Effect of Continuous Positive Airway Pressure (CPAP) Mode on Lung Function, Exercise Tolerance, Vital Signs, and Dyspnea After Acute SARS-CoV-2 Infection
Abstract
1. Introduction
2. Methods
2.1. Ethical Aspects
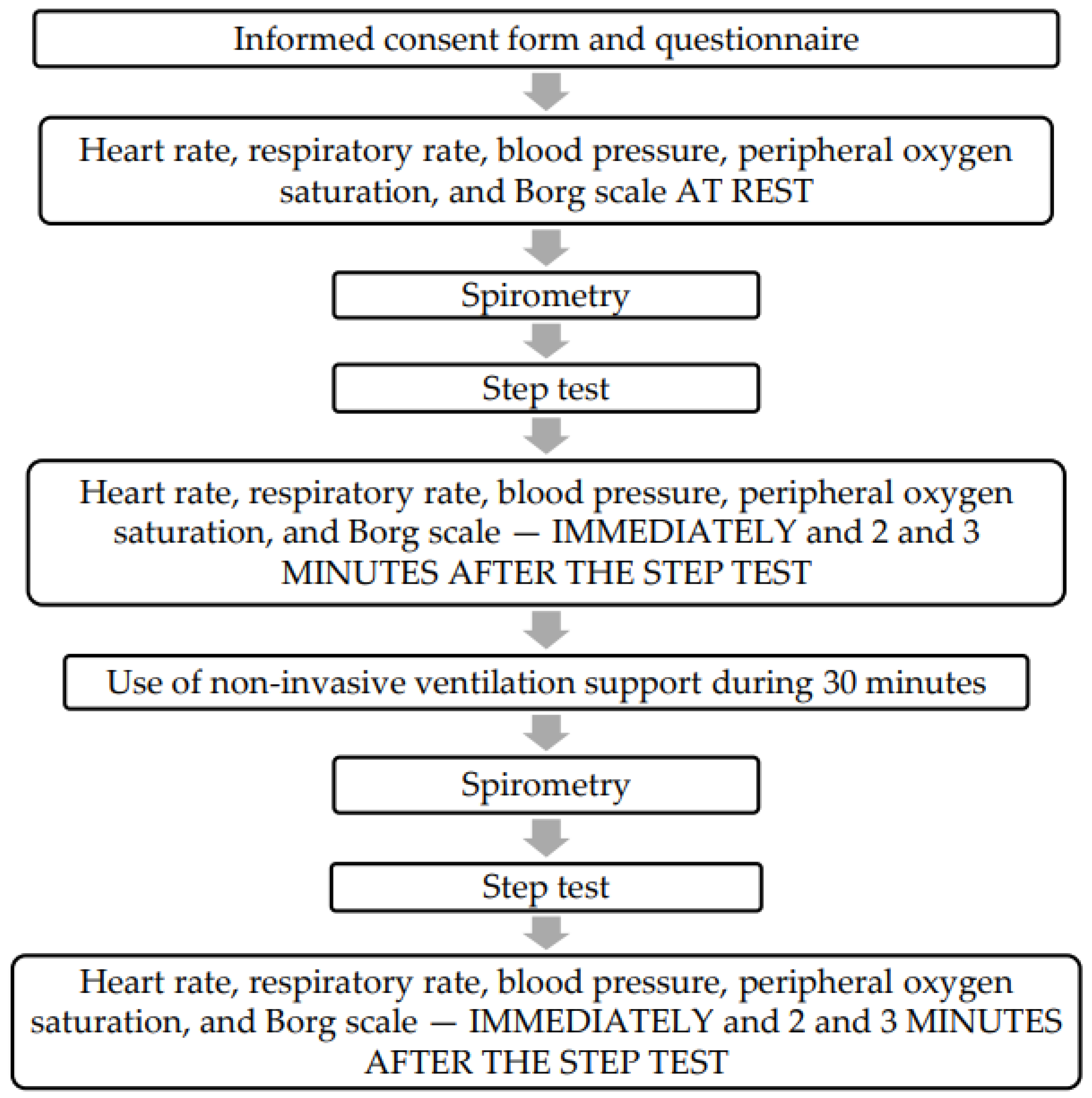
2.2. Study Population and Protocols Used in This Study to Measure Biomarkers
2.3. Submaximal Exercise Protocol of Two-Minute Step Test
2.4. Spirometry Test Protocol
2.5. Clinical Signs and Symptoms and Measurements on the Borg Scale
2.6. NIV Protocol
2.7. Statistical Analysis
3. Results
3.1. Spirometry Markers and Submaximal Exercise Protocol of a Two-Minute Step Test
3.2. Vital Signs and Sensation of Dyspnea (Borg Scale)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, P.; Baldwin, C.; Beach, L.; Bissett, B.; Boden, I.; Cruz, S.M.; Gosselink, R.; Granger, C.L.; Hodgson, C.; E Holland, A.; et al. Physiotherapy management for COVID-19 in the acute hospital setting and beyond: An update to clinical practice recommendations. J. Physiother. 2021, 68, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, J.C.; Moreno, M.R.; Piqueras-Sola, B.; Cortés-Martín, J.; Liñán-González, A.; Mellado-García, E.; Rodriguez-Blanque, R. Physical Therapies in the Treatment of Post-COVID Syndrome: A Systematic Review. Biomedicines 2023, 11, 2253. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Siqueira, B.A.; Sansone, N.M.S.; Marson, F.A.L. COVID-19 in Brazil: A Three-Year Update. Diagn. Microbiol. Infect. Dis. 2023, 107, 16074. [Google Scholar] [CrossRef] [PubMed]
- Katz, G.M.; Bach, K.; Bobos, P.; Cheung, A.; Décary, S.; Goulding, S.; Herridge, M.S.; McNaughton, C.D.; Palmer, K.S.; Razak, F.A.; et al. Understanding How Post–COVID-19 Condition Affects Adults and Health Care Systems. JAMA Health Forum 2023, 4, e231933. [Google Scholar] [CrossRef]
- Sommen, S.L.; Havdal, L.B.; Selvakumar, J.; Einvik, G.; Leegaard, T.M.; Lund-Johansen, F.; Michelsen, A.E.; Mollnes, T.E.; Stiansen-Sonerud, T.; Tjade, T.; et al. Inflammatory markers and pulmonary function in adolescents and young adults 6 months after mild COVID-19. Front. Immunol. 2023, 13, 1081718. [Google Scholar] [CrossRef] [PubMed]
- Scharf, R.E.; Anaya, J.-M. Post-COVID Syndrome in Adults—An Overview. Viruses 2023, 15, 675. [Google Scholar] [CrossRef]
- Najafi, M.B.; Javanmard, S.H. Post-COVID-19 Syndrome Mechanisms, Prevention and Management. Int. J. Prev. Med. 2023, 14, 59. [Google Scholar] [CrossRef]
