An Overview of the Etiopathogenic Mechanisms Involved in the Expression of the Oral Microbiota
Abstract
:1. Introduction
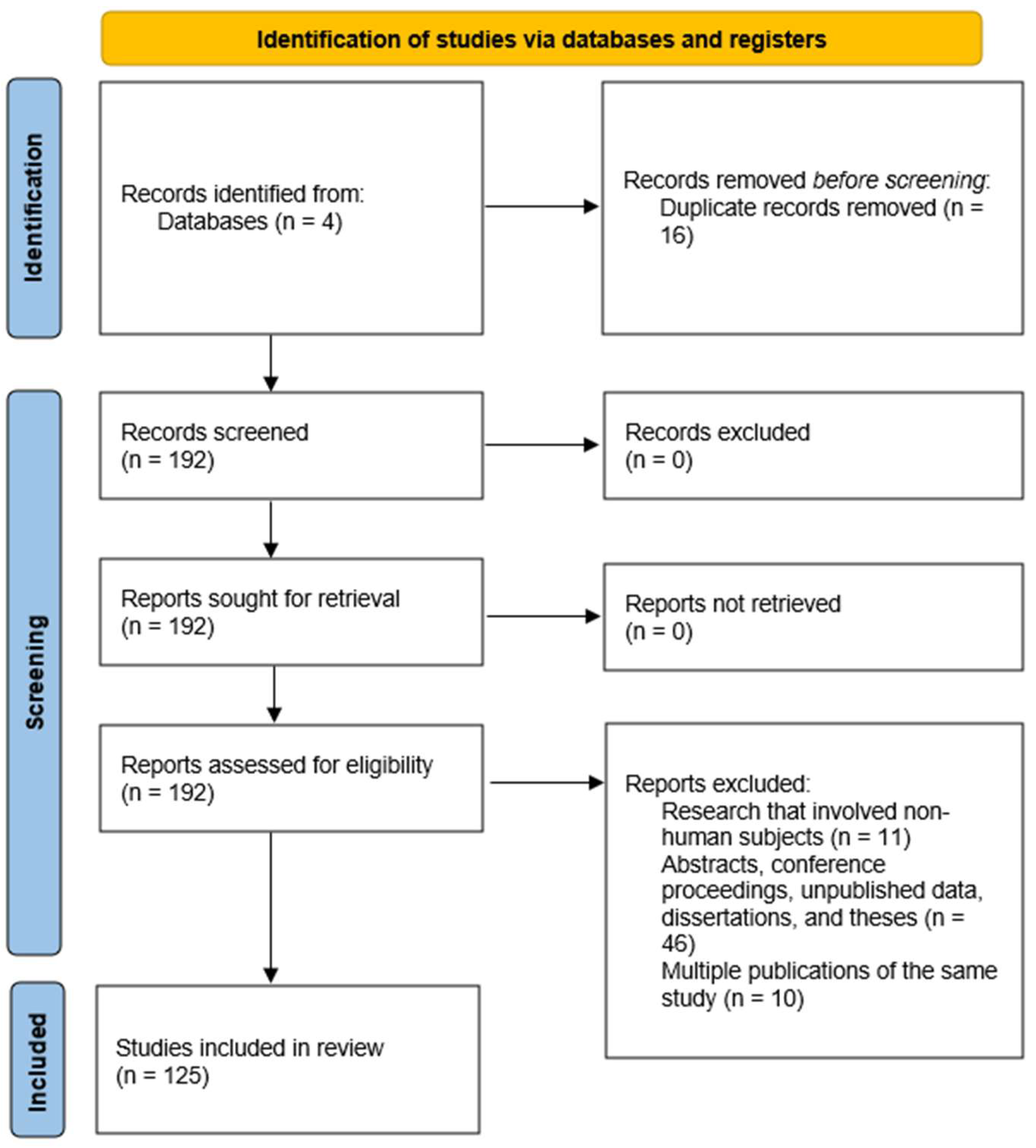
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Selection Process
3. Implications of the Oral Microbiota in Systemic and Dental Diseases
| Oral Disease | Study Focus | Key Findings | Implications |
|---|---|---|---|
| Periodontitis | Dysbiosis of subgingival plaque [4] | Increasing prevalence of Treponema denticola, Tannerella forsythia, and Porphyromonas gingivalis. Mechanisms for biofilm formation and sulfur metabolism. | Discovered possible diagnoses and therapy paths as well as microbiological indicators. |
| Dental caries | Microbial diversity in caries severity [9] | Enrichment of Streptococcus mutans, Lactobacillus spp., and Bifidobacterium dentium. Less diversity and more acidogenic metabolism. | Ideas for preventative plans emphasizing microbial diversity restoration and acidogenic activity. |
| Oral cancer (OSCC) | Role of Fusobacterium nucleatum in cancer development [10] | F. nucleatum enriched in tumors. Activation of NF-κB pathways, immune evasion, and resistance to apoptosis. | Highlights F. nucleatum as a therapeutic target in oral squamous cell carcinoma. |
| Halitosis | Microbial dysbiosis in tongue biofilm [11] | Increased Solobacterium moorei, Prevotella spp., and Fusobacterium nucleatum. Elevated sulfur-reduction pathways. | Discovers bacterial causes of halitosis for focused treatments. |
| Oral lichen planus (OLP) | Altered microbiome in buccal mucosa [12] | Decreased Streptococcus salivarius, increased Prevotella spp., and Porphyromonas spp. Upregulation of LPS biosynthesis and immune modulation genes. | Suggests a microbial involvement in the oral lichen planus inflammatory condition. |
| Diabetes and periodontitis | Salivary microbiome in comorbidities [13] | Enrichment of P. gingivalis and T. denticola. Increased oxidative stress and pro-inflammatory cytokine activation pathways. | Emphasizes oral-systemic health relationships and inflammatory routes. |
| Oral candidiasis | Fungal-bacterial interactions [14] | Co-occurrence of Candida albicans and Streptococcus mutans. Enhanced biofilm formation and quorum sensing. | Clarifies the function of microbial collaboration in biofilm resistance and degree of infection. |
| Sjögren’s Syndrome | Altered salivary microbiome [15] | Reduced microbial diversity. Increased Lactobacillus species, decreased Streptococcus. Altered oxidative stress and immune signaling pathways. | Makes recommendations on microbial dysbiosis in inflammation and autoimmune. |
| Systemic Disease | Study Focus | Key Findings | Implications |
|---|---|---|---|
| Cardiovascular disease | The link between Porphyromonas gingivalis and atherosclerosis [16] | Detection of P. gingivalis DNA in atherosclerotic plaques. Association with inflammation pathways and vascular damage. | Implies that oral bacteria cause systematic inflammation and vascular malfunction. |
