Harnessing Visual Neuroplasticity Through Auditory Biofeedback—Functional and Electrophysiological Gains Across Retinal, Optic-Nerve, and Cortical Visual Impairment: A Prospective Pilot Study
Abstract
1. Introduction
1.1. Theoretical Basis of Auditory Biofeedback and Neuroplasticity
1.2. Expanded Literature Review
2. Methods
2.1. Setting and Participants
2.2. Intervention
2.3. Outcome Measures and Follow-Up
2.4. Individual Case Summaries
2.5. Mechanistic Considerations in Individual Outcomes
3. Discussion
3.1. Interpretation and Implications
3.1.1. Context Within Current Rehabilitation Paradigms
3.1.2. Mechanistic Synthesis
3.1.3. Clinical Feasibility and Cost
3.1.4. Public Health and Equity Implications
3.1.5. Strengths and Methodological Limitations
3.2. Scientific Rationale for Expansion to Broader Populations
3.3. Problems, Gaps, and Future Plans
4. Results
Narrative of Individual Cases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Istituto Nazionale di Statistica. Condizioni di Salute e Ricorso ai Servizi Sanitari in Italia e nell’UE–EHIS 2019; ISTAT: Rome, Italy, 2022. [Google Scholar]
- Burton, M.J.; Ramke, J.; Marques, A.P.; A Bourne, R.R.; Congdon, N.; Jones, I.; Tong, B.A.M.A.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on global eye health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness & Vision Impairment Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar]
- van Nispen, R.M.; Virgili, G.; Hoeben, M.; Langelaan, M.; Klevering, J.; Keunen, J.E.; van Rens, G.H. Low-vision rehabilitation for better quality of life in visually impaired adults. Cochrane Database Syst. Rev. 2020, 2020, CD006543. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.D.; Li, W. Adult visual cortical plasticity. Neuron 2012, 75, 250–264. [Google Scholar] [CrossRef]