- Shah, B.; Ahmad, M.N.; Khalid, M.; Minhas, A.; Ali, R.; Sarfraz, Z.; Sarfraz, A. Long COVID and Wavering Incidence of Pulmonary Embolism: A Systematic Review. J. Community Hosp. Intern. Med. Perspect. 2023, 13, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Panei, P.; Arcieri, R.; Germinario, E.A.P.; Capuano, A.; Margari, L.; Chiarotti, F.; Curatolo, P. Safety of Methylphenidate and Atomoxetine in Children with Attention-Deficit/Hyperactivity Disorder (ADHD): Data from the Italian National ADHD Registry. CNS Drugs 2015, 29, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.H.; Branson, R.D.; Lipnick, M.S. Respiratory Drive, Dyspnea, and Silent Hypoxemia: A Physiological Review in the Context of COVID-19. Respir. Care 2022, 67, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Guinto, E.; Gerayeli, F.V.; Eddy, R.L.; Lee, H.; Milne, S.; Sin, D.D. Post-COVID-19 dyspnoea and pulmonary imaging: A systematic review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220253. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.J.; Hall, C.B. Clinical significance of pulmonary function tests. Alterations in pulmonary function following respiratory viral infection. Chest 1979, 76, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.K.; Afzal, S.; Ahlström, M.G.; Nordestgaard, B.G.; Schneider, U.V.; Nielsen, L.; Kofoed, K.; Benfield, T.; Ronit, A. Lung Function Decline in Relation to COVID-19 in the General Population: A Matched Cohort Study With Prepandemic Assessment of Lung Function. J. Infect. Dis. 2022, 225, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Hockele, L.F.; Affonso, J.V.S.; Rossi, D.; Eibel, B. Pulmonary and Functional Rehabilitation Improves Functional Capacity, Pulmonary Function and Respiratory Muscle Strength in Post COVID-19 Patients: Pilot Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 14899. [Google Scholar] [CrossRef]
- Tanni, S.E.; Fabro, A.T.; de Albuquerque, A.; Ferreira, E.V.M.; Verrastro, C.G.Y.; Sawamura, M.V.Y.; Ribeiro, S.M.; Baldi, B.G. Pulmonary fibrosis secondary to COVID-19: A narrative review. Expert Rev. Respir. Med. 2021, 15, 791–803. [Google Scholar] [CrossRef]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological-pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346-2021. