastwyk, M.; Louey, A.G.; Giummarra, J.; Robertson, J.; Darby, D.G.; Masters, C.L.; Rowe, C.C. Safety of Disclosing Amyloid Imaging Results to MCI and AD Patients. Ment. Health Fam. Med. 2018, 14, 748–756. [Google Scholar]
- Grill, J.D.; Raman, R.; Ernstrom, K.; Sultzer, D.L.; Burns, J.M.; Donohue, M.C.; Johnson, K.A.; Aisen, P.S.; Sperling, R.A.; Karlawish, J. Short-Term Psychological Outcomes of Disclosing Amyloid Imaging Results to Research Participants Who Do Not Have Cognitive Impairment. JAMA Neurol. 2020, 77, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Wake, T.; Tabuchi, H.; Funaki, K.; Ito, D.; Yamagata, B.; Yoshizaki, T.; Nakahara, T.; Jinzaki, M.; Yoshimasu, H.; Tanahashi, I.; et al. Disclosure of Amyloid Status for Risk of Alzheimer Disease to Cognitively Normal Research Participants with Subjective Cognitive Decline: A Longitudinal Study. Am. J. Alzheimers Dis. Other Demen 2020, 35, 1533317520904551. [Google Scholar] [CrossRef]
- Schaeffer, E.; Toedt, I.; Köhler, S.; Rogge, A.; Berg, D. Risk Disclosure in Prodromal Parkinson’s Disease. Mov. Disord. 2021, 36, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, D.; Cheng, Q.; Zhang, P.; Zhao, C.; Min, J.; Wang, F. Association of Levels of Physical Activity with Risk of Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2018, 1, e182421. [Google Scholar] [CrossRef]
- Portugal, B.; Artaud, F.; Degaey, I.; Roze, E.; Fournier, A.; Severi, G.; Canonico, M.; Proust-Lima, C.; Elbaz, A. Association of Physical Activity and Parkinson Disease in Women: Long-Term Follow-up of the E3N Cohort Study. Neurology 2023, 101, e386–e398. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.; Ryan, J.; Laws, M.; Devine, A. A Comprehensive Examination of the Evidence for Whole of Diet Patterns in Parkinson’s Disease: A Scoping Review. Nutr. Neurosci. 2024, 27, 547–565. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective Study of Dietary Pattern and Risk of Parkinson Disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Gu, Y.; Mejia-Santana, H.; Cote, L.; Marder, K.S.; Scarmeas, N. The Association between Mediterranean Diet Adherence and Parkinson’s Disease. Mov. Disord. 2012, 27, 771–774. [Google Scholar] [CrossRef]
- Cassani, E.; Barichella, M.; Ferri, V.; Pinelli, G.; Iorio, L.; Bolliri, C.; Caronni, S.; Faierman, S.A.; Mottolese, A.; Pusani, C.; et al. Dietary Habits in Parkinson’s Disease: Adherence to Mediterranean Diet. Park. Relat. Disord. 2017, 42, 40–46. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. MIND Diet Associated with Reduced Incidence and Delayed Progression of ParkinsonismA in Old Age. J. Nutr. Health Aging 2018, 22, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Molsberry, S.; Bjornevik, K.; Hughes, K.C.; Healy, B.; Schwarzschild, M.; Ascherio, A. Diet Pattern and Prodromal Features of Parkinson Disease. Neurology 2020, 95, e2095–e2108. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, Z.; Sheklabadi, E.; Derakhshan, Y.; Bagherniya, M.; Chitsaz, A. The Effect of the Mediterranean Diet on Cognitive Function in Patients with Parkinson’s Disease: A Randomized Clinical Controlled Trial. Complement. Ther. Med. 2020, 50, 102366. [Google Scholar] [CrossRef]
- Metcalfe-Roach, A.; Yu, A.C.; Golz, E.; Cirstea, M.; Sundvick, K.; Kliger, D.; Foulger, L.H.; Mackenzie, M.; Finlay, B.B.; Appel-Cresswell, S. MIND and Mediterranean Diets Associated with Later Onset of Parkinson’s Disease. Mov. Disord. 2021, 36, 977–984. [Google Scholar] [CrossRef]
- Strikwerda, A.J.; Dommershuijsen, L.J.; Ikram, M.K.; Voortman, T. Diet Quality and Risk of Parkinson’s Disease: The Rotterdam Study. Nutrients 2021, 13, 3970. [Google Scholar] [CrossRef]
- Yin, W.; Löf, M.; Pedersen, N.L.; Sandin, S.; Fang, F. Mediterranean Dietary Pattern at Middle Age and Risk of Parkinson’s Disease: A Swedish Cohort Study. Mov. Disord. 2021, 36, 255–260. [Google Scholar] [CrossRef]
- Paknahad, Z.; Sheklabadi, E.; Moravejolahkami, A.R.; Chitsaz, A.; Hassanzadeh, A. The Effects of Mediterranean Diet on Severity of Disease and Serum Total Antioxidant Capacity (TAC) in Patients with Parkinson’s Disease: A Single Center, Randomized Controlled Trial. Nutr. Neurosci. 2022, 25, 313–320. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Liu, Y.; Chen, S.; Wu, S.; Gao, X. Diet Quality Is Associated with Prodromal Parkinson’s Disease Features in Chinese Adults. Mov. Disord. 2022, 37, 2367–2375. [Google Scholar] [CrossRef]
- Lawrie, S.; Coe, S.; Mansoubi, M.; Welch, J.; Razzaque, J.; Hu, M.T.; Dawes, H. Dietary Patterns and Nonmotor Symptoms in Parkinson’s Disease: A Cross-Sectional Analysis. J. Am. Nutr. Assoc. 2023, 42, 393–402. [Google Scholar] [CrossRef]