- Cooke, S.F.; Bear, M.F. Visual experience induces long-term potentiation in primary visual cortex. J. Neurosci. 2010, 30, 16304–16313. [Google Scholar] [CrossRef]
- Chiari, M.; Savi, C.; Battagliola, E. Visual rehabilitation with Retimax Vision Trainer in severe acquired brain injury: Two-case report. Neuropsychol. Trends. 2014, 15, 35–40. [Google Scholar] [CrossRef]
- Verdina, T.; Piaggi, S.; Ferraro, V.; Russolillo, V.; Peschiera, R.; Chester, J.; Mastropasqua, R.; Cavallini, G.M. Biofeedback rehabilitation based on VEP analysis in advanced age-related macular degeneration. Sci. Rep. 2020, 10, 20886. [Google Scholar] [CrossRef]
- Esposito Veneruso, P.; Ziccardi, L.; Magli, G.; Falsini, B.; Magli, A. Short-term Vision-Trainer rehabilitation in anisometropic amblyopia. Doc. Ophthalmol. 2014, 129, 177–189. [Google Scholar] [CrossRef]
- DiMattina, C.; Zhang, K. How optimal stimuli for sensory neurons are constrained by network architecture. Neural Comput. 2008, 20, 668–708. [Google Scholar] [CrossRef]
- Nemes-Drăgan, I.; Hapca, M.C.; Nicoară, S.D.; Țîpcu, A.; Pașcalău, R. Reconnecting anisometropic amblyopic eyes to the cortex: VEP-based auditory biofeedback. Diagnostics 2024, 14, 1861. [Google Scholar] [CrossRef]
- Widihastha, S.H.; Satari, K.; Irfani, I.; Virgana, R.; Amiruddin, P.O. Vision rehabilitation using microperimetric biofeedback in age-related macular degeneration. Int. J. Ophthalmol. 2023, 16, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Malvasi, M.; Compagno, S.; Segnalini, A.; Malvasi, V.M.; Pacella, F.; Turchetti, P.; Pacella, E. Effectiveness of microperimetric biofeedback fixation training in retinal vein occlusions. Clin. Optom. 2024, 16, 131–142. [Google Scholar] [CrossRef]