| Diabetes mellitus | Oral microbiome shifts in type 2 diabetes [17] | Reduced microbial diversity. Increased abundance of Prevotella intermedia and Porphyromonas gingivalis. | Emphasizes how oral dysbiosis affects glucose metabolism and general inflammation. |
| Adverse pregnancy outcomes | Association of oral bacteria with preterm birth [18] | Fusobacterium nucleatum detected in the placenta of preterm birth cases. Bacteria are linked to inflammation and premature rupture of membranes. | Proposes translocation of oral bacteria as a risk factor for negative pregnancy results. |
| Alzheimer’s disease | Role of P. gingivalis in neurodegeneration [19] | P. gingivalis and its gingipain enzymes detected in brain tissue of Alzheimer’s patients. Associated with amyloid plaque formation and neuroinflammation. | Proposes oral infections as possible causes of neurological diseases and treatment targets. |
| Rheumatoid arthritis | Oral dysbiosis and autoimmune activation [20] | Elevated Aggregatibacter actinomycetemcomitans in patients. Association with hypercitrullination and autoantibody production. | Supports the role of oral bacteria in triggering autoimmune responses in rheumatoid arthritis. |
| Colorectal cancer | Impact of Fusobacterium nucleatum on colorectal tumor progression [21] | F. nucleatum promotes tumor growth via E-cadherin/β-catenin signaling. Detected in higher abundance in colorectal tumor tissues. | Highlights F. nucleatum as a potential biomarker and therapeutic target for colorectal cancer. |
| Chronic kidney disease | Oral microbiome changes in chronic kidney disease [22] | Increased abundance of Tannerella forsythia and Fusobacterium nucleatum. Association with systemic inflammation and uremic toxins. | Suggests that oral dysbiosis may exacerbate inflammation in chronic kidney disease. |
| Respiratory infections | Oral microbiome in aspiration pneumonia [23] | Increased prevalence of Streptococcus pneumoniae and Pseudomonas aeruginosa in oral samples of patients with pneumonia. | Highlights the oral cavity as a reservoir for respiratory pathogens in vulnerable individuals. |
4. Mechanisms of Pathogenesis
4.1. Alteration of the Microbial Equilibrium
4.2. Biofilm Production
4.3. Toxin Release and Proteolytic Enzyme Production
4.4. Modification of the Oral Mucosal Barrier
4.5. Chronic Inflammation and Systemic Impact
4.6. Dysfunctions of the Host Immunological Response
4.7. Interaction Between Oral Microbiota and Environmental Factors
5. The Contribution of Endogenous Factors in the Development and Occurrence of Oral Dysbiosis
5.1. Oral Microbiota and Immune System Interactions
5.2. Genetic Regulation of Collagen Synthesis and Degradation in Oral Health
5.3. Genes of Lipid Metabolism in the Context of Oral Dysbiosis
5.4. Epigenetic Changes Induced by Pathogenic Microbiota
6. Oncogenic Signaling Pathways Activated in the Context of Oral Dysbiosis
6.1. The NF-κB Pathway and Chronic Inflammation
6.2. The PI3K/AKT/mTOR Pathway and Cell Survival
6.3. The Wnt/β-Catenin Pathway and Genomic Instability
6.4. The MAPK/ERK Pathway and Cell Differentiation
6.5. MicroRNAs and Epigenetic Regulation of Signaling Pathways