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, G.H.J.; Park, S.B.; Lee, S.I.; Koh, J.S.; Brown, M.S.; Abtin, F.; McNitt-Gray, M.F.; Goldin, J.G.; Lee, J.S. Quantitative Computed Tomography Lung COVID Scores with Laboratory Markers: Utilization to Predict Rapid Progression and Monitor Longitudinal Changes in Patients with Coronavirus 2019 (COVID-19) Pneumonia. Biomedicines 2024, 12, 120. [Google Scholar] [CrossRef]
- Yazdi, N.A.; Ghadery, A.H.; SeyedAlinaghi, S.; Jafari, F.; Jafari, S.; Hasannezad, M.; Koochak, H.E.; Salehi, M.; Manshadi, S.A.D.; Meidani, M.; et al. Predictors of the chest CT score in COVID-19 patients: A cross-sectional study. Virol. J. 2021, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Kaymaz, D.; Lanini, B.; Vagheggini, G.; Ergün, P.; Gigliotti, F.; Ambrosino, N.; Paneroni, M. Non-invasive ventilation during cycle exercise training in patients with chronic respiratory failure on long-term ventilatory support: A randomized controlled trial. Respirology 2018, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Marrara, K.T.; Di Lorenzo, V.A.P.; Jaenisch, R.B.; Cabiddu, R.; Sato, T.d.O.; Mendes, R.G.; Oliveira, C.R.; Costa, D.; Borghi-Silva, A. Noninvasive Ventilation as an Important Adjunct to an Exercise Training Program in Subjects With Moderate to Severe COPD. Respir. Care 2018, 63, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Gloeckl, R.; Andrianopoulos, V.; Stegemann, A.; Oversohl, J.; Schneeberger, T.; Schoenheit-Kenn, U.; Hitzl, W.; Dreher, M.; Koczulla, A.R.; Kenn, K. High-pressure non-invasive ventilation during exercise in COPD patients with chronic hypercapnic respiratory failure: A randomized, controlled, cross-over trial. Respirology 2019, 24, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Borel, J.-C.; Wuyam, B.; Chouri-Pontarollo, N.; Deschaux, C.; Levy, P.; Pépin, J.-L. During exercise non-invasive ventilation in chronic restrictive respiratory failure. Respir. Med. 2008, 102, 711–719. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, N.R. Physiologic Effects of Noninvasive Ventilation. Respir. Care 2019, 64, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol. 2010, 199, 367–383. [Google Scholar] [CrossRef]
- Gama, G.; Farinatti, P.; Rangel, M.V.d.S.; Mira, P.A.d.C.; Laterza, M.C.; Crisafulli, A.; Borges, J.P. Muscle metaboreflex adaptations to exercise training in health and disease. Eur. J. Appl. Physiol. 2021, 121, 2943–2955. [Google Scholar] [CrossRef]