- Maraki, M.I.; Yannakoulia, M.; Xiromerisiou, G.; Stefanis, L.; Charisis, S.; Giagkou, N.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; et al. Mediterranean Diet Is Associated with a Lower Probability of Prodromal Parkinson’s Disease and Risk for Parkinson’s Disease/Dementia with Lewy Bodies: A Longitudinal Study. Eur. J. Neurol. 2023, 30, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Keramati, M.; Kheirouri, S.; Etemadifar, M. Dietary Approach to Stop Hypertension (DASH), but Not Mediterranean and MIND, Dietary Pattern Protects against Parkinson’s Disease. Food Sci. Nutr. 2024, 12, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, X.; Park, Y.; Wang, J.; Huang, X.; Hollenbeck, A.; Blair, A.; Chen, H. Alcohol Consumption, Types of Alcohol, and Parkinson’s Disease. PLoS ONE 2013, 8, e66452. [Google Scholar] [CrossRef]
- Shao, C.; Wang, X.; Wang, P.; Tang, H.; He, J.; Wu, N. Parkinson’s Disease Risk and Alcohol Intake: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Front. Nutr. 2021, 8, 709846. [Google Scholar] [CrossRef]
- Mitchell, E.; Chohan, H.; Bestwick, J.P.; Noyce, A.J. Alcohol and Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Park. Dis. 2022, 12, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.M.; Hernán, M.A.; Willett, W.C.; Ascherio, A. Diet and Parkinson’s Disease: A Potential Role of Dairy Products in Men. Ann. Neurol. 2002, 52, 793–801. [Google Scholar] [CrossRef]
- Park, M.; Ross, G.W.; Petrovitch, H.; White, L.R.; Masaki, K.H.; Nelson, J.S.; Tanner, C.M.; Curb, J.D.; Blanchette, P.L.; Abbott, R.D. Consumption of Milk and Calcium in Midlife and the Future Risk of Parkinson Disease. Neurology 2005, 64, 1047–1051. [Google Scholar] [CrossRef]
- Chen, H.; O’Reilly, E.; McCullough, M.L.; Rodriguez, C.; Schwarzschild, M.A.; Calle, E.E.; Thun, M.J.; Ascherio, A. Consumption of Dairy Products and Risk of Parkinson’s Disease. Am. J. Epidemiol. 2007, 165, 998–1006. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary Fat Intake and Risk of Parkinson’s Disease: A Case-Control Study in Japan. J. Neurol. Sci. 2010, 288, 117–122. [Google Scholar] [CrossRef]
- Kyrozis, A.; Ghika, A.; Stathopoulos, P.; Vassilopoulos, D.; Trichopoulos, D.; Trichopoulou, A. Dietary and Lifestyle Variables in Relation to Incidence of Parkinson’s Disease in Greece. Eur. J. Epidemiol. 2013, 28, 67–77. [Google Scholar] [CrossRef]
- Sääksjärvi, K.; Knekt, P.; Lundqvist, A.; Männistö, S.; Heliövaara, M.; Rissanen, H.; Järvinen, R. A Cohort Study on Diet and the Risk of Parkinson’s Disease: The Role of Food Groups and Diet Quality. Br. J. Nutr. 2013, 109, 329–337. [Google Scholar] [CrossRef]
- Jiang, W.; Ju, C.; Jiang, H.; Zhang, D. Dairy Foods Intake and Risk of Parkinson’s Disease: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Epidemiol. 2014, 29, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of Dairy Foods and Risk of Parkinson Disease. Neurology 2017, 89, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Domenighetti, C.; Sugier, P.-E.; Ashok Kumar Sreelatha, A.; Schulte, C.; Grover, S.; Mohamed, O.; Portugal, B.; May, P.; Bobbili, D.R.; Radivojkov-Blagojevic, M.; et al. Dairy Intake and Parkinson’s Disease: A Mendelian Randomization Study. Mov. Disord. 2022, 37, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Hajji-Louati, M.; Portugal, B.; Correia, E.; Laouali, N.; Lee, P.-C.; Artaud, F.; Roze, E.; Mancini, F.R.; Elbaz, A. Consumption of Milk and Other Dairy Products and Incidence of Parkinson’s Disease: A Prospective Cohort Study in French Women. Eur. J. Epidemiol. 2024, 39, 1023–1036. [Google Scholar] [CrossRef]
- Gröninger, M.; Sabin, J.; Kaaks, R.; Amiano, P.; Aune, D.; Castro, N.C.; Guevara, M.; Hansen, J.; Homann, J.; Masala, G.; et al. Associations of Milk, Dairy Products, Calcium and Vitamin D Intake with Risk of Developing Parkinson’s Disease within the EPIC4ND Cohort. Eur. J. Epidemiol. 2024, 39, 1251–1265. [Google Scholar] [CrossRef]
- Qi, H.; Li, S. Dose-Response Meta-Analysis on Coffee, Tea and Caffeine Consumption with Risk of Parkinson’s Disease. Geriatr. Gerontol. Int. 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Hong, C.T.; Chan, L.; Bai, C.-H. The Effect of Caffeine on the Risk and Progression of Parkinson’s Disease: A Meta-Analysis. Nutrients 2020, 12, 1860. [Google Scholar] [CrossRef]
- De Rijk, M.C.; Breteler, M.M.; den Breeijen, J.H.; Launer, L.J.; Grobbee, D.E.; van der Meché, F.G.; Hofman, A. Dietary Antioxidants and Parkinson Disease. The Rotterdam Study. Arch. Neurol. 1997, 54, 762–765. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.M.; Schwarzschild, M.A.; Hernán, M.A.; Logroscino, G.; Willett, W.C.; Ascherio, A. Folate Intake and Risk of Parkinson’s Disease. Am. J. Epidemiol. 2004, 160, 368–375. [Google Scholar] [CrossRef]
- Etminan, M.; Gill, S.S.; Samii, A. Intake of Vitamin E, Vitamin C, and Carotenoids and the Risk of Parkinson’s Disease: A Meta-Analysis. Lancet Neurol. 2005, 4, 362–365. [Google Scholar] [CrossRef]
