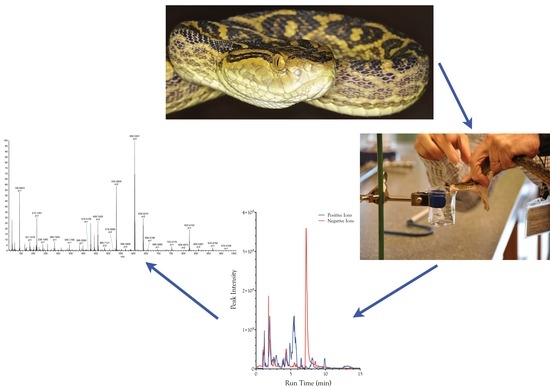
Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined
Abstract
1. Introduction
2. Results and Discussion
2.1. Compounds Isolated from Snake Venoms
2.2. Carboxylic Acids That Chelate Divalent Cations
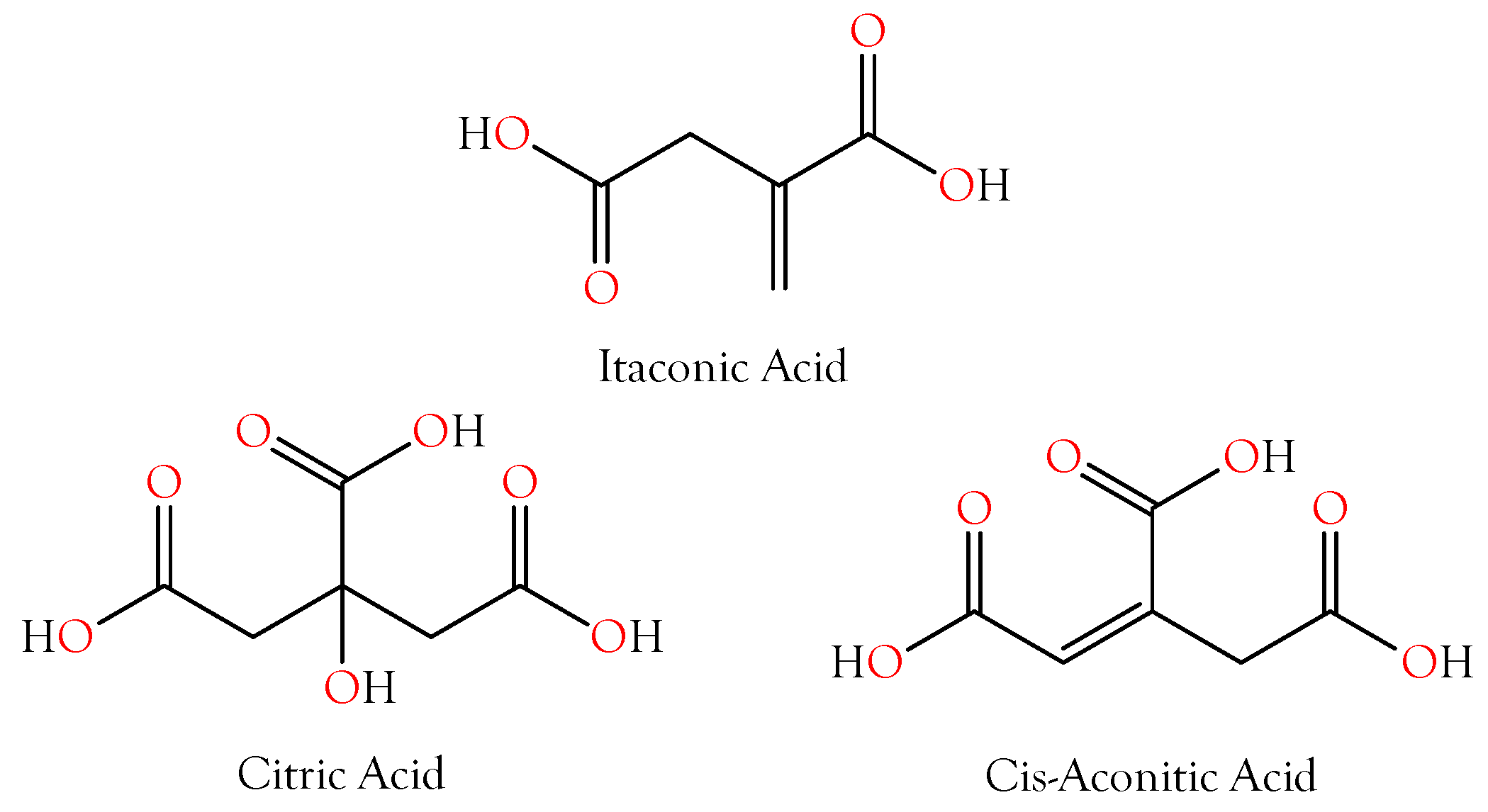
2.2.1. Citric Acid
2.2.2. Itaconic and cis-Aconitic Acids
2.3. Other Carboxylic Acids
2.3.1. 4-Guanidinobutyric Acid
2.3.2. 5-Guanidino-2-oxopentanoic Acid
2.3.3. Imidazole-4-acetic Acid
2.3.4. 4-Hydroxyphenylacetic and 4-Hydroxyphenylpyruvic Acids
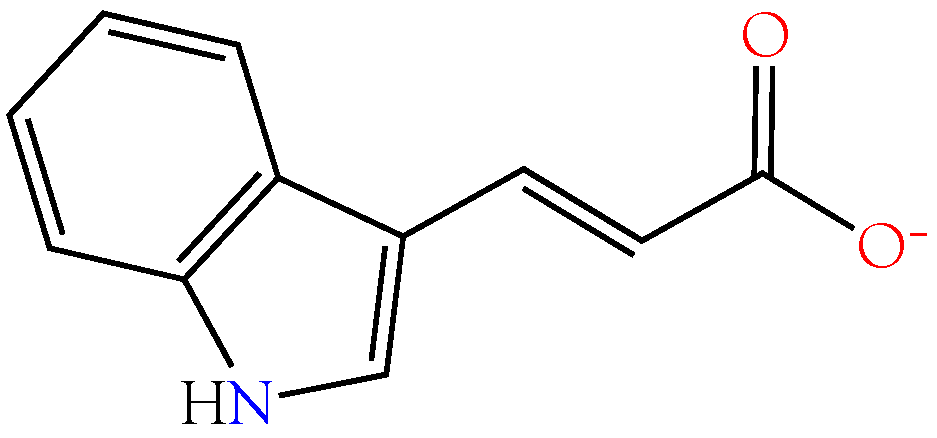
2.3.5. Indole-3-acrylic Acid
2.3.6. 5-Aminolevulinic Acid
2.4. Purine Nucleosides
2.4.1. Adenosine
2.4.2. Inosine
2.4.3. Guanosine
2.4.4. Ethyl Adenosine Carboxylate (EAC)
2.4.5. Minor Purines
2.5. Neurotransmitters
2.5.1. Acetylcholine
2.5.2. γ-Aminobutyric Acid
2.6. Amines and Alkaloids
2.6.1. Creatine and Creatinine
2.6.2. Carnitines
2.6.3. Cholines
2.6.4. Betaines
2.6.5. Taurine
2.6.6. Carnosine (β-Alanyl-l-histidine)
2.6.7. Lesser Amines and Alkaloids
2.7. Amino Acids
2.8. Carbohydrates
2.9. Metabolite Biosynthetic Pathways
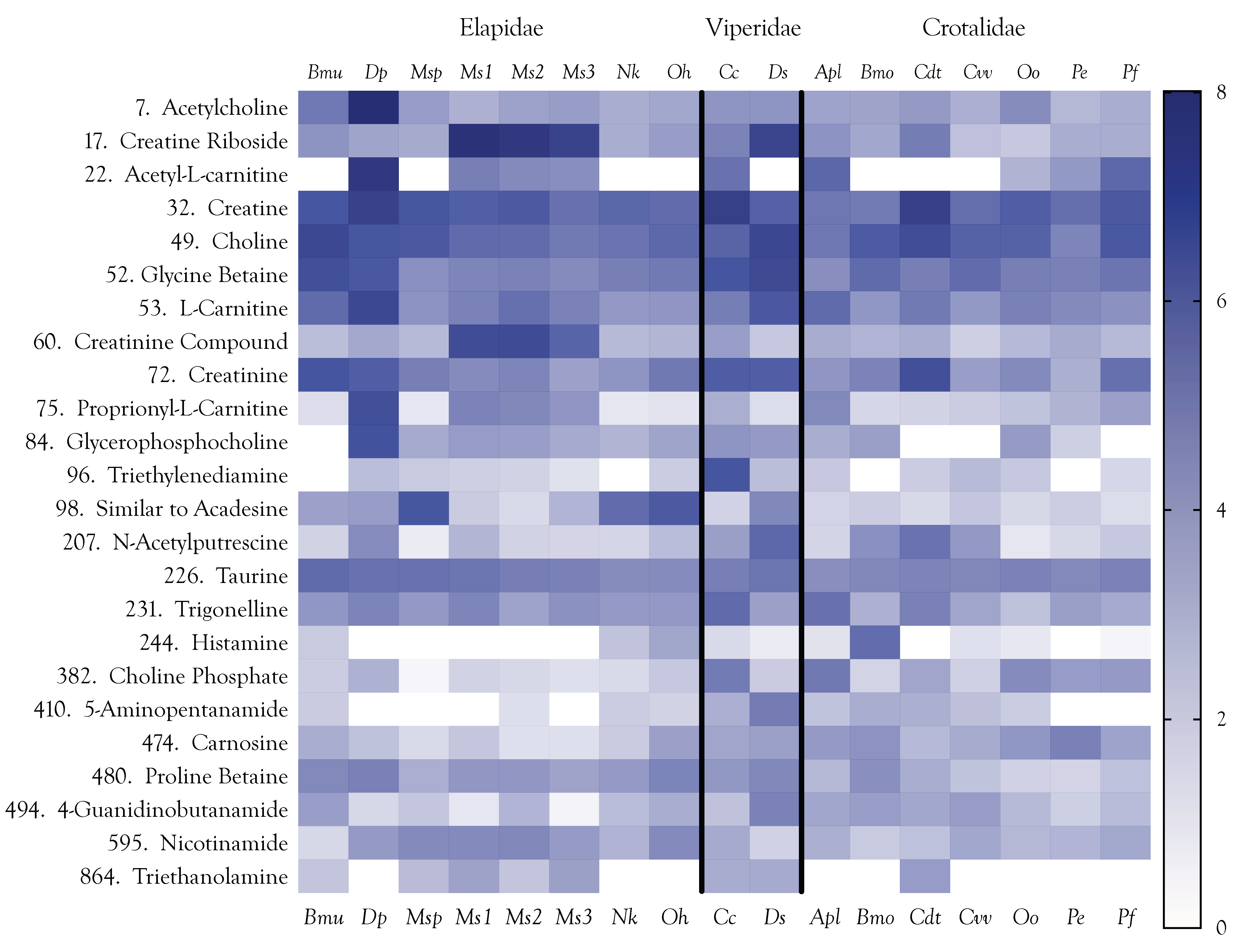
2.10. Final Considerations about Organic Metabolites
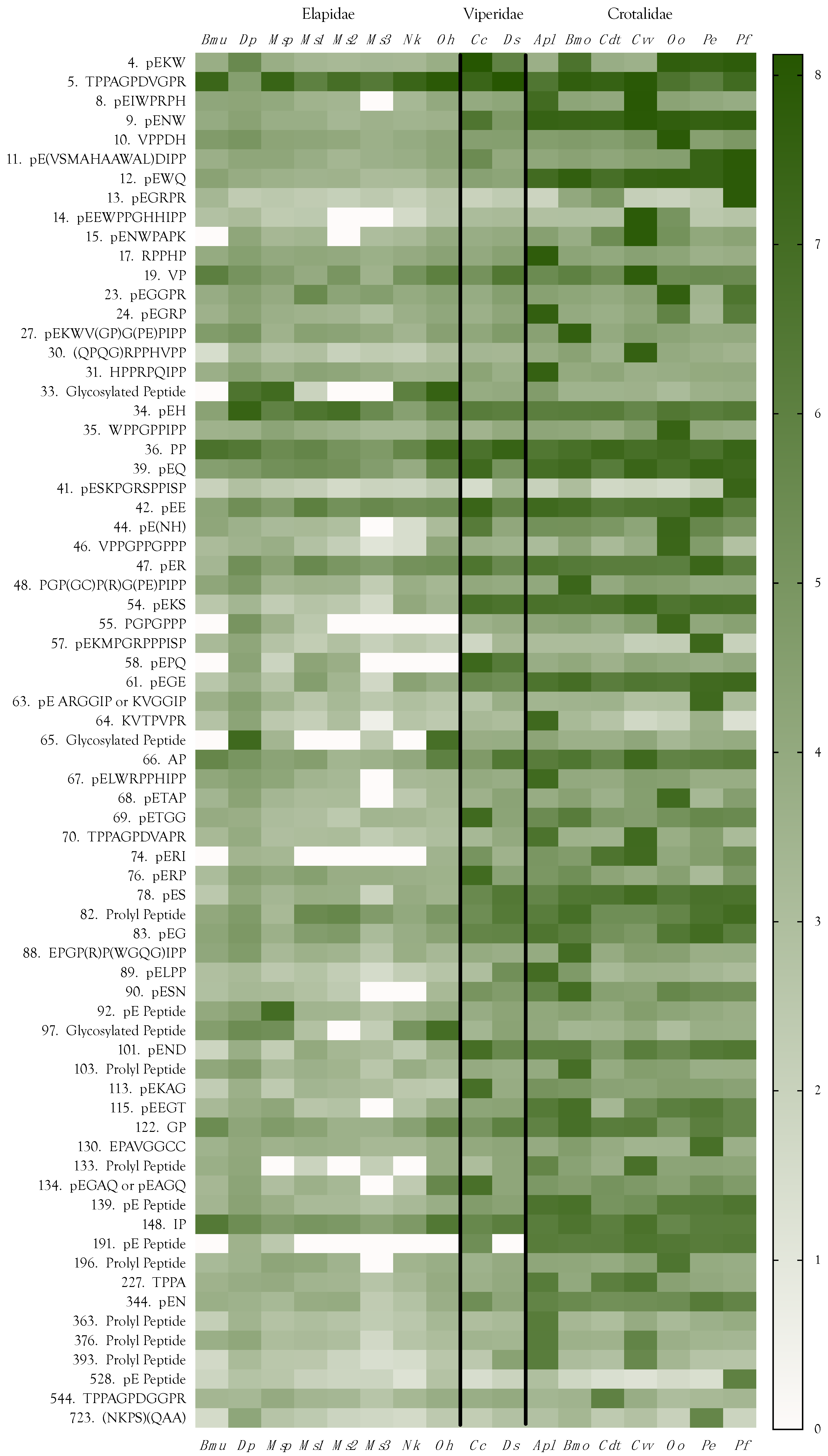
2.11. Peptides
2.11.1. Dipeptides
Prolyl Dipeptides
Pyroglutamyl Dipeptides
2.11.2. Pyroglutamyl Tripeptides
pEKW
pENW
pEKS
pEPQ, pEGE, pERI, pERP, pE(NH), pESN, and pEND
2.11.3. Tetrapeptides
2.11.4. Longer Oligopeptides
2.11.5. Oligopeptides
pEGRPR
pESKPGRSPPISP
TPPAGPDVGPR
pEEWPPCHHIPP
(QPGQ)RPPHVPP
pENWPAPK
pETGG
pEGRP
RPPHP
pEKAG
pELPP
EPAVGGCC
Final Considerations about Peptides
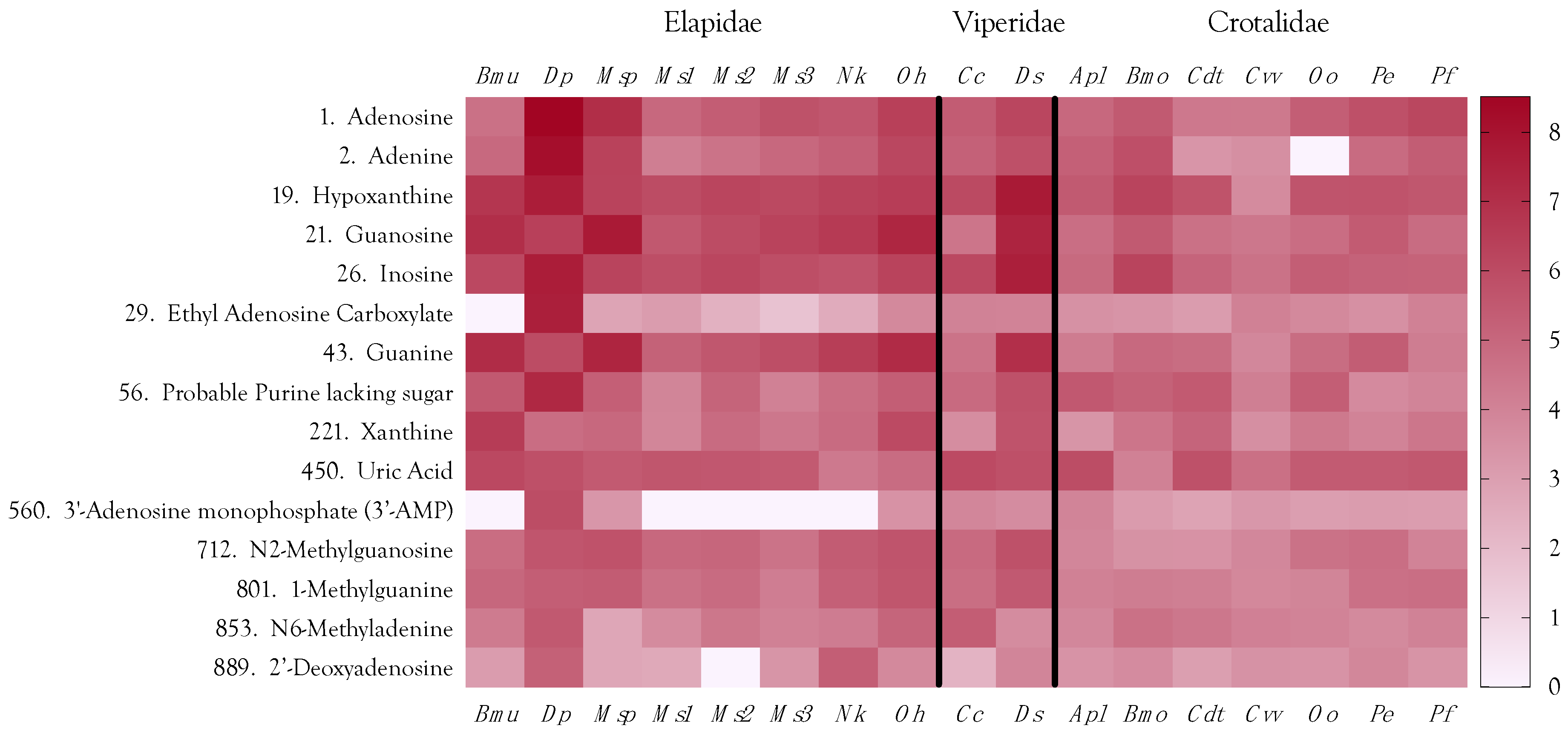
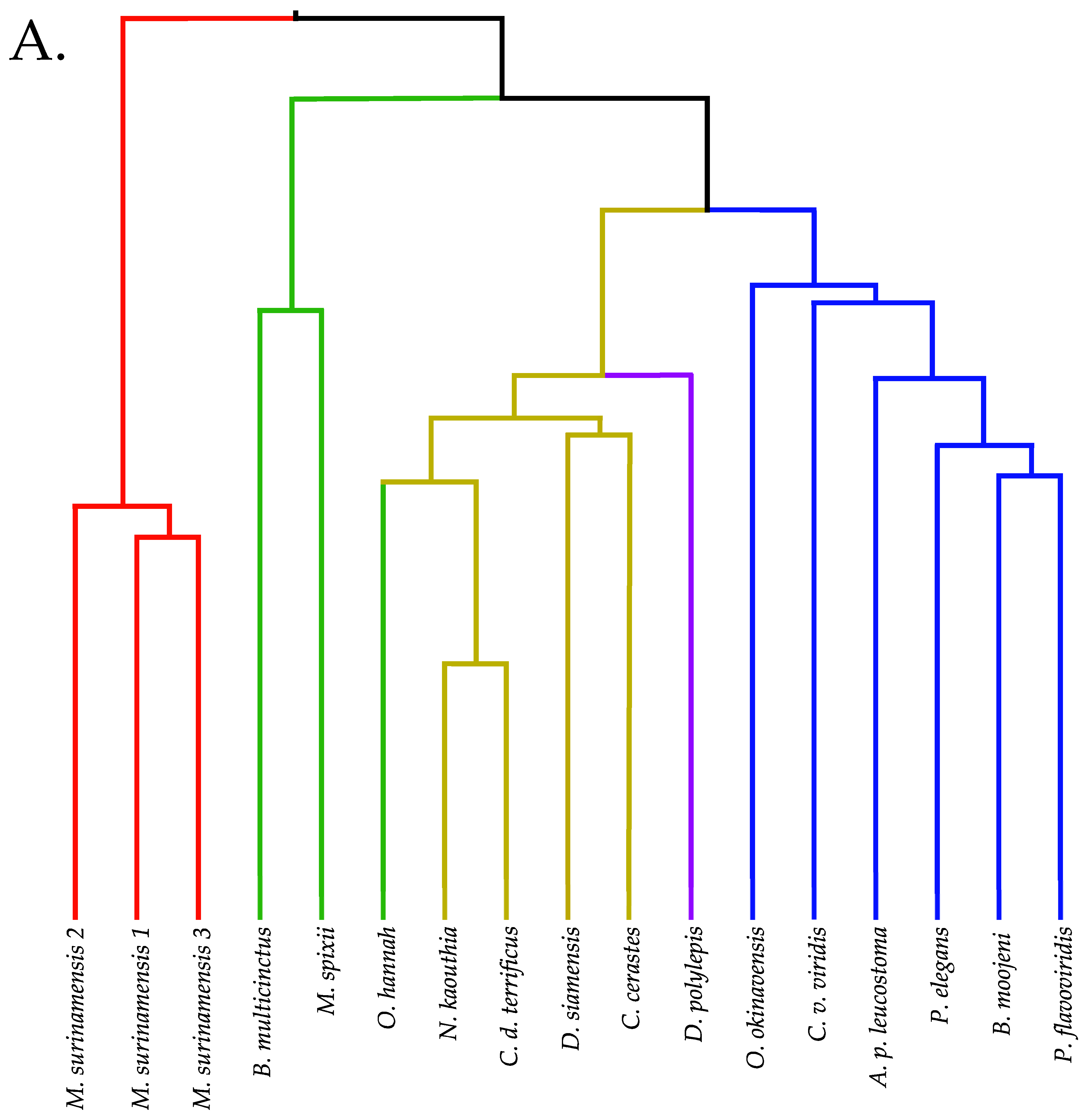
2.12. Venom Similarities in Terms of Metabolite and Peptide Content
3. Materials and Methods