7. Strengths and Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Bihan, M.; Yooseph, S.; Methé, B.A. Analyses of the microbial diversity across the human microbiome. PLoS ONE 2012, 7, e32118. [Google Scholar] [CrossRef]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project: Dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 2014, 16, 276–289. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Monteiro-Silva, F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent. Res. J. 2014, 11, 291–301. [Google Scholar]
- D’Agostino, S.; Ferrara, E.; Valentini, G.; Stoica, S.A.; Dolci, M. Exploring Oral Microbiome in Healthy Infants and Children: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11403. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Crielaard, W.; Zaura, E.; Schuller, A.A.; Huse, S.M.; Montijn, R.C.; Keijser, B.J. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med. Genomics 2011, 4, 22. [Google Scholar] [CrossRef]
- Spatafora, G.; Li, Y.; He, X.; Cowan, A.; Tanner, A.C.R. The Evolving Microbiome of Dental Caries. Microorganisms 2024, 12, 121. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and oral cancer: A critical review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef]
- Seerangaiyan, K.; van Winkelhoff, A.J.; Harmsen, H.J.M.; Rossen, J.W.A.; Winkel, E.G. The tongue microbiome in healthy subjects and patients with intra-oral halitosis. J. Breath Res. 2017, 11, 036010. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, W.; Tu, Q.; Ge, Y.; He, J.; Zhou, Y.; Gou, Y.; Van Nostrand, J.D.; Qin, Y.; Li, J.; et al. Preliminary analysis of salivary microbiome and their potential roles in oral lichen planus. Sci. Rep. 2016, 6, 22943. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhao, Q.; Deng, J.; Chen, K.; Jiang, X.; Ma, F.; Ma, S.; Li, Z. Salivary Microbiome Profile of Diabetes and Periodontitis in a Chinese Population. Front. Cell. Infect. Microbiol. 2022, 12, 933833. [Google Scholar] [CrossRef] [PubMed]
- Montelongo-Jauregui, D.; Lopez-Ribot, J.L. Candida Interactions with the Oral Bacterial Microbiota. J. Fungi 2018, 4, 122. [Google Scholar] [CrossRef]
- Mieliauskaitė, D.; Kontenis, V. Insights into Microbiota in Sjögren’s Syndrome. Medicina 2023, 59, 1661. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4592–4598. [Google Scholar] [CrossRef]
- Shaalan, A.; Lee, S.; Feart, C.; Garcia-Esquinas, E.; Gomez-Cabrero, D.; Lopez-Garcia, E.; Morzel, M.; Neyraud, E.; Rodriguez-Artalejo, F.; Streich, R.; et al. Alterations in the Oral Microbiome Associated With Diabetes, Overweight, and Dietary Components. Front. Nutr. 2022, 9, 914715. [Google Scholar] [CrossRef]
- Vander Haar, E.L.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe 2018, 50, 55–59. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Mikuls, T.R.; Payne, J.B.; Yu, F.; Thiele, G.M.; Reynolds, R.J.; Cannon, G.W.; Markt, J.; McGowan, D.; Kerr, G.S.; Redman, R.S.; et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 1090–1100. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.M.; Willmott, T.; Summers, A.; Knight, C.G.; Humphreys, G.J.; Konkel, J.E.; Augustine, T.; McBain, A.J. Investigating oral microbiome dynamics in chronic kidney disease and post-transplantation in continuous culture. Microbiol. Spectr. 2024, 12, e00598-24. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Nicu, E.A.; Krom, B.P.; Keijser, B.J. Acquiring and maintaining a normal oral microbiome: Current perspective. Front. Cell. Infect. Microbiol. 2014, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A. Microbiota of the Human Body: Implications in Health and Disease. Preface. Adv. Exp. Med. Biol. 2016, 902, v. [Google Scholar]