- Cortés-Telles, A.; López-Romero, S.; Figueroa-Hurtado, E.; Pou-Aguilar, Y.N.; Wong, A.W.; Milne, K.M.; Ryerson, C.J.; Guenette, J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021, 288, 103644. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, A.; Misuraca, C.; Bianchi, A.; Borsa, N.; Limonta, S.; Maggiolini, S.; Bonardi, D.R.; Corsonello, A.; Di Rosa, M.; Soraci, L.; et al. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection 2020, 49, 153–157. [Google Scholar] [CrossRef]
- Postolache, P.; Săndulache, Ș.; Ghimuș, C.; Nechifor, A. Assessment of Exercise Capacity: A key element in Pulmonary Rehabilitation. In Cardiorespiratory Fitness; Sözen, H., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ling, Y.; Bai, T.; Xie, Y.; Huang, J.; Li, J.; Xiong, W.; Yang, D.; Chen, R.; Lu, F.; et al. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am. J. Respir. Crit. Care Med. 2020, 201, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; Jennings, G.; Xue, F.; Duggan, E.; Gormley, J.; Monaghan, A. Predictors of Submaximal Exercise Test Attainment in Adults Reporting Long COVID Symptoms. J. Clin. Med. 2022, 11, 2376. [Google Scholar] [CrossRef]
- Reis, H.V.; Borghi-Silva, A.; Catai, A.M.; Reis, M.S. Impact of CPAP on physical exercise tolerance and sympathetic-vagal balance in patients with chronic heart failure. Braz. J. Phys. Ther. 2014, 18, 218–227. [Google Scholar] [CrossRef]
- Torres-Castro, R.; Núñez-Cortés, R.; Larrateguy, S.; Alsina-Restoy, X.; Barberà, J.A.; Gimeno-Santos, E.; García, A.R.; Sibila, O.; Blanco, I. Assessment of Exercise Capacity in Post-COVID-19 Patients: How Is the Appropriate Test Chosen? Life 2023, 13, 621. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir Crit Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Parazzi, P.L.F.; Marson, F.A.L.; Ribeiro, M.A.G.O.; Schivinski, C.I.S.; Ribeiro, J.D. Correlation between parameters of volumetric capno-graphy and spirometry during a submaximal exercise protocol on a treadmill in patients with cystic fibrosis and healthy controls. Pulmonology 2019, 25, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Neto, M.G.; Duarte, L.F.G.; de Sousa Rodrigues, E., Jr.; Bittencourt, H.S.; Dos Santos, N.G.; David, B.C.; da Silva Lima, E.; Dos Reis, H.F.C. Effects of noninvasive ventilation with bilevel positive airway pressure on exercise tolerance and dyspnea in heart failure patients. Hellenic J. Cardiol. 2018, 59, 317–320. [Google Scholar] [CrossRef]