3.1. Venoms
3.2. Initial Sample Preparation Using Ultrafiltration
3.3. Mass Spectrometry
3.3.1. Hydrophobic Interaction Chromatography
3.3.2. Reverse Phase Chromatography
3.3.3. Mass Spectrometry Parameters
3.3.4. Identification and Quantification of Metabolites and Peptides
3.3.5. Clustering and Hierarchical Classification
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Accessibility
References
- Ganguly, S.N.; Malkana, M.T. Indian snake venoms. II. Cobra venom: Its chemical composition, protein fractions and their physiological actions. Indian J. Med. Res. 1936, 24, 281–286. [Google Scholar]
- Devi, A. The protein and nonprotein constituents of snake venoms. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E.E., Deulofeu, V., Eds.; Academic Press: New York, NY, USA, 1968; Volume 1, pp. 119–165. [Google Scholar]
- Bieber, A.L. Metal and Nonprotein Constituents in Snake Venoms. In Snake Venoms; Md, C.-Y.L., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1979; pp. 295–306. ISBN 9783642669156. [Google Scholar]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Fischer, F.G.; Dorfel, H. Adenosine in the venom of puffadder Bitis arietans. Snake venoms. I. Hoppe Seylers Z. Physiol. Chem. 1954, 296, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Doery, H.M. Purine Compounds in Snake Venoms. Nature 1956, 177, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Doery, H.M. Additional purine compounds in the venom of the tiger snake (Notechis scutatus). Nature 1957, 180, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.-L.; Lee, C.Y. A nucleoside isolated from the venom of Bungarus multicinctus. Toxicon 1965, 3, 1–4. [Google Scholar] [CrossRef]