- Ganesan, S.M.; Joshi, V.; Fellows, M.; Dabdoub, S.M.; Nagaraja, H.N.; O’Donnell, B.; Deshpande, N.R.; Kumar, P.S. A tale of two risks: Smoking, diabetes and the subgingival microbiome. ISME J. 2017, 11, 2075–2089. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Katz, J.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef]
- Dinis, M.; Agnello, M.; Cen, L.; Shokeen, B.; He, X.; Shi, W.; Wong, D.T.W.; Lux, R.; Tran, N.C. Oral Microbiome: Streptococcus mutans/Caries Concordant-Discordant Children. Front. Microbiol. 2022, 13, 782825. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef]
- Bertolini, M.; Costa, R.C.; Barão, V.A.R.; Villar, C.C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J.G. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Mallo, N.; Otero-Casal, P.; Pose-Rodríguez, J.M.; Otero, A. Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral Dis. 2022, 28, 307–313. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Tanner, A.C.R. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef]
- Luo, T.L.; Vanek, M.E.; Gonzalez-Cabezas, C.; Marrs, C.F.; Foxman, B.; Rickard, A.H. In vitro model systems for exploring oral biofilms: From single-species populations to complex multi-species communities. J. Appl. Microbiol. 2022, 132, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Bamford, C.V.; Francescutti, T.; Cameron, C.E.; Jenkinson, H.F.; Dymock, D. Characterization of a novel family of fibronectin-binding proteins with M23 peptidase domains from Treponema denticola. Mol. Oral Microbiol. 2010, 25, 369–383. [Google Scholar] [CrossRef]
- Frederick, J.R.; Sarkar, J.; McDowell, J.V.; Marconi, R.T. Molecular signaling mechanisms of the periopathogen, Treponema denticola. J. Dent. Res. 2011, 90, 1155–1163. [Google Scholar] [CrossRef]
- Fenno, J.C. Treponema denticola interactions with host proteins. J. Oral Microbiol. 2012, 4, 9929. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chan, Y.; Gao, W.; Leung, W.K.; Watt, R.M. Diversity of Treponema denticola and Other Oral Treponeme Lineages in Subjects with Periodontitis and Gingivitis. Microbiol. Spectr. 2021, 9, e00701-21. [Google Scholar] [CrossRef] [PubMed]
- Awany, D.; Allali, I.; Dalvie, S.; Hemmings, S.; Mwaikono, K.S.; Thomford, N.E.; Gomez, A.; Mulder, N.; Chimusa, E.R. Host and Microbiome Genome-Wide Association Studies: Current State and Challenges. Front. Genet. 2019, 9, 637. [Google Scholar] [CrossRef]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Kretschmar, S.; Yin, L.; Roberts, F.; London, R.; Flemmig, T.T.; Arushanov, D.; Kaiyala, K.; Chung, W.O. Protease inhibitor levels in periodontal health and disease. J. Periodontal Res. 2012, 47, 228–235. [Google Scholar] [CrossRef]