- De Lau, L.M.L.; Koudstaal, P.J.; Witteman, J.C.M.; Hofman, A.; Breteler, M.M.B. Dietary Folate, Vitamin B12, and Vitamin B6 and the Risk of Parkinson Disease. Neurology 2006, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kilkkinen, A.; Rissanen, H.; Marniemi, J.; Sääksjärvi, K.; Heliövaara, M. Serum Vitamin D and the Risk of Parkinson Disease. Arch. Neurol. 2010, 67, 808–811. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Lack of Association of Dairy Food, Calcium, and Vitamin D Intake with the Risk of Parkinson’s Disease: A Case-Control Study in Japan. Park. Relat. Disord. 2011, 17, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Qi, H.; Wang, L.; Fan, X.; Han, F.; Wang, H.; Bi, S. Vitamin D Status and Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neurol. Sci. 2014, 35, 1723–1730. [Google Scholar] [CrossRef]
- Takeda, A.; Nyssen, O.P.; Syed, A.; Jansen, E.; Bueno-de-Mesquita, B.; Gallo, V. Vitamin A and Carotenoids and the Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2014, 42, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. Associations between B Vitamins and Parkinson’s Disease. Nutrients 2015, 7, 7197–7208. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Rimm, E.B.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of Antioxidant Vitamins and Risk of Parkinson’s Disease. Mov. Disord. 2016, 31, 1909–1914. [Google Scholar] [CrossRef]
- Shrestha, S.; Lutsey, P.L.; Alonso, A.; Huang, X.; Mosley, T.H.J.; Chen, H. Serum 25-Hydroxyvitamin D Concentrations in Mid-Adulthood and Parkinson’s Disease Risk. Mov. Disord. 2016, 31, 972–978. [Google Scholar] [CrossRef]
- Luo, X.; Ou, R.; Dutta, R.; Tian, Y.; Xiong, H.; Shang, H. Association Between Serum Vitamin D Levels and Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2018, 9, 909. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Ying, A.F.; Khan, S.; Wu, Y.; Jin, A.; Wong, A.S.Y.; Tan, E.-K.; Yuan, J.-M.; Koh, W.-P.; Tan, L.C.S. Dietary Antioxidants and Risk of Parkinson’s Disease in the Singapore Chinese Health Study. Mov. Disord. 2020, 35, 1765–1773. [Google Scholar] [CrossRef]
- Chang, M.C.; Kwak, S.G.; Kwak, S. Effect of Dietary Vitamins C and E on the Risk of Parkinson’s Disease: A Meta-Analysis. Clin. Nutr. 2021, 40, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Hantikainen, E.; Trolle Lagerros, Y.; Ye, W.; Serafini, M.; Adami, H.-O.; Bellocco, R.; Bonn, S. Dietary Antioxidants and the Risk of Parkinson Disease: The Swedish National March Cohort. Neurology 2021, 96, e895–e903. [Google Scholar] [CrossRef]
- Talebi, S.; Ghoreishy, S.M.; Jayedi, A.; Travica, N.; Mohammadi, H. Dietary Antioxidants and Risk of Parkinson’s Disease: A Systematic Review and Dose–Response Meta-Analysis of Observational Studies. Adv. Nutr. 2022, 13, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Y.; Chen, J.-X.; Chen, G.-S.; Gao, H.; Huo, J.-H.; Pang, Y.-F.; Gao, Q.-H. Dietary β-Carotene and Vitamin A and Risk of Parkinson Disease: A Protocol for Systematic Review and Meta-Analysis. Medicine 2022, 101, e31002. [Google Scholar] [CrossRef] [PubMed]
- Flores-Torres, M.H.; Christine, C.W.; Bjornevik, K.; Molsberry, S.A.; Hung, A.Y.; Healy, B.C.; Blacker, D.; Schwarzschild, M.A.; Ascherio, A. Long-Term Intake of Folate, Vitamin B6, and Vitamin B12 and the Incidence of Parkinson’s Disease in a Sample of U.S. Women and Men. Mov. Disord. 2023, 38, 866–879. [Google Scholar] [CrossRef]
- Hao, X.; Li, H.; Li, Q.; Gao, D.; Wang, X.; Wu, C.; Wang, Q.; Zhu, M. Dietary Vitamin E Intake and Risk of Parkinson’s Disease: A Cross-Sectional Study. Front. Nutr. 2023, 10, 1289238. [Google Scholar] [CrossRef]
- Niu, F.; Xie, W.; Zhang, W.; Kawuki, J.; Yu, X. Vitamin C, Vitamin E, β-Carotene and Risk of Parkinson’s Disease: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Nutr. Neurosci. 2024, 27, 329–341. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, H.; Ding, Y.; Lu, R.; Yang, X. No Association between Genetically Predicted Vitamin D Levels and Parkinson’s Disease. PLoS ONE 2024, 19, e0313631. [Google Scholar] [CrossRef]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco Smoking and the Risk of Parkinson Disease: A 65-Year Follow-up of 30,000 Male British Doctors. Neurology 2020, 94, e2132–e2138. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Perez, X.A.; Bordia, T. Nicotine as a Potential Neuroprotective Agent for Parkinson’s Disease. Mov. Disord. 2012, 27, 947–957. [Google Scholar] [CrossRef]
- Derkinderen, P.; Shannon, K.M.; Brundin, P. Gut Feelings about Smoking and Coffee in Parkinson’s Disease. Mov. Disord. 2014, 29, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Sohail, S.; Yu, L.; Schneider, J.A.; Bennett, D.A.; Buchman, A.S.; Lim, A.S.P. Sleep Fragmentation and Parkinson’s Disease Pathology in Older Adults without Parkinson’s Disease. Mov. Disord. 2017, 32, 1729–1737. [Google Scholar] [CrossRef]