- Lo, T.-B.; Chen, Y.-H. Chemical Studies of Formosan Cobra (Naja Naja Atra) Venom: Part II. Isolation and Characterization of Guanosine, Adenosine, and Inosine from Cobra Venom. J. Chin. Chem. Soc. 1966, 13, 195–202. [Google Scholar] [CrossRef]
- Lin, S.-Y.S.; Lee, C.Y. Are neurotoxins from elapid venoms glycoproteins? Toxicon 1971, 9, 295–296. [Google Scholar] [CrossRef]
- Eterovic, V.A.; Hebert, M.S.; Hanley, M.R.; Bennett, E.L. The lethality and spectroscopic properties of toxins from Bungarus multicinctus (Blyth) venom. Toxicon 1975, 13, 37–48. [Google Scholar] [CrossRef]
- Aird, S.D. Taxonomic distribution and quantitative analysis of free purine and pyrimidine nucleosides in snake venoms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D. Nucleoside composition of Heloderma venoms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; Watanabe, Y.; Villar-Briones, A.; Roy, M.C.; Terada, K.; Mikheyev, A.S. Quantitative high-throughput profiling of snake venom gland transcriptomes and proteomes (Ovophis okinavensis and Protobothrops flavoviridis). BMC Genom. 2013, 14, 790. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; Aggarwal, S.; Villar-Briones, A.; Tin, M.M.-Y.; Terada, K.; Mikheyev, A.S. Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly. BMC Genom. 2015, 16, 647. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; da Silva, N.J., Jr.; Qiu, L.; Villar-Briones, A.; Saddi, V.A.; Pires de Campos Telles, M.; Grau, M.L.; Mikheyev, A.S. Coralsnake Venomics: Analyses of Venom Gland Transcriptomes and Proteomes of Six Brazilian Taxa. Toxins 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.A.; Geno, P.W.; Sumner, L.W.; Cooke, M.E.; Hudiburg, S.A.; Ownby, C.L.; Kaiser, I.I.; Odell, G.V. Citrate is a major component of snake venoms. Toxicon 1992, 30, 461–464. [Google Scholar] [CrossRef]