- Ruggiero, S.; Cosgarea, R.; Potempa, J.; Potempa, B.; Eick, S.; Chiquet, M. Cleavage of extracellular matrix in periodontitis: Gingipains differentially affect cell adhesion activities of fibronectin and tenascin-C. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 517–526. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Kozak, M.; Dabrowska-Zamojcin, E.; Mazurek-Mochol, M.; Pawlik, A. Cytokines and Their Genetic Polymorphisms Related to Periodontal Disease. J. Clin. Med. 2020, 9, 4045. [Google Scholar] [CrossRef]
- Liu, X.; Li, H. A Systematic Review and Meta-Analysis on Multiple Cytokine Gene Polymorphisms in the Pathogenesis of Periodontitis. Front. Immunol. 2022, 12, 713198. [Google Scholar] [CrossRef]
- Mazurek-Mochol, M.; Bonsmann, T.; Mochol, M.; Poniewierska-Baran, A.; Pawlik, A. The Role of Interleukin 6 in Periodontitis and Its Complications. Int. J. Mol. Sci. 2024, 25, 2146. [Google Scholar] [CrossRef]
- Vitiello, F.; Bourgeois, D.; Orilisi, G.; Orsini, G.; Carrouel, F. Non-Cariogenic Effect of Milk and Dairy Products on Oral Health in Children and Adolescents: A Scoping Review. Children 2024, 11, 149. [Google Scholar] [CrossRef]
- Shkembi, B.; Huppertz, T. Impact of Dairy Products and Plant-Based Alternatives on Dental Health: Food Matrix Effects. Nutrients 2023, 15, 1469. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, M.; Gasparro, R.; D’Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- de Nies, L.; Kobras, C.M.; Stracy, M. Antibiotic-induced collateral damage to the microbiota and associated infections. Nat. Rev. Microbiol. 2023, 21, 789–804. [Google Scholar] [CrossRef]
- Ng, K.M.; Aranda-Díaz, A.; Tropini, C.; Frankel, M.R.; Van Treuren, W.; O’Loughlin, C.T.; Merrill, B.D.; Yu, F.B.; Pruss, K.M.; Oliveira, R.A.; et al. Recovery of the Gut Microbiota after Antibiotics Depends on Host Diet, Community Context, and Environmental Reservoirs. Cell Host Microbe 2020, 28, 628. [Google Scholar] [CrossRef]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H.; Eliakim, R.; Herrera-deGuise, C.; Karakan, T.; Merenstein, D.; Piscoya, A.; Ramakrishna, B.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and Prebiotics. J. Clin. Gastroenterol. 2024, 58, 533–553. [Google Scholar] [CrossRef]
- Oliver, A.; Xue, Z.; Villanueva, Y.T.; Durbin-Johnson, B.; Alkan, Z.; Taft, D.H.; Liu, J.; Korf, I.; Laugero, K.D.; Stephensen, C.B.; et al. Association of Diet and Antimicrobial Resistance in Healthy U.S. Adults. mBio 2022, 13, e00101-22. [Google Scholar] [CrossRef] [PubMed]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef]
- Blaser, M.J. The microbiome revolution. J. Clin. Investig. 2014, 124, 4162–4165. [Google Scholar] [CrossRef]
- Gomez, A.; Espinoza, J.L.; Harkins, D.M.; Leong, P.; Saffery, R.; Bockmann, M.; Torralba, M.; Kuelbs, C.; Kodukula, R.; Inman, J.; et al. Host Genetic Control of the Oral Microbiome in Health and Disease. Cell Host Microbe 2017, 22, 269–278.e3. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd Allah, E.F. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2019, 9, 2868. [Google Scholar] [CrossRef] [PubMed]