- Lysen, T.S.; Darweesh, S.K.L.; Ikram, M.K.; Luik, A.I.; Ikram, M.A. Sleep and Risk of Parkinsonism and Parkinson’s Disease: A Population-Based Study. Brain 2019, 142, 2013–2022. [Google Scholar] [CrossRef]
- Chen, H.; Schernhammer, E.; Schwarzschild, M.A.; Ascherio, A. A Prospective Study of Night Shift Work, Sleep Duration, and Risk of Parkinson’s Disease. Am. J. Epidemiol. 2006, 163, 726–730. [Google Scholar] [CrossRef]
- Sieurin, J.; Andel, R.; Tillander, A.; Valdes, E.G.; Pedersen, N.L.; Wirdefeldt, K. Occupational Stress and Risk for Parkinson’s Disease: A Nationwide Cohort Study. Mov. Disord. 2018, 33, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-L.E.; Bai, Y.-M.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Pan, T.-L.; Chen, T.-J.; Tsai, S.-J.; et al. Post-Traumatic Stress Disorder and Risk of Parkinson Disease: A Nationwide Longitudinal Study. Am. J. Geriatr. Psychiatry 2017, 25, 917–923. [Google Scholar] [CrossRef]
- Espay, A.J.; McFarthing, K. Alpha-Synuclein and the Parkinson’s Disease Drug Pipeline. Park. Relat. Disord. 2023, 111, 105432. [Google Scholar] [CrossRef]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef]
- Karabayir, I.; Gunturkun, F.; Butler, L.; Goldman, S.M.; Kamaleswaran, R.; Davis, R.L.; Colletta, K.; Chinthala, L.; Jefferies, J.L.; Bobay, K.; et al. Externally Validated Deep Learning Model to Identify Prodromal Parkinson’s Disease from Electrocardiogram. Sci. Rep. 2023, 13, 12290. [Google Scholar] [CrossRef] [PubMed]
- Vaish, A. A Machine Learning Approach for Early Identification of Prodromal Parkinson’s Disease. Cureus 2024, 16, e63240. [Google Scholar] [CrossRef] [PubMed]
- Warden, M.N.; Searles Nielsen, S.; Camacho-Soto, A.; Garnett, R.; Racette, B.A. A Comparison of Prediction Approaches for Identifying Prodromal Parkinson Disease. PLoS ONE 2021, 16, e0256592. [Google Scholar] [CrossRef]
- Tabashum, T.; Snyder, R.C.; O’Brien, M.K.; Albert, M.V. Machine Learning Models for Parkinson Disease: Systematic Review. JMIR Med. Inform. 2024, 12, e50117. [Google Scholar] [CrossRef] [PubMed]
- Makarious, M.B.; Leonard, H.L.; Vitale, D.; Iwaki, H.; Sargent, L.; Dadu, A.; Violich, I.; Hutchins, E.; Saffo, D.; Bandres-Ciga, S.; et al. Multi-Modality Machine Learning Predicting Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 35. [Google Scholar] [CrossRef]
- Koo, Y.; Kim, M.; Lee, W.-W. Predicting Parkinson’s Disease Using a Deep-Learning Algorithm to Analyze Prodromal Medical and Prescription Data. J. Clin. Neurol. 2025, 21, 21–30. [Google Scholar] [CrossRef]
- Prashanth, R.; Dutta Roy, S. Early Detection of Parkinson’s Disease through Patient Questionnaire and Predictive Modelling. Int. J. Med. Inform. 2018, 119, 75–87. [Google Scholar] [CrossRef]
- Dehsarvi, A.; Smith, S.L. Classification of Resting-State fMRI Using Evolutionary Algorithms: Towards a Brain Imaging Biomarker for Parkinson’s Disease. arXiv 2019, arXiv:1910.05378. [Google Scholar]
- Tran, C.; Shen, K.; Liu, K.; Ashok, A.; Ramirez-Zamora, A.; Chen, J.; Li, Y.; Fang, R. Deep Learning Predicts Prevalent and Incident Parkinson’s Disease from UK Biobank Fundus Imaging. Sci. Rep. 2024, 14, 3637. [Google Scholar] [CrossRef]
- Arnaldi, D.; Mattioli, P.; Famà, F.; Girtler, N.; Brugnolo, A.; Pardini, M.; Donniaquio, A.; Massa, F.; Orso, B.; Raffa, S.; et al. Stratification Tools for Disease-Modifying Trials in Prodromal Synucleinopathy. Mov. Disord. 2022, 37, 52–61. [Google Scholar] [CrossRef]
- Lang, A.E.; Siderowf, A.D.; Macklin, E.A.; Poewe, W.; Brooks, D.J.; Fernandez, H.H.; Rascol, O.; Giladi, N.; Stocchi, F.; Tanner, C.M.; et al. Trial of Cinpanemab in Early Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 408–420. [Google Scholar] [CrossRef] [PubMed]


| Reference | Year (Start) | Country | Participants | Incident PD | Follow-Up (Years) | Incidence a | Comments |
|---|---|---|---|---|---|---|---|
| Studies Designed for Investigating Prodromal PD | |||||||
| Kasten et al. (2013) [7] | 2010–continue | Germany (EPIPARK) | 715 | NA | 7 | NA | Ongoing study with updates in dataset every two years. |
| Gaenslen et al. (2014) [8] | 2009–2010 | Germany (TREND) | 698 | 16 | 7 | 3.27 | Identified 23 clinical prodromal markers. |
| Lerche et al. (2014) [9] | NA | Germany and Italy (PRIPS) | 1847 | 21 | 5 | 2.27 | Patients developing PD after two years have a similar pattern of those with three years. |
| Jennings et al. (2017) [10] | NA | USA (PARS) | 303 | 26 | 6 | 14.30 | Hyposmia and abnormal dopamine transporter imaging are predictive of PD conversion. |
| Hughes et al. (2018) [11] | 2012 | USA (HFPS/NHS ProPD) | 20,726 | 86 | 3 | 1.38 | Constipation, RBD, and hyposmia had sensitivity 29% and PPV of 35%. |