- Huxlin, K.R.; Martin, T.; Kelly, K.; Riley, M.; Friedman, D.I.; Burgin, W.S.; Hayhoe, M. Perceptual relearning of complex visual motion after V1 damage in humans. J. Neurosci. 2009, 29, 3981–3991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahli, E.; Altinbay, D.; Bingol-Kiziltunc, P.; Idil, A. Micro-perimetric acoustic biofeedback in central scotoma. Curr. Eye Res. 2021, 46, 731–738. [Google Scholar] [CrossRef]
- Ventura, L.M.; Porciatti, V. Restoration of retinal ganglion-cell function in early glaucoma after intra-ocular pressure reduction. Ophthalmology 2005, 112, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Park, A.S.Y.; Wong, G.H.T.; Tan, K.W.S.; Cheung, B.W.S.; Oremus, M.; Cheong, A.M.Y.; Thompson, B. Efficacy of perceptual learning in low vision: A systematic review and meta-analysis. Optom Vis Sci. 2024, 101, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Lapajne, L.; Roškar, S.; Pompe, M.T.; Svetina, M.; Jarc-Vidmar, M.; Hawlina, M. Vision training with VEP biofeedback in amblyopia after the critical period. Doc. Ophthalmol. 2020, 141, 269–278. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, J.; Li, S.; Zhang, J. Effect of duration between sessions on microperimetric biofeedback training in maculopathies. Sci. Rep. 2024, 14, 12524. [Google Scholar] [CrossRef]
- Brodie, S.E. 2023–2024 Basic and Clinical Science Course, Section 3: Clinical Optics and Vision Rehabilitation; American Academy of Ophthalmology: San Francisco, CA, USA, 2023. [Google Scholar]
- Bernard, J.B.; Chung, S.T.L. What is a preferred retinal locus? Annu. Rev. Vis. Sci. 2023, 9, 201–220. [Google Scholar] [CrossRef]
- Tarita-Nistor, L.; Brent, M.H.; Steinbach, M.J.; González, E.G. Fixation stability during binocular viewing in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1887–1893. [Google Scholar] [CrossRef]
- Saionz, E.L.; Tadin, D.; Melnick, M.D.; Huxlin, K.R. Functional preservation and enhanced capacity for visual restoration in subacute occipital stroke. Brain 2020, 143, 1857–1872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayer, A.U.; Maag, K.P.; Erb, C. Detection of optic neuropathy with pattern electroretinography in ocular hypertension and glaucoma. Ophthalmology 2002, 109, 1350–1361. [Google Scholar] [CrossRef]