- Chermont, S.; Quintão, M.M.; Mesquita, E.T.; Rocha, N.N.; Nóbrega, A.C.L. Noninvasive Ventilation With Continuous Positive Airway Pressure Acutely Improves 6-Minute Walk Distance in Chronic Heart Failure. J. Cardiopulm. Rehabil. Prev. 2009, 29, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Wu, Q.; Wu, X.; Hao, S.; Xie, L.; Li, S. Non-invasive ventilation intervention during exercise training in individuals with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2021, 64, 101460. [Google Scholar] [CrossRef]
- Baffa, G.S.; da Luz Goulart, C.; Caruso, F.R.; de Araújo, A.S.G.; Dos Santos, P.B.; Roscani, M.G.; Prone, F.R.; Bonjorno, J.C.; Mendes, R.G.; Borghi-Silva, A. Non-invasive ventilation can modulate heart rate variability during high-intensity exercise in COPD-CHF patients. Heart Lung. 2021, 50, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Okan, S.; Okan, F.; Yücesoy, F.D. Evaluation of pulmonary function and exercise capacity after COVID-19 pneumonia. Hear. Lung 2022, 54, 1–6. [Google Scholar] [CrossRef]
- Lin, M.; Stewart, M.T.; Zefi, S.; Mateti, K.V.; Gauthier, A.; Sharma, B.; Martinez, L.R.; Ashby, C.R.; Mantell, L.L. Dual effects of supplemental oxygen on pulmonary infection, inflammatory lung injury, and neuromodulation in aging and COVID-19. Free. Radic. Biol. Med. 2022, 190, 247–263. [Google Scholar] [CrossRef]
- Palot, A.; Nguyên, X.-L.; Launois, S.; Prigent, A.; Graml, A.; Aversenq, E.; Koltes, C.; Recart, D.; Lavergne, F. Effect of switching from continuous to bilevel positive airway pressure on sleep quality in patients with obstructive sleep apnea: The prospective POP IN VAuto study. J. Thorac. Dis. 2023, 15, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chen, S.; Zhang, Y.; Dong, F.; Zhang, Z.; Hu, B.; Zhu, Z.; Li, F.; Wang, X.; Wang, Y.; et al. Diffusion Capacity Abnormalities for Carbon Monoxide in Patients with COVID-19 At Three-Month Follow-up. Eur. Respir. J. 2021, 58, 2003677. [Google Scholar] [CrossRef] [PubMed]
- Barisione, G.; Brusasco, V. Lung diffusing capacity for nitric oxide and carbon monoxide following mild-to-severe COVID-19. Physiol. Rep. 2021, 9, e14748. [Google Scholar] [CrossRef]