- Abdul, N.S.; Shenoy, M.; Reddy, N.R.; Sangappa, S.B.; Shivakumar, G.C.; Di Blasio, M.; Cicciù, M.; Minervini, G. Gene sequencing applications to combat oral-cavity related disorders: A systematic review with meta-analysis. BMC Oral Health 2024, 24, 103. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chung, S.W.; Auh, Q.S.; Hong, S.J.; Lee, Y.A.; Jung, J.; Lee, G.J.; Park, H.J.; Shin, S.I.; Hong, J.Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef]
- Bhandary, R.; Venugopalan, G.; Ramesh, A.; Tartaglia, G.M.; Singhal, I.; Khijmatgar, S. Microbial Symphony: Navigating the Intricacies of the Human Oral Microbiome and Its Impact on Health. Microorganisms 2024, 12, 571. [Google Scholar] [CrossRef]
- Wankhede, A.N.; Wankhede, S.A.; Wasu, S.P. Role of Genetic in Periodontal Disease. J. Int. Clin. Dent. Res. Organ. 2017, 9, 53–58. [Google Scholar] [CrossRef]
- Brodzikowska, A.; Górski, B. Polymorphisms in Genes Involved in Inflammation and Periodontitis: A Narrative Review. Biomolecules 2022, 12, 552. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova, P.; Fassmann, A.; Dusek, L.; Izakovicova Holla, L. Glutathione S-transferase M1, T1, and P1 polymorphisms and periodontitis in a Caucasian population: A case-control study. BMC Oral Health 2024, 24, 288. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, K.-H.; Wu, H.-P. Unraveling the Complexities of Toll-like Receptors: From Molecular Mechanisms to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 5037. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. (Elite Ed.) 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Wang, Y.; Ren, X.; Han, J. Molecular mechanisms and clinical manifestations of rare genetic disorders associated with type I collagen. Intractable Rare Dis. Res. 2019, 8, 98–107. [Google Scholar] [CrossRef]
- Mallikarjunappa, A.S.; George, S.; Aghanashini, S.; Bhat, D.; Mundinamane, D.B.; Nadiger, S. Collagen—The Skeleton of the Periodontium: A Review. J. Sci. Dent. 2021, 11, 31–36. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Atanasova, T.; Stankova, T.; Bivolarska, A.; Vlaykova, T. Matrix Metalloproteinases in Oral Health-Special Attention on MMP-8. Biomedicines 2023, 11, 1514. [Google Scholar] [CrossRef]
- Zalewska, E.A.; Ławicka, R.; Grygorczuk, P.; Nowosielska, M.; Kicman, A.; Ławicki, S. Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. Int. J. Mol. Sci. 2024, 25, 2721. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Oton-Gonzalez, L.; Mazziotta, C.; Iaquinta, M.R.; Mazzoni, E.; Nocini, R.; Trevisiol, L.; D’Agostino, A.; Tognon, M.; Rotondo, J.C.; Martini, F. Genetics and Epigenetics of Bone Remodeling and Metabolic Bone Diseases. Int. J. Mol. Sci. 2022, 23, 1500. [Google Scholar] [CrossRef] [PubMed]
- Ck, A.A.; Tholupunuri, H.; Reddy, M.R.; Muralidhar, M.; Jayyarapu, D.; Nair, S. Genetic Impact on Bone Modulation—A Review Bridging Bioscience to Genetic Engineering. Glob. Med. Genet. 2021, 8, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gadi, L.S.A.; Chau, D.Y.S.; Parekh, S. Morphological and Ultrastructural Collagen Defects: Impact and Implications in Dentinogenesis Imperfecta. Dent. J. 2023, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Raka, F.; Adeli, K. The Role of the Gut Microbiota in Lipid and Lipoprotein Metabolism. J. Clin. Med. 2019, 8, 2227. [Google Scholar] [CrossRef]