- Elgohary, A.M.; Elbedewy, H.A.; Saad, H.A.; Eid, T.M. Pattern electroretinogram changes in primary open-angle glaucoma. Eur. J. Ophthalmol. 2020, 30, 1362–1369. [Google Scholar]
- Chen, S.; Lu, H.; Cheng, C.; Ye, Z.; Hua, T. Rapidly repeated visual stimulation induces long-term potentiation of VEPs and increased content of membrane AMPA and NMDA receptors in the V1 cortex of cats. Front. Neurosci. 2024, 18, 1386801. [Google Scholar] [CrossRef]
- Polat, U.; Ma-Naim, T.; Belkin, M.; Sagi, D. Improving vision in adult amblyopia by perceptual learning. Proc. Natl. Acad. Sci. USA 2004, 101, 6692–6697. [Google Scholar] [CrossRef]
- Lorenzini, M.C.; Wittich, W. Virtual-reality applications in low-vision rehabilitation. In Virtual Reality in Health and Rehabilitation; CRC Press: Boca Raton, FL, USA, 2020; pp. 267–282. [Google Scholar]
- Kasowski, J.; Beyeler, M. Immersive virtual-reality simulations of bionic vision. In Proceedings of the Augmented Humans ’22, Kashiwa, Japan, 13–15 March 2022; ACM: New York, NY, USA, 2022. [Google Scholar]
- He, Q.; Yang, X.Y.; Gong, B.; Bi, K.; Fang, F. Alpha-frequency transcranial alternating-current stimulation boosts visual perceptual learning. Brain Stimul. 2022, 15, 546–553. [Google Scholar]
- Bolognini, N.; Diana, L.; Rossetti, A.; Melzi, L.; Basso, G.; Manzo, V.; Cruz-Sanabria, F.; Cammarata, G.; Cernigliaro, F.; Bianchi Marzoli, S.; et al. Telerehabilitation for visual-field defects with a multisensory training: Feasibility study. J. Neuroeng. Rehabil. 2025, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.H.; Bae, S.H.; Lee, S.H.; Moon, N.J. Customisable smartphone low-vision aid: Clinical performance. Sci. Rep. 2022, 12, 14489. [Google Scholar]