- Francis, B.; Seebart, C.; Kaiser, I.I. Citrate is an endogenous inhibitor of snake venom enzymes by metal-ion chelation. Toxicon 1992, 30, 1239–1246. [Google Scholar] [CrossRef]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pajor, A.M. Functional Characterization of CitM, the Mg2+-Citrate Transporter. J. Membr. Biol. 2002, 185, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Odell, G.V.; Ferry, P.C.; Vick, L.M.; Fenton, A.W.; Decker, L.S. Citrate inhibition of snake venom proteases. Toxicon 1998, 36, 1801–1806. [Google Scholar] [CrossRef]
- Sunnerhagen, M.; Drakenberg, T.; Forsen, S.; Stenflo, J. Effect of Ca2+ on the structure of vitamin K-dependent coagulation factors. Haemostasis 1996, 26 (Suppl. 1), 45–53. [Google Scholar]
- Losner, S.; Volk, B.W. Citrate clotting time in anticoagulant therapy. Am. J. Clin. Pathol. 1953, 23, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Huijgens, P.C.; van den Berg, C.A.; Voetdijk, A.M.; Imandt, L.M. The influence of citrate on platelet aggregation and malondialdehyde production. Scand. J. Haematol. 1983, 31, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.N.; Spain, S.; Goldsmith, H.L. Extracellular-free Ca++ accounts for the sex difference in the aggregation of human platelets in citrated platelet-rich plasma. Thromb. Res. 1990, 58, 47–60. [Google Scholar] [CrossRef]
- Bashir, W.; Tyrrell, E.; Feeney, O.; Paull, B. Retention of alkali, alkaline earth and transition metals on an itaconic acid cation-exchange column. Eluent pH, ionic strength and temperature effects upon selectivity. J. Chromatogr. A 2002, 964, 113–122. [Google Scholar] [CrossRef]