- Ekbom, E.; Frithiof, R.; Öi, E.; Larson, I.M.; Lipcsey, M.; Rubertsson, S.; Wallin, E.; Janson, C.; Hultström, M.; Malinovschi, A. Impaired diffusing capacity for carbon monoxide is common in critically ill Covid-19 patients at four months post-discharge. Respir Med. 2021, 182, 106394. [Google Scholar]
- Ricotta, A.C.G.; Nunes, G.B.; Almeida AF de Gonzaga, F.M.G.; Licurci M das, G.B.; Nogueira, D.V. Post-Covid effects on respiratory mechanics, pulmonary function, response to physical exercise and quality of life. Res. Soc. Dev. 2022, 11, e324111537053. [Google Scholar]
- da Cruz, M.R.; Camilo, L.M.; Xavier, T.B.d.C.; Ribeiro, G.C.d.M.; Medeiros, D.M.; Reis, L.F.d.F.; Guimarães, B.L.d.S.; Japiassú, A.M.; Carvalho, A.R.S. Positive end-expiratory pressure induced changes in airway driving pressure in mechanically ventilated COVID-19 Acute Respiratory Distress Syndrome patients. Crit. Care 2023, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Freund, O.; Breslavsky, A.; Fried, S.; Givoli-Vilensky, R.; Cohen-Rubin, S.; Zacks, N.; Kleinhendler, E.; Unterman, A.; Frydman, S.; Wand, O.; et al. Interactions and clinical implications of serological and respiratory variables 3 months after acute COVID-19. Clin. Exp. Med. 2023, 23, 3729–3736. [Google Scholar] [CrossRef]

| Marker | Data | Mild COVID-19 * | Severe COVID-19 * | p-Value |
|---|---|---|---|---|
| Sex | Female | 20 (80%) | 12 (48%) | 0.038 a |
| Male | 5 (20%) | 13 (52%) | ||
| Age during hospitalization (years) | 34 (29.00 to 40.50) | 46 (34 to 54) | 0.008 c | |
| Race | White people | 22 (88%) | 23 (92%) | 1.000 b |
| Mixed people | 3 (12%) | 2 (8%) | ||
| Vaccine against coronavirus disease (COVID-19) | No information | 0 | 1 (4%) | 0.235 b |
| 1 dose | 0 | 2 (8%) | ||
| 2 doses | 25 (100%) | 22 (88%) | ||
| Comorbidities | Absent (none) | 18 (72%) | 10 (40%) | 0.045 b |
| Present (≥1) | 7 (28%) | 15 (60%) | ||
| Pulmonary involvement during active infection | No information | NA | 2 (8%) | NA |
| >50% | NA | 16 (60%) | ||
| ≤50% | NA | 7 (32%) | ||
| Hospitalization (days) | NA | 7.00 (5.00 to 12.00) | NA | |
| Non-invasive ventilation (NIV, days) | NA | 4.00 (2.50 to 7.00) | NA | |
| NIV interface during hospitalization | NIV | NA | 16 (60%) | NA |
| NIV + others | NA | 10 (40%) |