- Saionz, E.L.; Feldon, S.E.; Huxlin, K.R. Rehabilitation of cortically induced visual-field loss. Curr. Opin. Neurol. 2021, 34, 67–74. [Google Scholar]
- Xu, D.; Yu, M.; Zheng, C.; Ji, S.; Dai, J. The effects of an electronic head-mounted display in vision rehabilitation for patients with tunnel vision. Int. Ophthalmol. 2024, 44, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabel, B.A.; Gudlin, J. Vision restoration training for homonymous visual field loss: A randomized controlled home-based study. JAMA Ophthalmol. 2014, 132, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.B.; Vanicek, T.; Seiger, R.; Klöbl, M.; Spurny, B.; Handschuh, P.; Ritter, V.; Unterholzner, J.; Godbersen, G.M.; Gryglewski, G.; et al. Neuroplastic effects of a selective serotonin reuptake inhibitor in relearning and retrieval. Neuroimage 2021, 236, 118039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
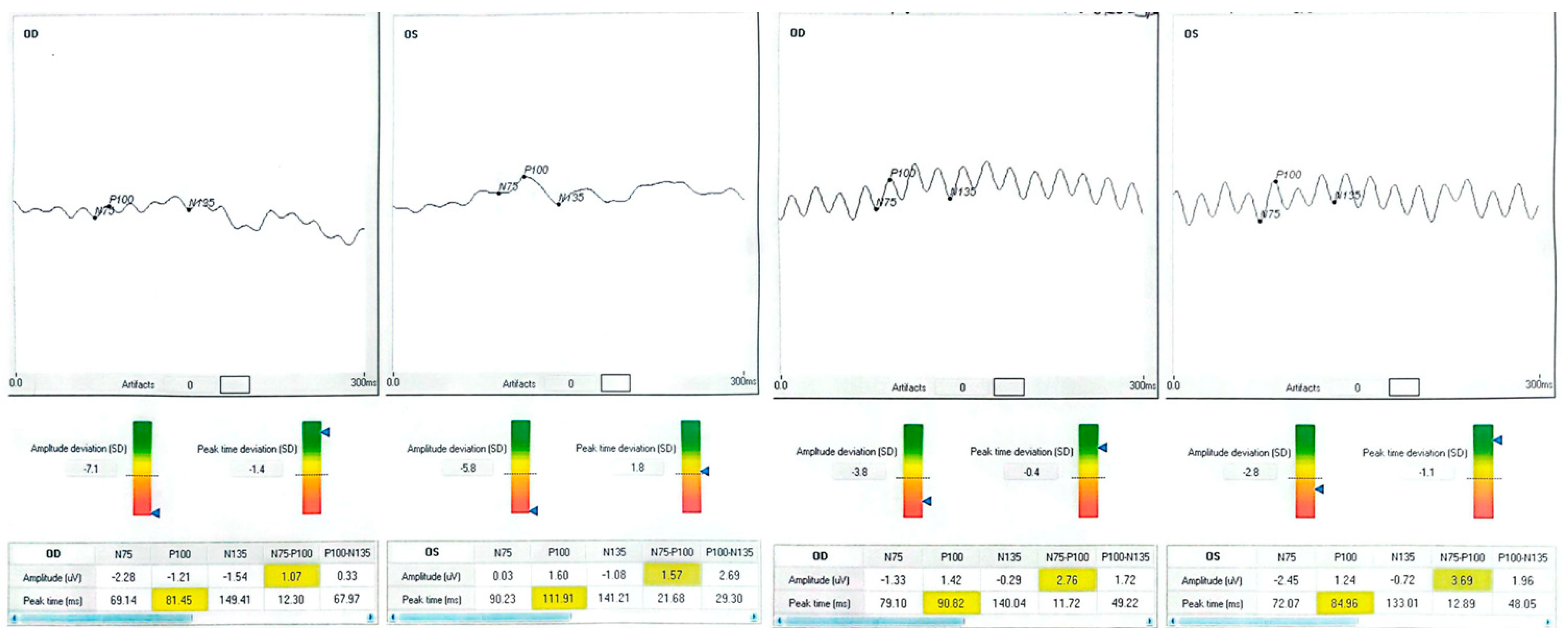
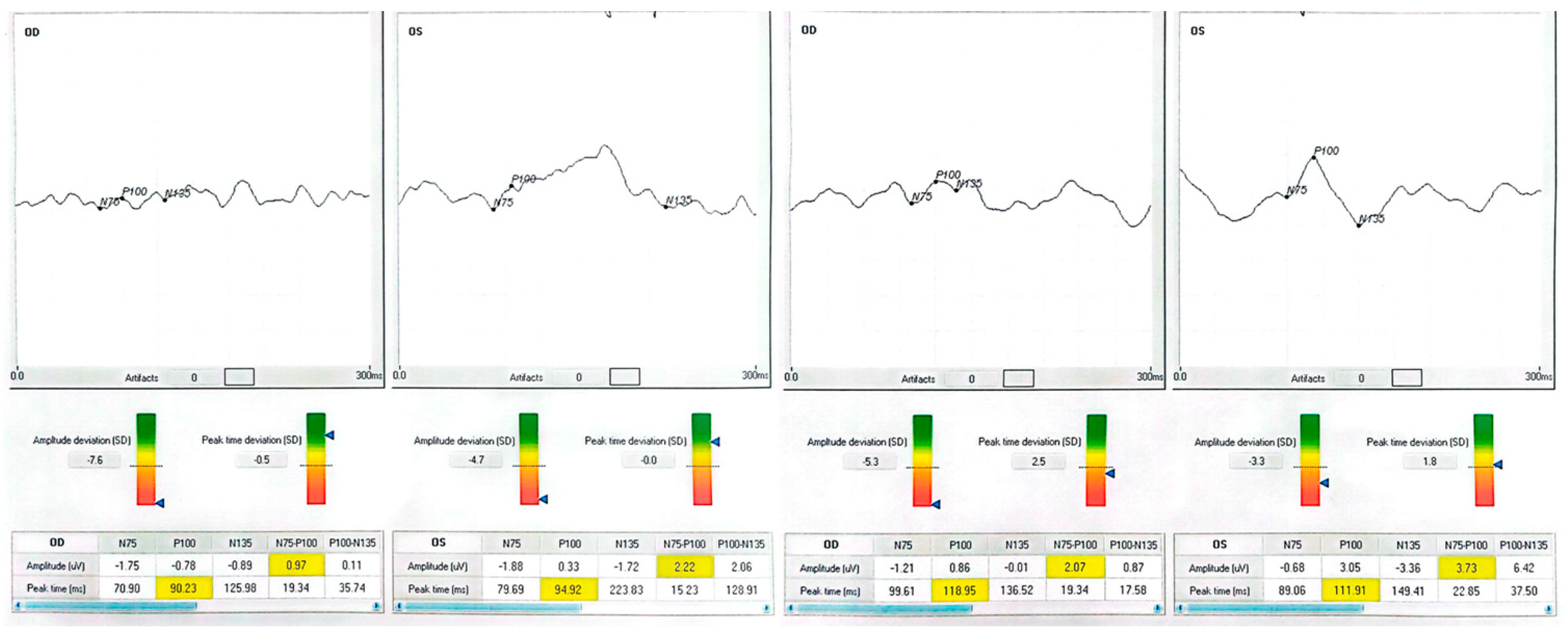
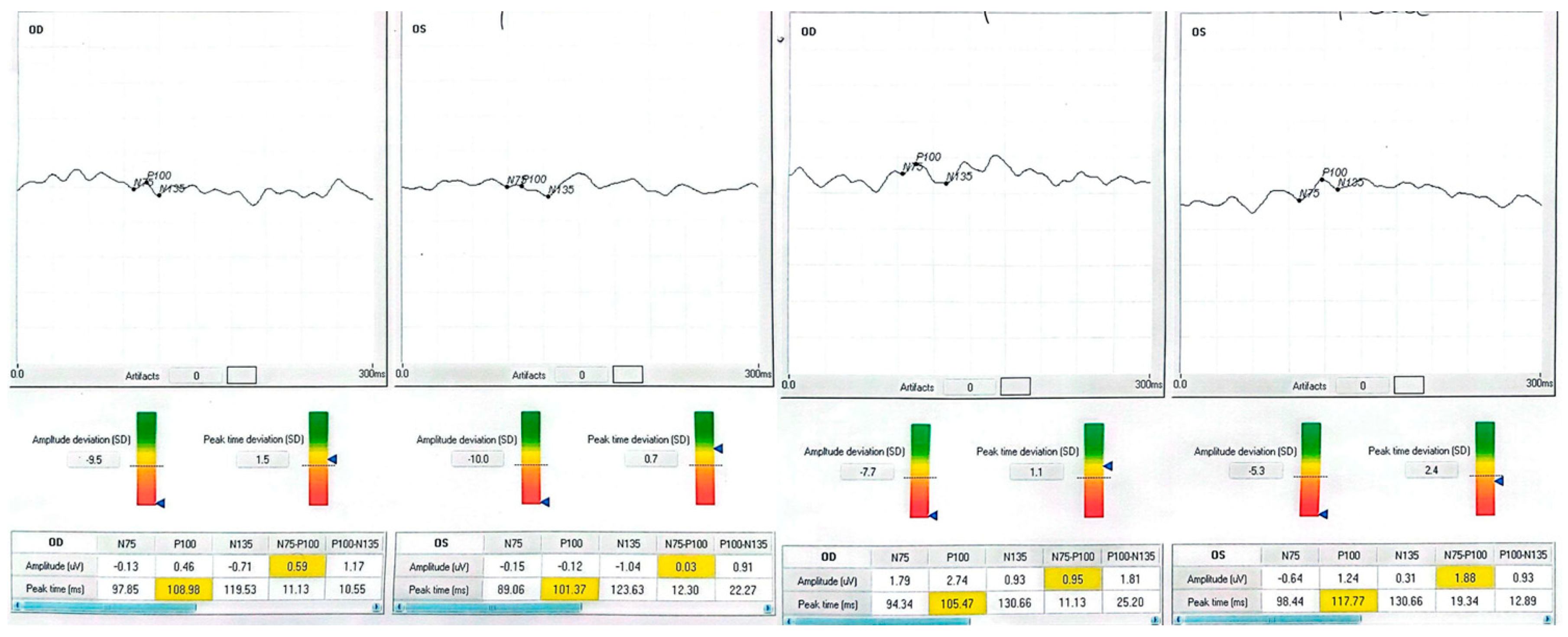
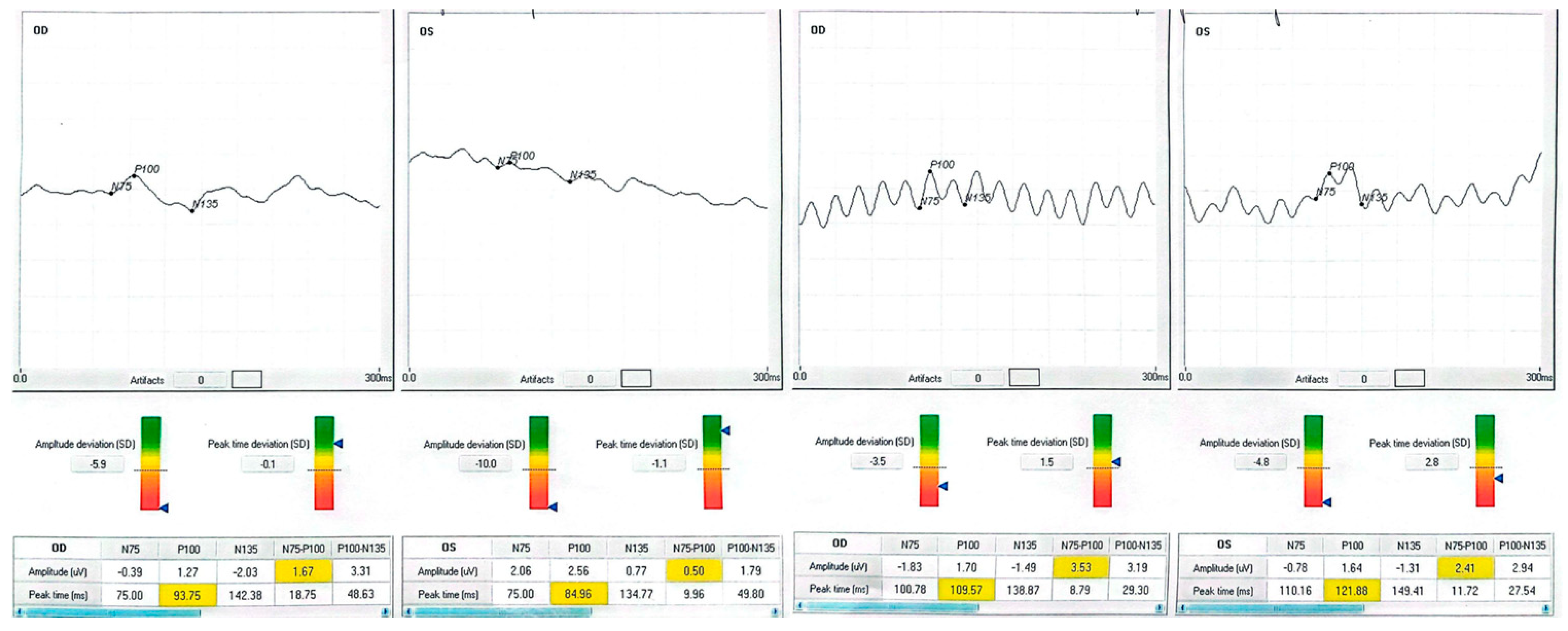
| Patient | Eye | Diagnosis | BCVA Pre (ETDRS) | BCVA Post | Δ Letters | P100 amp (µV) Pre → Post | P100 l at (ms) Pre → Post |
|---|---|---|---|---|---|---|---|
| 1 (70-year-old male with systemic hypertension) | OD | Occipital stroke (right hemianopia) | 63 | 79 | +16 | −1.21 → 1.42 | 81.5 → 90.0 |
| 1 | OS | — | 80 | 80 | 0 | 1.60 → 1.24 | 111.9 → 84.9 |
| 2 (59-year-old male) | OD | Macular hole (Stage IV) | 35 | 35 | 0 | −0.78 → 0.86 | 90.2 → 119.0 |
| 2 | OS | — | 35 | 60 | +25 | 0.33 → 3.05 | 94.9 → 111.9 |
| 3 63-year-old male with systemic hypertension) | OD | End-stage open-angle glaucoma | 25 | 40 | +15 | 0.46 → 2.74 | 109.0 → 105.5 |
| 3 | OS | — | 5 | 5 | 0 | −0.12 → 1.24 | 101.4 → 117.8 |