- Curtis, D.R.; Watkins, J.C. The excitation and depression of spinal neurones by structurally related amino acids. J. Neurochem. 1960, 6, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, W.I. ATP-sensitive potassium channels in the cerebral circulation. Stroke 2003, 34, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, M. A role for guanidino compounds in the brain. Mol. Cell. Biochem. 2003, 244, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, M.; Hosoya, K.-I. Transport characteristics of guanidino compounds at the blood-brain barrier and blood-cerebrospinal fluid barrier: Relevance to neural disorders. Fluids Barriers CNS 2011, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Jinnai, D.; Sawai, A.; Mori, A. Gamma-guanidinobutyric acid as a convulsive substance. Nature 1966, 212, 617. [Google Scholar] [CrossRef] [PubMed]
- Constanti, A. The GABA dose/conductance relationship on lobster muscle. J. Physiol. 1979, 75, 645–649. [Google Scholar]
- De Deyn, P.P.; Marescau, B.; MacDonald, R.L. Epilepsy and the GABA-hypothesis a brief review and some examples. Acta Neurol. Belg. 1990, 90, 65–81. [Google Scholar] [PubMed]
- De Deyn, P.P.; Macdonald, R.L. Guanidino compounds that are increased in cerebrospinal fluid and brain of uremic patients inhibit GABA and glycine responses on mouse neurons in cell culture. Ann. Neurol. 1990, 28, 627–633. [Google Scholar] [CrossRef] [PubMed]
- De Deyn, P.P.; Marescau, B.; Macdonald, R.L. Effects of alpha-keto-delta-guanidinovaleric acid on inhibitory amino acid responses on mouse neurons in cell culture. Brain Res. 1988, 449, 54–60. [Google Scholar] [CrossRef]
- De Deyn, P.P.; Marescau, B.; Macdonald, R.L. Guanidino compounds that are increased in hyperargininemia inhibit GABA and glycine responses on mouse neurons in cell culture. Epilepsy Res. 1991, 8, 134–141. [Google Scholar] [CrossRef]