| Biomarkers | Groups * | Before Receiving NIV | After Receiving NIV | p-Value a |
|---|---|---|---|---|
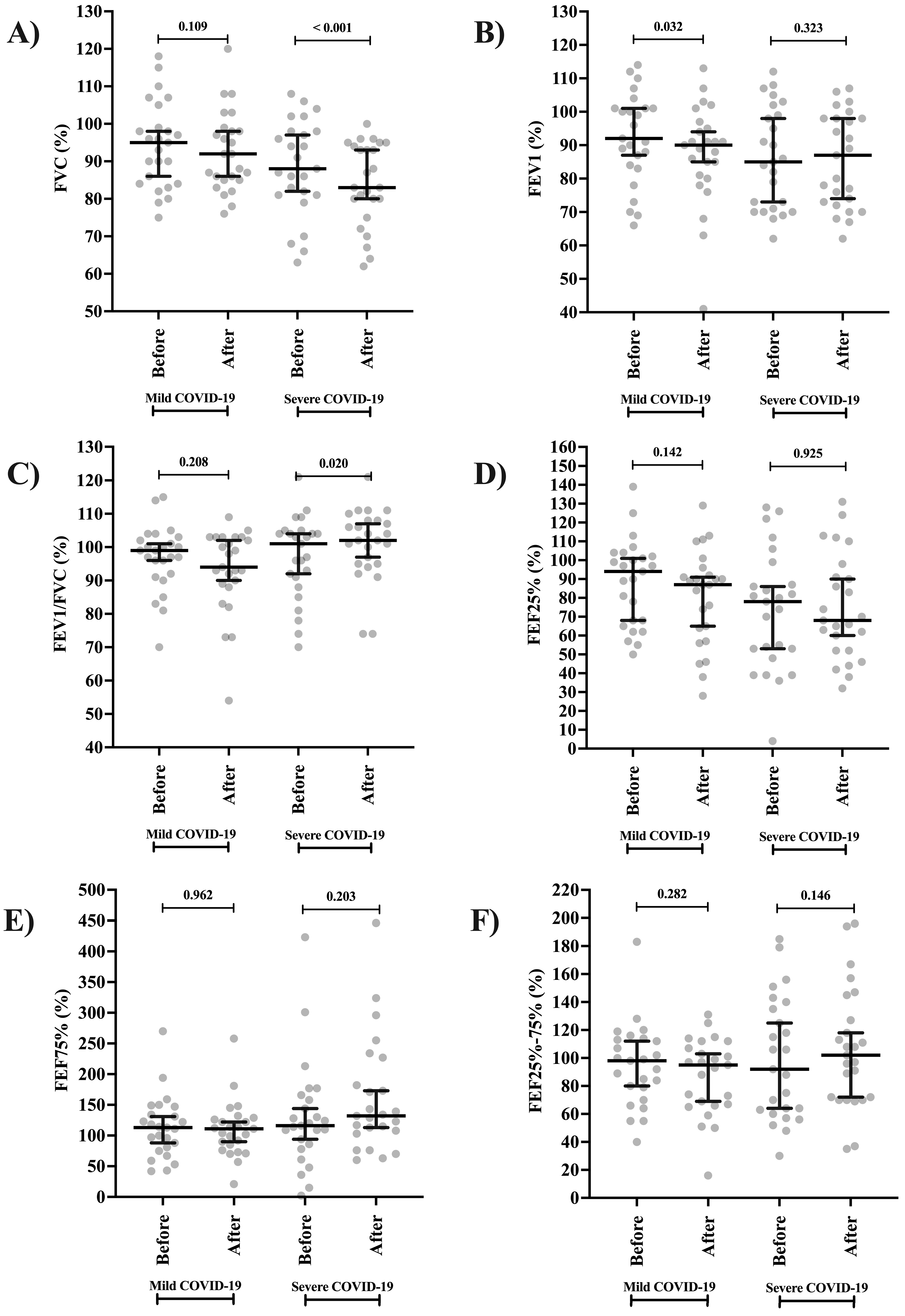
| FVC | Mild | 95.00 (84.00 to 102.00) | 92.00 (83.50 to 101.00) | 0.109 |
| Severe | 88.00 (81.00 to 98.00) | 83.00 (77.50 to 94.50) | <0.001 | |
| p-value b | 0.187 | 0.021 | ||
| FEV1 | Mild | 92.00 (85.00 to 98.50) | 90.00 (80.50 to 96.00) | 0.032 |
| Severe | 85.00 (70.50 to 100.50) | 87.00 (72.50 to 98.00) | 0.323 | |
| p-value b | 0.171 | 0.580 | ||
| FEV1/FVC | Mild | 99.00 (91.50 to 101.50) | 94.00 (88.67 to 103.00) | 0.208 |
| Severe | 101.20 (89.50 to 104.50) | 102.00 (95.00 to 108.00) | 0.020 | |
| p-value b | 0.634 | 0.008 | ||
| FEF25% | Mild | 94.00 (66.50 to 103.00) | 87.00 (60.50 to 94.00) | 0.142 |
| Severe | 78.00 (50.50 to 93.00) | 68.00 (52.00 to 94.61) | 0.925 | |
| p-value b | 0.064 | 0.382 | ||
| FEF75% | Mild | 113.00 (78.00 to 140.50) | 111.00 (80.50 to 127.50) | 0.968 |
| Severe | 116.00 (82.00 to 162.00) | 132.20 (105.50 to 204.50) | 0.023 | |
| p-value b | 0.628 | 0.029 | ||
| FEF25–75% | Mild | 98.00 (74.50 to 113.50) | 95.00 (66.50 to 109.50) | 0.282 |
| Severe | 92.00 (61.50 to 137.50) | 102.00 (71.00 to 136.00) | 0.146 | |
| p-value b | 0.900 | 0.130 | ||
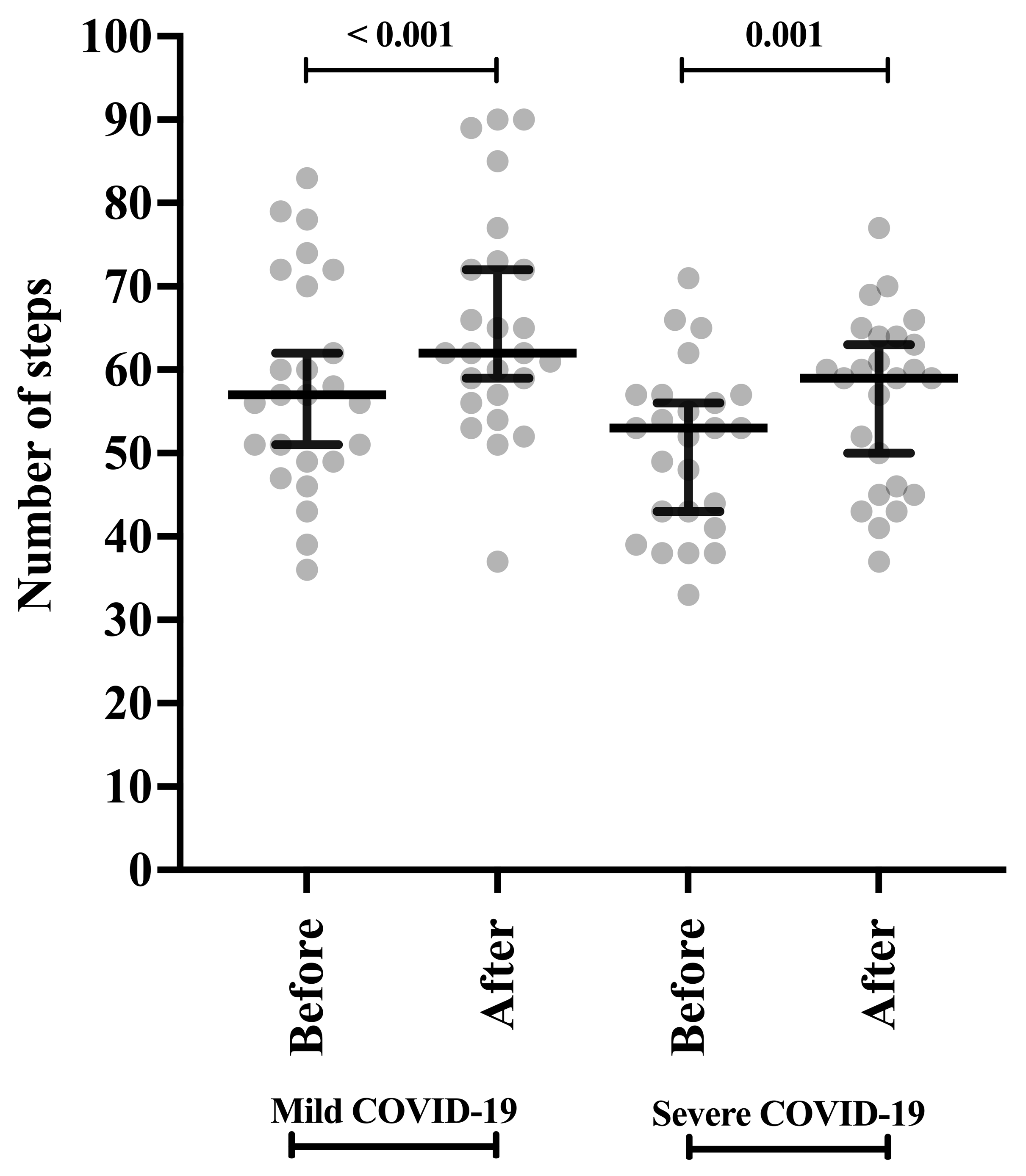
| Number of steps | Mild | 57.00 (49.00 to 71.00) | 62.00 (56.50 to 72.50) | <0.001 |
| Severe | 53.00 (42.00 to 57.00) | 59.00 (45.50 to 64.05) | <0.001 | |
| p-value b | 0.042 | 0.042 |
| Biomarkers | Groups * | Rest | Before Receiving NIV | After Receiving NIV | p-Value a | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 min | 3 min | Post | 2 min | 3 min | ||||
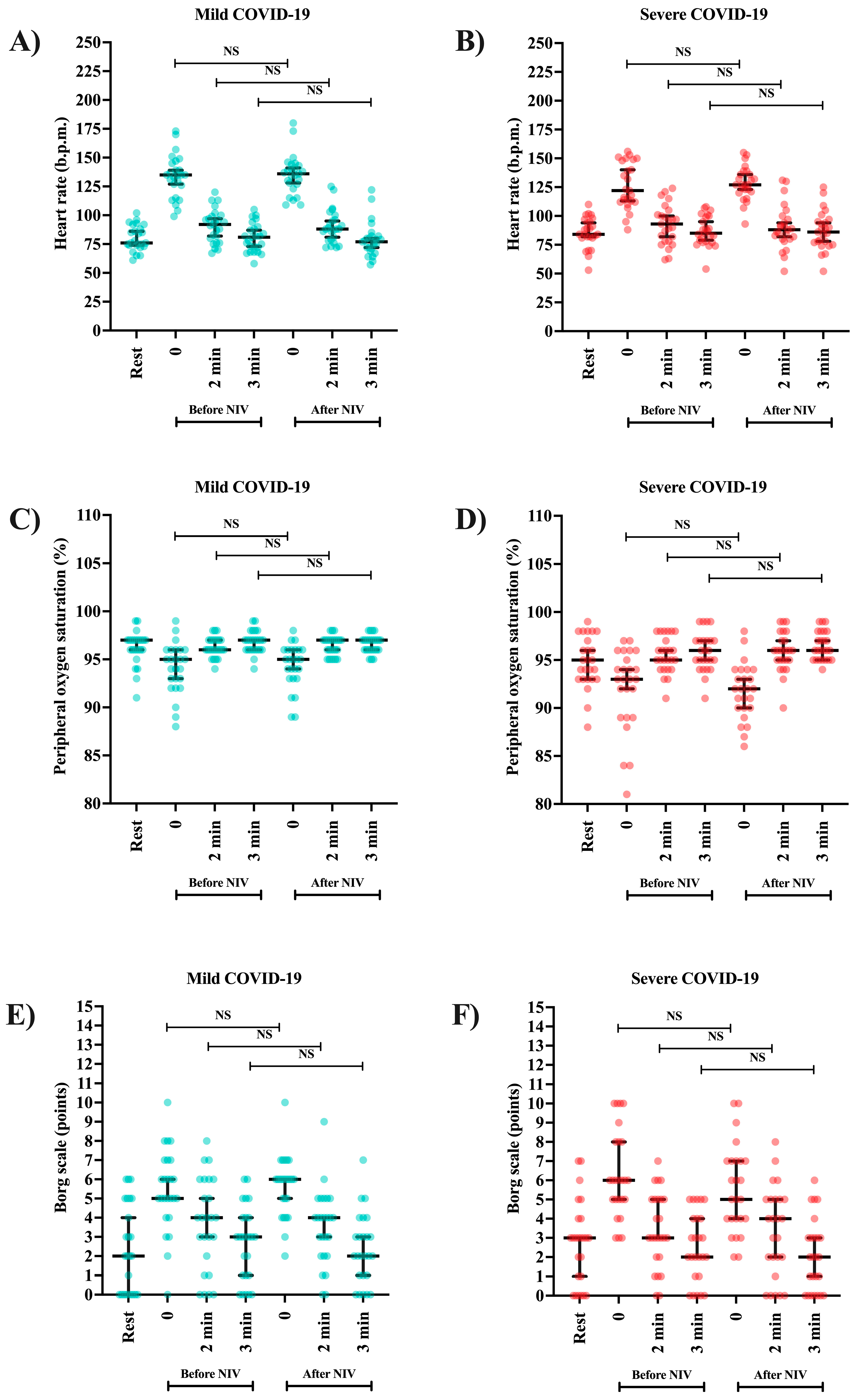
| Heart frequency | Mild | 76 (73.5 to 90.0) | 135 (120.5 to 146.0) | 92 (78.00 to 99.50) | 81 (70.0 to 89.0) | 136 (125.0 to 143.5) | 88 (78.5 to 98.0) | 77 (70.0 to 82.0) | <0.001 |
| Severe | 84 (81.0 to 96.0) | 122 (112.0 to 148.5) | 93 (78.0 to 103.0) | 85 (77.5 to 99.5) | 127 (120.5 to 137.5) | 88 (80.0 to 98.5) | 86 (76.0 to 96.0) | <0.001 | |
| p-value b | 0.114 | 0.277 | 0.705 | 0.048 | 0.130 | 0.938 | 0.035 | ||
| SpO2 | Mild | 97 (96.0 to 97.0) | 95 (92.5 to 96.0) | 97 (95.5 to 97.0) | 97 (96.0 to 97.0) | 95 (93.0 to 95.0) | 96 (96.0 to 97.0) | 97 (96.0 to 97.5) | <0.001 |
| Severe | 95.00 (93.0 to 97.5) | 93.00 (89.0 to 95.5) | 96.00 (95.0 to 97.5) | 96 (95.0 to 98.0) | 92 (90.0 to 93.93) | 95 (94.0 to 97.4) | 96 (94.5 to 97.0) | <0.001 | |