| 4 (40-year-old male with type 2 diabetes mellitus) | OD | Diabetic maculopathy (with cystoid edema) | 40 | 50 | +10 | 1.27 → 1.70 | 93.8 → 109.6 |
| 4 | OS | — | 50 | 63 | +13 | 2.56 → 1.64 | 85.0 → 121.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeppieri, M.; Amato, R.; Catania, D.; Musa, M.; Avitabile, A.; D’Esposito, F.; Gagliano, C.; Capobianco, M.; Nicolosi, S.G. Harnessing Visual Neuroplasticity Through Auditory Biofeedback—Functional and Electrophysiological Gains Across Retinal, Optic-Nerve, and Cortical Visual Impairment: A Prospective Pilot Study. Clin. Pract. 2025, 15, 170. https://doi.org/10.3390/clinpract15090170
Zeppieri M, Amato R, Catania D, Musa M, Avitabile A, D’Esposito F, Gagliano C, Capobianco M, Nicolosi SG. Harnessing Visual Neuroplasticity Through Auditory Biofeedback—Functional and Electrophysiological Gains Across Retinal, Optic-Nerve, and Cortical Visual Impairment: A Prospective Pilot Study. Clinics and Practice. 2025; 15(9):170. https://doi.org/10.3390/clinpract15090170
Chicago/Turabian StyleZeppieri, Marco, Roberta Amato, Daniela Catania, Mutali Musa, Alessandro Avitabile, Fabiana D’Esposito, Caterina Gagliano, Matteo Capobianco, and Simonetta Gaia Nicolosi. 2025. "Harnessing Visual Neuroplasticity Through Auditory Biofeedback—Functional and Electrophysiological Gains Across Retinal, Optic-Nerve, and Cortical Visual Impairment: A Prospective Pilot Study" Clinics and Practice 15, no. 9: 170. https://doi.org/10.3390/clinpract15090170
APA StyleZeppieri, M., Amato, R., Catania, D., Musa, M., Avitabile, A., D’Esposito, F., Gagliano, C., Capobianco, M., & Nicolosi, S. G. (2025). Harnessing Visual Neuroplasticity Through Auditory Biofeedback—Functional and Electrophysiological Gains Across Retinal, Optic-Nerve, and Cortical Visual Impairment: A Prospective Pilot Study. Clinics and Practice, 15(9), 170. https://doi.org/10.3390/clinpract15090170