- Garcia, C.; Andersen, C.J.; Blesso, C.N. The Role of Lipids in the Regulation of Immune Responses. Nutrients 2023, 15, 3899. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Kaur, G.; Grover, V.; Bhaskar, N.; Kaur, R.K.; Jain, A. Periodontal Infectogenomics. Inflamm. Regen. 2018, 38, 8. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Laberge, S.; Akoum, D.; Wlodarczyk, P.; Massé, J.D.; Fournier, D.; Semlali, A. The Potential Role of Epigenetic Modifications on Different Facets in the Periodontal Pathogenesis. Genes 2023, 14, 1202. [Google Scholar] [CrossRef]
- Lavu, V.; Venkatesan, V.; Rao, S.R. The epigenetic paradigm in periodontitis pathogenesis. J. Indian Soc. Periodontol. 2015, 19, 142–149. [Google Scholar] [CrossRef]
- Khouly, I.; Braun, R.S.; Ordway, M.; Ghassib, I.; Larsson, L.; Asa’ad, F. The Role of Epigenetics in Periodontal and Systemic Diseases and Smoking: A Systematic Review. Appl. Sci. 2021, 11, 5269. [Google Scholar] [CrossRef]
- Vyhnalova, T.; Danek, Z.; Gachova, D.; Linhartova, P.B. The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma. Microorganisms 2021, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Špiljak, B.; Ozretić, P.; Andabak Rogulj, A.; Lončar Brzak, B.; Brailo, V.; Škerlj, M.; Vidović Juras, D. Oral Microbiome Research in Biopsy Samples of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma and Its Challenges. Appl. Sci. 2024, 14, 11405. [Google Scholar] [CrossRef]
- Muñoz-Medel, M.; Pinto, M.P.; Goralsky, L.; Cáceres, M.; Villarroel-Espíndola, F.; Manque, P.; Pinto, A.; Garcia-Bloj, B.; de Mayo, T.; Godoy, J.A.; et al. Porphyromonas gingivalis, a bridge between oral health and immune evasion in gastric cancer. Front. Oncol. 2024, 14, 1403089. [Google Scholar] [CrossRef]
- Wang, B.; Deng, J.; Donati, V.; Merali, N.; Frampton, A.E.; Giovannetti, E.; Deng, D. The Roles and Interactions of Porphyromonas gingivalis and Fusobacterium nucleatum in Oral and Gastrointestinal Carcinogenesis: A Narrative Review. Pathogens 2024, 13, 93. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, T.; Ma, T.; Huang, W.; Wang, Y. Epigenetics and environmental health. Front. Med. 2024, 18, 571–596. [Google Scholar] [CrossRef]
- La Rosa, G.R.M.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of oral dysbiosis with oral cancer development. Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Erić, S.; Meštrović, T.; Stipetić, M.M.; Juzbašić, M.; Katalinić, D.; Bekić, S.; Muršić, D.; Flam, J.; Belić, D.; et al. The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer. Cancers 2024, 16, 2997. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Kandalai, S.; Li, H.; Zhang, N.; Peng, H.; Zheng, Q. The human microbiome and cancer: A diagnostic and therapeutic perspective. Cancer Biol. Ther. 2023, 24, 2240084. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef]