| p-value b | 0.060 | 0.105 | 0.272 | 0.676 | <0.001 | 0.079 | 0.046 | ||
| Borg scale | Mild | 2 (0.0 to 5.0) | 5 (4.5 to 7.0) | 4 (1.5 to 6.0) | 3 (1.0 to 4.0) | 6 (4.5 to 6.7) | 4 (2.0 to 5.0) | 2 (1.0 to 3.5) | <0.001 |
| Severe | 3 (0.50 to 4.0) | 6 (5.0 to 8.0) | 3 (2.0 to 5.0) | 2 (1.0 to 4.0) | 5 (4.00 to 7.0) | 3.75 (1.5 to 7.0) | 2.0 (0.0 to 3.0) | <0.001 | |
| p-value b | 0.533 | 0.283 | 0.624 | 0.738 | 0.666 | 0.769 | 0.602 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, E.R.; Parazzi, P.L.F.; Marson, F.A.L.; Ribeiro, M.Â.G.O.; Gomez, C.C.S.; Conti, P.B.M.; Siqueira, B.A.; Silva, E.A.B.A.; Ribeiro, J.D., on behalf of UNICOVIDS Group. Effect of Continuous Positive Airway Pressure (CPAP) Mode on Lung Function, Exercise Tolerance, Vital Signs, and Dyspnea After Acute SARS-CoV-2 Infection. Clin. Pract. 2025, 15, 73. https://doi.org/10.3390/clinpract15040073
Nascimento ER, Parazzi PLF, Marson FAL, Ribeiro MÂGO, Gomez CCS, Conti PBM, Siqueira BA, Silva EABA, Ribeiro JD on behalf of UNICOVIDS Group. Effect of Continuous Positive Airway Pressure (CPAP) Mode on Lung Function, Exercise Tolerance, Vital Signs, and Dyspnea After Acute SARS-CoV-2 Infection. Clinics and Practice. 2025; 15(4):73. https://doi.org/10.3390/clinpract15040073
Chicago/Turabian StyleNascimento, Emilia Raposo, Paloma Lopes Francisco Parazzi, Fernando Augusto Lima Marson, Maria Ângela Gonçalves Oliveira Ribeiro, Carla Cristina Sousa Gomez, Patrícia Blau Margosian Conti, Bianca Aparecida Siqueira, Edvane Aparecida Braz Araújo Silva, and José Dirceu Ribeiro on behalf of UNICOVIDS Group. 2025. "Effect of Continuous Positive Airway Pressure (CPAP) Mode on Lung Function, Exercise Tolerance, Vital Signs, and Dyspnea After Acute SARS-CoV-2 Infection" Clinics and Practice 15, no. 4: 73. https://doi.org/10.3390/clinpract15040073
APA StyleNascimento, E. R., Parazzi, P. L. F., Marson, F. A. L., Ribeiro, M. Â. G. O., Gomez, C. C. S., Conti, P. B. M., Siqueira, B. A., Silva, E. A. B. A., & Ribeiro, J. D., on behalf of UNICOVIDS Group. (2025). Effect of Continuous Positive Airway Pressure (CPAP) Mode on Lung Function, Exercise Tolerance, Vital Signs, and Dyspnea After Acute SARS-CoV-2 Infection. Clinics and Practice, 15(4), 73. https://doi.org/10.3390/clinpract15040073