- Olsen, R.W. The GABA postsynaptic membrane receptor-ionophore complex. Site of action of convulsant and anticonvulsant drugs. Mol. Cell. Biochem. 1981, 39, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.W.; Prestwich, S.A.; Meldrum, B.S. gamma-Aminobutyric acid and benzodiazepine binding sites in audiogenic seizure-susceptible mice. J. Neurochem. 1982, 39, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Schwartz-Porsche, D. Low levels of gamma-aminobutyric acid in cerebrospinal fluid of dogs with epilepsy. J. Neurochem. 1986, 46, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, A.; DiMicco, J.A. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology 1987, 26, 407–417. [Google Scholar] [CrossRef]
- Kása, P.; Joó, F.; Dobó, E.; Wenthold, R.J.; Ottersen, O.P.; Storm-Mathisen, J.; Wolff, J.R. Heterogeneous distribution of GABA-immunoreactive nerve fibers and axon terminals in the superior cervical ganglion of adult rat. Neuroscience 1988, 26, 635–644. [Google Scholar] [CrossRef]
- Wolff, J.R.; Kasa, P.; Dobo, E.; Wenthold, R.J.; Joo, F. Quantitative analysis of the number and distribution of neurons richly innervated by GABA-immunoreactive axons in the rat superior cervical ganglion. J. Comp. Neurol. 1989, 282, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kenny, S.L.; Ariano, M.A. The immunofluorescence localization of glutamate decarboxylase in the rat superior cervical ganglion. J. Auton. Nerv. Syst. 1986, 17, 211–215. [Google Scholar] [CrossRef]
- Häppölä, O.; Päivärinta, H.; Soinila, S.; Wu, J.Y.; Panula, P. Localization of l-glutamate decarboxylase and GABA transaminase immunoreactivity in the sympathetic ganglia of the rat. Neuroscience 1987, 21, 271–281. [Google Scholar] [CrossRef]