- Reyes, M.; Flores, T.; Betancur, D.; Peña-Oyarzún, D.; Torres, V.A. Wnt/β-Catenin Signaling in Oral Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 4682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, J.; Shi, Y.; Fang, X.; Tang, Z. MAPK Signaling Pathway in Oral Squamous Cell Carcinoma: Biological Function and Targeted Therapy. Cancers 2022, 14, 4625. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.; Lopez, A.; Lin, E.; Sales, D.; Perets, R.; Jain, P. Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers. Cancers 2021, 13, 5059. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Park, J.I. MAPK-ERK Pathway. Int. J. Mol. Sci. 2023, 24, 9666. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Chen, C.; Chu, X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol. Cancer 2018, 17, 22. [Google Scholar] [CrossRef]
- Wang, J.; Lv, N.; Lu, X.; Yuan, R.; Chen, Z.; Yu, J. Diagnostic and therapeutic role of microRNAs in oral cancer (Review). Oncol. Rep. 2021, 45, 58–64. [Google Scholar] [CrossRef]
- Nikolaieva, N.; Sevcikova, A.; Omelka, R.; Martiniakova, M.; Mego, M.; Ciernikova, S. Gut Microbiota–MicroRNA Interactions in Intestinal Homeostasis and Cancer Development. Microorganisms 2023, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Alabasi, G.; Sandaltzopoulos, R.; Marcu, K.B.; Kolettas, E. Roles of NF-κB Signaling in the Regulation of miRNAs Impacting on Inflammation in Cancer. Biomedicines 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, I.; Ciccarese, F.; Sharova, E.; Urso, L.; Raimondi, V.; Silic-Benussi, M.; D’Agostino, D.M.; Ciminale, V. The miR-200 Family of microRNAs: Fine Tuners of Epithelial-Mesenchymal Transition and Circulating Cancer Biomarkers. Cancers 2021, 13, 5874. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, I.A.; Orasanu, C.I.; Cozaru, G.-C.; Ionescu, A.-C.; Kajanto, L.; Cimpineanu, B.; Chisoi, A.; Mitroi, A.N.; Poinareanu, I.; Voda, R.I.; et al. An Overview of the Etiopathogenic Mechanisms Involved in the Expression of the Oral Microbiota. Clin. Pract. 2025, 15, 80. https://doi.org/10.3390/clinpract15040080
Popovici IA, Orasanu CI, Cozaru G-C, Ionescu A-C, Kajanto L, Cimpineanu B, Chisoi A, Mitroi AN, Poinareanu I, Voda RI, et al. An Overview of the Etiopathogenic Mechanisms Involved in the Expression of the Oral Microbiota. Clinics and Practice. 2025; 15(4):80. https://doi.org/10.3390/clinpract15040080
Chicago/Turabian StylePopovici, Ion Alexandru, Cristian Ionut Orasanu, Georgeta-Camelia Cozaru, Anita-Cristina Ionescu, Lidia Kajanto, Bogdan Cimpineanu, Anca Chisoi, Adrian Nelutu Mitroi, Ionut Poinareanu, Raluca Ioana Voda, and et al. 2025. "An Overview of the Etiopathogenic Mechanisms Involved in the Expression of the Oral Microbiota" Clinics and Practice 15, no. 4: 80. https://doi.org/10.3390/clinpract15040080
APA StylePopovici, I. A., Orasanu, C. I., Cozaru, G.-C., Ionescu, A.-C., Kajanto, L., Cimpineanu, B., Chisoi, A., Mitroi, A. N., Poinareanu, I., Voda, R. I., Ursica, O. A., & Pundiche, M. B. (2025). An Overview of the Etiopathogenic Mechanisms Involved in the Expression of the Oral Microbiota. Clinics and Practice, 15(4), 80. https://doi.org/10.3390/clinpract15040080