| Mahlknecht et al. (2018) [12] | 1990–2005 | Italy (Bruneck study) | 574 | 20 | 10 | 3.48 | MDS prodromal PD has PPV of 78%. |
| Studies Not Designed for Investigating Prodromal PD | |||||||
| Ross et al. (2012) [13] | 1965 | Japan and USA (HAAS) | 8000 | 137 | 30 | 0.57 | Evaluation of olfactory function, bowel movements, sleep, attention, and executive function are associated with PD. |
| Hofman et al. (2015) [14] | 1990 | The Netherlands (Rotterdam study) | 14,926 | 122 | 31 | 0.26 | NA |
| Shrestha et al. (2017) [15] | 1993 | USA (Agricultural health study) | 52,394 | 191 | 24 | 0.15 | Only in the male sex non-motor symptoms were associated with dose–response to PD. |
| Healthcare and Claims Databases | |||||||
| Schrag et al. (2015) [16] | 1996 | UK (THIN UK) | 11 million | 8166 | 10 | 0.07 | Constipation was associated with PD. |
| Searles Nielsen et al. (2017) [17] | 2004 | USA (Medicare) | 22 million | 89,790 | 5 | 0.81 | Using administrative claims data to predict PD. |
| Neuroimaging | Descriptions | |
|---|---|---|
| Dopamine transporter/fluorodeoxyphenylalanine (18F-DOPA) | Striatal dopaminergic denervation | |
| Fluorodeoxyglucose F 18 (18F-FDG) | Disease -related network pattern (PD, LBD), disease-specific metabolism (LBD), and compensatory hypermetabolism (PD) | |
| Technetium Tc 99m hexamethylpropyleneamine oxime single-photon emission computed tomography (99mTc-HMPAO SPECT) | Perfusion changes (prodromal-LBD) | |
| 18F-fluoroethoxybenzovesamicol positron emission tomography (18F-FEOBV PET) | Central cholinergic terminal loss (LBD), and possibly cholinergic denervation in the brainstem and pancreas/colon (early LBD study) | |
| Neuromelanin | Loss in the substantia nigra and locus coeruleus | |
| Magnetic resonance imaging | Gray matter | Gray matter atrophy in orbital frontal cortex and amygdaloid body in LBD |
| Cortical | Thinning and shape change | |
| Other | Iron sensitive (T2-weighted), free water (diffusion-weighted), diffusion tensor imaging along the perivascular space | |
| Retinal | Optical coherence tomography and optical coherence tomography angiography | |
| Cardiac (123I-metaiodobenzylguanidine) | Postganglionic sympathetic denervation | |
| Colonic | Transit time and colonic volume | |
| Reference | Patient Disease (n) | Controls (n) | Matrix | Sensitivity (%) | Specificity (%) | Comment |
|---|---|---|---|---|---|---|
| Fairfoul et al. (2016) [90] | RBD (3) | HC (20) | CSF | 100 | 95 | NA |
| Rossi et al. (2020) [91] | RBD (18) | HC (62) | CSF | 100 | 98 | NA |
| Iranzo et al. (2021) [92] | RBD (52) | HC (40) | CSF | 90 | 90 | SAA positivity 10 years before conversion |
| Stefani et al. (2021) [93] | RBD (63) | HC (40) | OM | 44 | 90 | NA |
| Poggiolini et al. (2022) [94] | RBD (54) | HC (55) | CSF | 64 | 96 | NA |
| Concha-Marambio et al. (2023) [95] | RBD (29) | HC (64) | CSF | 93 | 97 | SAA positivity 8.2 years before conversion |
| Iranzo et al. (2023) [96] | RBD (88) | HC (40) | CSF | 75 | 97 | NA |
| Liguori et al. (2023) [97] | RBD (41) | HC (40) | SB | 59 | 82 | NA |
| Okuzumi et al. (2023) [98] | RBD (9) | HC (128) | Serum | 44 | 91 | NA |
| Siderowf et al. (2023) [99] | RBD (33) | HC (157) | CSF | 84 | 96 | NA |
| Hyposmia (18) | HC (157) | CSF | 88 | 96 | NA | |
| Dam et al. (2024) [100] | RBD (61) | NA | CSF | 76 | NA | Data from the PPMI, PASADENA, and SPARK studies |
| Hyposmia (40) | NA | CSF | 72 | NA |
| Matrix | Technique | Sensitivity | Specificity | ||
|---|---|---|---|---|---|
| Clinical Overt | Prodromal | Clinical Overt | Prodromal | ||
| CSF | Oligomeric αSyn | Moderate | Low | Moderate | Moderate |
| RT-QuIC αSyn | High | Moderate | High | High | |
| Total αSyn | Intermediate | Unknown | Low | Unknown | |
| Blood | Oligomeric αSyn | Low | Unknown | Moderate | Unknown |
| Skin | RT-QuIC αSyn | High | Intermediate | High | High |
| Olfactory mucosa | RT-QuIC αSyn | Low | Low | High | High |
| Plasma | RT-QuIC αSyn | High | Intermediate | High | High |
| Reference | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Niu et al. (2020) [105] | 97 | 54 |
| Jiang et al. (2020) [106] | 94 | 72 |
| Yan et al. (2022) [107] | 61 | 81 |
| Sharafeldin et al. (2023) [108] | 69 | 100 |
| Yan et al. (2024) [104] | 86 | 87 |
| Reference | Country | Condition | Participants | Considerations |
|---|---|---|---|---|
| Lim et al. (2016) [128] | USA | Subjective cognitive decline | 11 | Disclosure did not affect mood. Those with elevated amyloid were more likely to make lifestyle changes. |
| Burns et al. (2017) [129] | USA | Cognitive normal | 97 | No sustained difference in depression and anxiety. Distress predicted by baseline anxiety and depression. |
| Taswell et al. (2018) [130] | Australia | Mild cognitive impairment | 99 | No difference in depression and anxiety. |