- Galvan, M.; Grafe, P.; ten Bruggencate, G. Presynaptic actions of 4-aminopyridine and gamma-aminobutyric acid on rat sympathetic ganglia in vitro. Naunyn Schmiedebergs Arch. Pharmacol. 1980, 314, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bowery, N.G.; Brown, D.A. Depolarizing actions of γ-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br. J. Pharmacol. 1974, 50, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.; Schubert, K. Neurotransmitters regulating feline aggressive behavior. Rev. Neurosci. 1995, 6, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tiba, M.; Iino, M.; Takayasu, T. The effect of gamma-aminobutyric acid on blood pressure. Jpn. J. Physiol. 1955, 5, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.A.; Hobbiger, F. gamma Aminobutyric acid; circulatory and respiratory effects in different species; re-investigation of the anti-strychnine action in mice. J. Physiol. 1959, 146, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Stanton, H.C.; Woodhouse, F.H. The effect of gamma-amino-n-butyric acid and some related compounds on the cardiovascular system of anesthetized dogs. J. Pharmacol. Exp. Ther. 1960, 128, 233–242. [Google Scholar] [PubMed]
- Maggi, C.A.; Giuliani, S.; Meli, A. The effect of peripherally administered GABA on noradrenaline-induced reflex vagal bradycardia in urethane anaesthetized rats. Gen. Pharmacol. 1985, 16, 579–584. [Google Scholar] [CrossRef]
- Serafin, B.; Borkowska, G.; Główczyk, J.; Kowalska, I.; Rump, S. Potential antihypertensive benzimidazole derivatives. Pol. J. Pharmacol. Pharm. 1989, 41, 89–96. [Google Scholar] [PubMed]
- Thomas, G.; Farhat, M.Y.; Heim, K.F.; Ramwell, P.W. Guanidino compounds and endothelium dependent relaxation. Adv. Prostaglandin Thromboxane Leukot. Res. 1991, 21B, 637–643. [Google Scholar] [PubMed]
- Cook, E.; Fujii, A. Methods and Compositions for Inducing Resistance to Bacterial Infections 1974. US Patent US3843798A. Available online: https://patents.google.com/patent/US3843798A/en (accessed on 25 September 2018).