| Alzheimer’s disease | 34 | |||
| Grill et al. (2020) [131] | USA | Cognitive normal | 1705 | No difference in depression and anxiety. |
| Wake et al. (2020) [132] | Japan | Subjective cognitive decline | 42 | No difference in depression and anxiety. |
| Reference | Country | Population | Diet Type | Consideration |
|---|---|---|---|---|
| Gao et al. (2007) [138] | USA | 131,368 HC | MedDiet | RR 0.75 |
| Alcalay et al. (2012) [139] | USA | 257 PD, 198 HC | MedDiet | Hight-MedDiet (OR 0.86), and low-MedDiet associated with earlier onset PD |
| Cassani et al. (2017) [140] | Italy | 600 PD, 600 HC | MedDiet and others | No association with PD progression |
| Mischley et al. (2017) [141] | USA | 1053 PD | MedDiet | MedDiet-related foods slow PD progression |
| Agarwal et al. (2018) [142] | USA | 706 HC | MedDiet, MIND, and others | MedDiet HR 0.89 (if adjusted for depression) |
| Molsberry et al. (2020) [143] | USA | 17,400 HC | MedDiet, AHEI, and others | ≥3 prodromal features of PD OR 0.82 |
| Paknahad et al. (2020) [144] | Iran | 70 PD, 35 HC | MedDiet | MedDiet improves cognitive and motor outcomes |
| Metcalfe-Roach et al. (2021) [145] | Canada | 167 PD, 119 HC | MedDiet, MIND, and others | MedDiet and MIND were associated with later onset of PD |
| Strikwerda et al. (2021) [146] | Netherlands | 9414 HC | MedDiet | HR 0.89 |
| Yin et al. (2021) [147] | Sweden | 47,128 HC | MedDiet | HR 0.54 (adjust for age > 65 years-old) |
| Paknahad et al. (2022) [148] | Iran | 70 PD, 34 HC | MedDiet | Motor function improvement |
| Zhang et al. (2022) [149] | China | 71,640 HC | MedDiet | ≥2 prodromal features of PD OR 0.74 |
| Lawrie et al. (2023) [150] | UK | 162 PD | MIND | No statistically significant effect |
| Maraki et al. (2023) [151] | Greece | 1047 HC | MedDiet | 60–70% lower risk for possible/probable prodromal PD |
| Keramati et al. (2024) [152] | Iran | 120 PD and 50 HC | MedDiet | No statistically significant effect |
| Reference | Country | Population | Consideration |
|---|---|---|---|
| Chen et al. (2002) [156] | USA | 135,894 HC | (+) dairy, low-fat milk, cheese; (−) whole milk, yogurt, ice-cream, butter |
| Park et al. (2005) [157] | Japan | 8006 HC | (+) whole and low-fat milk; (−) cheese, ice-cream, butter |
| Chen et al. (2007) [158] | USA | 130,864 HC | (+) dairy, milk, sour cream; (−) cheese, yogurt, ice-cream, butter, cream |
| Miyake et al. (2010) [159] | Japan | 249 PD, 368 HC | (+) None; (−) dairy, milk, cheese, yogurt, ice-cream |
| Kyrozis et al. (2013) [160] | Greece | 26,716 HC | (+) dairy, milk; (−) cheese, yogurt |
| Sääksjärvi et al. (2013) [161] | Finland | 4524 HC | (+) milk, low-fat milk; (−) cheese, yogurt, butter |
| Jiang et al. (2014) [162] | Meta-analysis | (+) dairy, milk, cheese; (−) yogurt, butter | |
| Hughes et al. (2017) [163] | USA | 129,346 | (−) low-fat milk |
| Domenighetti et al. (2022) [164] | European | 9823 PD, 368 HC | (+) dairy |
| Hajji-Louati et al. (2024) [165] | France | 71,542 HC | (+) total milk (HR/1-SD 1.09) |
| Gröninger et al. (2024) [166] | European | 183,225 HC | No statistically significant effect |
| Reference | Country | Population | Vitamin | Consideration |
|---|---|---|---|---|
| de Rijk et al. (1997) [169] | The Netherland | 5342 HC | E | (−): Vit E OR 0.5 |
| Chen et al. (2004) [170] | USA | 415 PD | Folate, B6, B12 | No statistically significant effect |
| Etminan et al. (2005) [171] | Meta-analysis | NA | C, E, carotenoids | (−): Vit E RR 0.81 Vit C and carotenoids: no statistically significant effect |
| de Lau et al. (2006) [172] | The Netherland | 5289 HC | Folate, B6, B12 | (−): B6 HR 0.69 Folate and B12: no statistically significant effect |
| Knekt et al. (2010) [173] | Finland | 7217 HC | D | (−): Vit D RR 0.33 |
| Miyake et al. (2011) [174] | Japan and UK | 249 PD, 368 HC | D | No statistically significant effect |
| Lv et al. (2014) [175] | Meta-analysis | NA | D | Vit D < 75 nmol/mL (insufficiency): OR 1.5 Vit D < 50 nmol/mL (deficiency): OR 2.2 |
| Takeda et al. (2014) [176] | Meta-analysis | NA | A, carotenoids | No statistically significant effect, except by lutein OR 1.85 |
| Shen et al. (2015) [177] | Meta-analysis | NA | Folate, B6, B12 | No statistically significant effect |
| Hughes et al. (2016) [178] | USA | 173,229 HC | β-carotene, C, E | No statistically significant effect |
| Shrestha et al. (2016) [179] | USA | 12,762 HC | D | No statistically significant effect |
| Luo et al. (2018) [180] | Meta-analysis | NA | D | Vit D 20–30 ng/mL (insufficiency): OR 1.73 Vit D < 20 ng/mL (deficiency): OR 2.08 |
| Wei et al. (2018) [181] | Meta-analysis | NA | E | No statistically significant effect |
| Ying et al. (2020) [182] | Singapore, China | 63,257 HC | A, C, E, carotenoids | No statistically significant effect |
| Chang et al. (2021) [183] | Meta-analysis | NA | C, E | (−): Vit E OR 0.79 Vit C: no statistically significant effect |
| Hantikainen et al. (2021) [184] | Sweden | 43,865 HC | C, E, β-carotene | (−): Vit C HR 0.68; Vit E HR 0.68 β-carotene: no statistically significant effect |
| Talebi et al. (2022) [185] | Meta-analysis | NA | C, E, carotenoids | (−): Vit E RR 0.84; Vit C RR 0.94; β-carotene RR 0.94 (+): lutein RR 1.86 |
| Wu et al. (2022) [186] | Meta-analysis | NA | A, β-carotene | (−): β-carotene OR 0.83 Vit A: no statistically significant effect |
| Flores-Torres et al. (2023) [187] | USA | 129,802 HC | Folate, B6, B12 | (−): B12 HR 0.80 Folate and B16: no statistically significant effect |
| Hao et al. (2023) [188] | USA | 13,340 | E | (−): Vit E OR 0.91 |
| Gröninger et al. (2024) [166] | European | 183,225 HC | D | No statistically significant effect |
| Niu et al. (2024) [189] | Meta-analysis | NA | C, E, β-carotene | (−): Vit E RR 0.87 Vit C and β-carotene: no statistically significant effect |
| Wang et al. (2024) [190] | European | 1.2 million HC | D | No statistically significant effect |
| Study Start to Completion | Identifier | Condition | Intervention | N Enrolled | Comment |
|---|---|---|---|---|---|
| 27 September 2018 to 27 April 2020 | NCT03671772 | RBD | NA | 170 | Progression of Prodromal Markers of α-synucleinopathy Neurodegeneration in the FDRs of Patients With RBD |
| June 2010 to 30 June 2020 | NCT01141023 | PD | DatScan | 952 | Study to Identify Clinical, Imaging and Biologic Markers of Parkinson Disease Progression (PPMI) |
| 1 September 2021 to 1 September 20212 | NCT04266457 | RBD, PD, LBD | NA | NA | Establishing Alpha-synuclein RT-QuIC Assay as a Diagnostic Technique in REM Sleep Behaviour Disorder |
| 15 May 2019 to 1 October 2022 | NCT04048603 | RBD | NA | 182 | Search for Biomarkers of Neurodegenerative Diseases in Idiopathic REM Sleep Behavior Disorder |
| 1 January 2020 to 1 January 2023 | NCT04152655 | RBD, PD | Idebenone | 180 | A Study of Efficacy and Safety of Idebenone vs. Placebo in Prodromal Parkinson Disease (SEASEiPPD) |
| 16 May 2017 to 16 January 2024 | NCT05253560 | GBA1 Mutation Carriers | NA | 600 | Prodromal Parkinsonian Features in GBA1 Mutation Carriers |
| 3 January 2022 to 30 June 2024 | NCT05353881 | RBD | NA | 102 | Prodromal Markers in Recurrent Dream Enactment Behaviors Without REM Sleep Without Atonia |
| 6 November 2014 to 6 November 2024 | NCT02305147 | PD | Clinical, biological and imaging follow-up | 360 | Cohort Study to Identify Predictor Factors of Onset and Progression of Parkinson’s Disease (ICEBERG) |
| 12 August 2022 to 1 May 2025 | NCT05826457 | LBD, PD, MSA, RBD | NA | 500 | North American Prodromal Synucleinopathy Consortium Stage 2 (NAPS2) |
| 3 January 2022 to 2 January 2022 | NCT05353959 | PD | NA | 400 | Progression Follow up of the First-degree Relatives of Patients with REM Sleep Behavior Disorder |
| 30 April 2021 to March 2025 | NCT05677529 | PD | NA | 8000 | Prodromal and Overt Parkinson’s Disease Epidemiological Study in Brazil (PROBE-PD) |
| 10 September 2020 to June 2025 | NCT04507139 | PD | NA | 50 | Early Longitudinal Imaging in Parkinson’s Progression Markers Initiative Using [¹⁸F] AV-133 and DaTscan™ |
| 4 May 2021 to 1 July 2025 | NCT04588285 | LBD | Ambroxol | 180 | Ambroxol in New and Early DLB, A Phase IIa Multicentre Randomized Controlled Double Blind Clinical Trial (ANeED) |
| 15 September 2022 to 31 August 2025 | NCT05757206 | RBD | Syn-One Test | 80 | The Syn-Sleep Study |
| 1 May 2023 to 30 April 2026 | NCT05934188 | PD | NA | 200 | Exploring the Gut–Brain Axis in Ageing and Neurodegeneration (GutBrain) |
| 29 August 2018 to 31 July 2026 | NCT03623672 | LBD, PD, MSA, RBD | NA | 500 | North American Prodromal Synucleinopathy (NAPS) Consortium |
| October 2024 to November 2026 | NCT06582121 | RBD, PD | Polysomnography | 457 | Study of Sleep Disorders in Prodromal and Definite Parkinsons Disease (SOMPARK) |
| 1 July 2021 to December 2026 | NCT04724941 | PD | NA | 2000 | Prodromal Alpha-Synuclein Screening in Parkinson’s Disease Study (PASS-PD) |
| 15 January 2024 to 1 December 2026 | NCT06193252 | PD, RBD | Physical activity | 110 | Slowing Parkinson’s Early Through Exercise Dosage-Netherlands (Slow-SPEED-NL) |
| 1 January 2023 to 31 December 2026 | NCT05611372 | RBD, PD | Rasagiline | 732 | Efficacy and Safety of Rasagiline in Prodromal Parkinson’s Disease |
| 12 April 2024 to 31 December 2026 | NCT06456684 | PD | Fluoro [18F]promethazine | 76 | AV133 Longitudinal Imaging Study in Patients With Early and Prdromal Parkinson’s Disease |
| 8 February 2024 to 30 December 2028 | NCT06467461 | LBD, PD, RBD | Skin biopsy, speech testing, ultra-high field 7T MRI | 60 | Identification of Prodromal Neurodegeneration in Serotonergic-Induced REM Sleep Behavior Disorder |
| 1 April 2024 to 30 December 2028 | NCT06420310 | PD | Esposure to pesticide | 260 | Pesticides and Parkinson’s Disease (Pest-PD) |
| 1 February 2023 to December 2032 | NCT05740683 | Anosmia, hyposmia, olfactory dysfunction | RT-QuiC | 100 | Alpha-synuclein Rt-quic and Neurologic Symptoms in Persons With idiOpathic anosMiA (AROMA) |
| 1 July 2020 to December 2033 | NCT04477785 | PD | NA | 4500 | PPMI Clinical—Establishing a Deeply Phenotyped PD Cohort |
| 28 July 2021 to December 2041 | NCT05065060 | PD | NA | 500,000 | Parkinson Progression Marker Initiative Online (PPMI Online) |
| Study | N | Type of Data | Machine Learning Models | Technique | Key Findings | Comparison with Other Models | AUC, SN, SP |
|---|---|---|---|---|---|---|---|
| Karabayir et al. (2023) [201] | 1189 | ECG data | Deep Learning Model | Convolutional Neural Networks | Developed a deep learning model to identify prodromal PD with high accuracy from ECG data. | Compared with logistic regression (ML), outperforming it. | AUC 0.74 |
| Vaish et al. (2024) [202] | NA | Clinical Data | Machine Learning Approach | NA | Developed an ML prediction model to improve risk prediction for PD, enabling early intervention and resource prioritization. | NA | NA |
| Warden et al. (2021) [203] | 88,265 | Administrative claims data | Various Prediction Approaches | Logistic Regression, Random Forest | Compared different ML-based prediction approaches for identifying prodromal PD using claims data. | Compared multiple ML models (logistic regression, decision trees, random forest). | Combined approach was the best model with AUC 0.83; SN 0.76; SP 0.76 |
| Tabashum et al. (2024) [204] | NA | Various data sources | Systematic Review of ML Models | Multiple Techniques | Systematic review highlighting the effectiveness of ML in predicting PD, but with variation in reported metrics. | Compared multiple ML models (overview study). | NA |
| Makarious et al. (2022) [205] | PPMI study | Multimodal data (genetic, clinical) | Automated ML Framework (GenoML) | Ensemble Learning | Multimodal model combining genetic and clinical data to predict PD risk systematically. | Compared with single-modality models, demonstrating superior accuracy. | AUC 0.85; SN 0.93; SP 0.43 |
| Koo et al. (2025) [206] | 9020 | Diagnostic and medication codes | Deep Learning Algorithm | Recurrent Neural Networks | Developed a deep learning model using diagnostic and medication data to screen for prodromal PD. | Compared with traditional statistical models, outperforming them. | AUC 0.92; SN 0.81; SP 0.94 |
| Prashant et al. (2018) [207] | PPMI study | Patient Questionnaire Data | Logistic Regression, Random Forests, Boosted Trees, SVM | Supervised Learning | Developed models to classify early PD from healthy controls using patient questionnaire data. | Compared multiple ML models (SVM, boosted trees, logistic regression). | SVM was the best model with AUC 0.96–0.98; SN 0.95–0.97; SP 0.82–0.94. But all the models had AUC from 0.96 to 0.98 |
| Dehsarvi et al. (2019) [208] | 128 | Resting-State fMRI Data | Evolutionary Algorithms | Cartesian Genetic Programming | Developed automatic methods for detecting brain imaging preclinical biomarkers for PD, achieving high classification accuracies. | Compared with Artificial Neural Networks (ANN) and Support Vector Machines (SVM); CGP provided comparable performance. | SN was 0.75 for differentiating prodromal PD from healthy controls |
| Tran et al. (2023) [209] | 296 | Retinal Fundus Imaging | Deep Learning Models | Transfer Learning | Predicted prevalent and incident PD from fundus imaging using deep learning. | Compared with conventional feature extraction models, showing improvement. | AlexNet was the best model with AUC 0.77; SN 0.76; SP 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissardo, J.P.; Caprara, A.L.F. A Narrative Review on Biochemical Markers and Emerging Treatments in Prodromal Synucleinopathies. Clin. Pract. 2025, 15, 65. https://doi.org/10.3390/clinpract15030065
Rissardo JP, Caprara ALF. A Narrative Review on Biochemical Markers and Emerging Treatments in Prodromal Synucleinopathies. Clinics and Practice. 2025; 15(3):65. https://doi.org/10.3390/clinpract15030065
Chicago/Turabian StyleRissardo, Jamir Pitton, and Ana Leticia Fornari Caprara. 2025. "A Narrative Review on Biochemical Markers and Emerging Treatments in Prodromal Synucleinopathies" Clinics and Practice 15, no. 3: 65. https://doi.org/10.3390/clinpract15030065
APA StyleRissardo, J. P., & Caprara, A. L. F. (2025). A Narrative Review on Biochemical Markers and Emerging Treatments in Prodromal Synucleinopathies. Clinics and Practice, 15(3), 65. https://doi.org/10.3390/clinpract15030065