- Mizutani, N.; Hayakawa, C.; Ohya, Y.; Watanabe, K.; Watanabe, Y.; Mori, A. Guanidino compounds in hyperargininemia. Tohoku J. Exp. Med. 1987, 153, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Dowling, J.E. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J. Neurosci. 1994, 14, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Dowling, J.E. GABAA and GABAC receptors on hybrid bass retinal bipolar cells. J. Neurophysiol. 1995, 74, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.H.; Lipton, S.A. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J. Neurosci. 1995, 15, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G. GABAA receptor pharmacology. Pharmacol. Ther. 1996, 69, 173–198. [Google Scholar] [CrossRef]
- Lukasiewicz, P.D.; Shields, C.R. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J. Neurophysiol. 1998, 79, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.E.; Xu, J.; Jain, S.; Moulder, K.; Mennerick, S.; Crowley, J.R.; Townsend, R.R.; Gordon, J.I. An integrated functional genomics and metabolomics approach for defining poor prognosis in human neuroendocrine cancers. Proc. Natl. Acad. Sci. USA. 2005, 102, 9901–9906. [Google Scholar] [CrossRef] [PubMed]
- Curtis, D.R.; Duggan, A.W.; Felix, D.; Johnston, G.A.R. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971, 32, 69–96. [Google Scholar] [CrossRef]
- Krnjević, K.; Phillis, J.W. Actions of certain amines on cerebral cortical neurones. Br. J. Pharmacol. Chemother. 1963, 20, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Simonsen, D.G. A hypnotic and possible analgesic effect of imidazoleacetic acid in mice. Biochem. Pharmacol. 1966, 15, 1875–1877. [Google Scholar] [CrossRef]
- Marcus, R.J.; Winters, W.D.; Roberts, E.; Simonsen, D.G. Neuropharmacological studies of imidazole-4-acetic acid actions in the mouse and rat. Neuropharmacology 1971, 10, 203–215. [Google Scholar] [CrossRef]
- Tunnicliff, G.; Wein, J.; Roberts, E. Effects of imidazoleaceticacid on brain amino acids and body temperature in mice. J. Neurochem. 1972, 19, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Sooriyamoorthy, T.; Livingston, A.; Taberner, P.V. A comparison of the effect of some sedative and anaesthetic drugs on cerebral blood flow in the rat. Gen. Pharmacol. Vasc. Syst. 1975, 6, 27–30. [Google Scholar] [CrossRef]
- Roberts, E. Toward a rational pharmacology of the GABA system. In Neurotransmitters; Simon, P., Ed.; Advances in Pharmacology and Therapeutics; Pergamon Press: Paris, France, 1979; Volume 2, pp. 43–68. ISBN 9780080231921. [Google Scholar]
- Khandelwal, J.K.; Prell, G.D.; Morrishow, A.M.; Green, J.P. Presence and measurement of imidazoleacetic acid, a gamma-aminobutyric acid agonist, in rat brain and human cerebrospinal fluid. J. Neurochem. 1989, 52, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Prell, G.D.; Morrishow, A.M.; Duoyon, E.; Lee, W.S. Inhibitors of histamine methylation in brain promote formation of imidazoleacetic acid, which interacts with GABA receptors. J. Neurochem. 1997, 68, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kusama, T.; Wang, T.L.; Guggino, W.B.; Cutting, G.R.; Uhl, G.R. GABA rho 2 receptor pharmacological profile: GABA recognition site similarities to rho 1. Eur. J. Pharmacol. 1993, 245, 83–84. [Google Scholar] [CrossRef]
- Tunnicliff, G. Pharmacology and function of imidazole 4-acetic acid in brain. Gen. Pharmacol. 1998, 31, 503–509. [Google Scholar] [CrossRef]
- Chebib, M.; Mewett, K.N.; Johnston, G.A. GABA(C) receptor antagonists differentiate between human rho1 and rho2 receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 1998, 357, 227–234. [Google Scholar] [CrossRef]
- Vien, J.; Duke, R.K.; Mewett, K.N.; Johnston, G.A.R.; Shingai, R.; Chebib, M. Trans-4-Amino-2-methylbut-2-enoic acid (2-MeTACA) and (+/−)-trans-2-aminomethylcyclopropanecarboxylic acid ((+/−)-TAMP) can differentiate rat rho3 from human rho1 and rho2 recombinant GABA(C) receptors. Br. J. Pharmacol. 2002, 135, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Kurdziel, K.; Głowiak, T.; Materazzi, S.; Jezierska, J. Crystal structure and physico-chemical properties of cobalt(II) and manganese(II) complexes with imidazole-4-acetate anion. Polyhedron 2003, 22, 3123–3128. [Google Scholar] [CrossRef]
- Bormann, J.; Feigenspan, A. GABAC receptors. Trends Neurosci. 1995, 18, 515–519. [Google Scholar] [CrossRef]
- Lukasiewicz, P.D.; Shields, C.R. A diversity of GABA receptors in the retina. Semin. Cell Dev. Biol. 1998, 9, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A.R.; Chebib, M.; Hanrahan, J.R.; Mewett, K.N. Neurochemicals for the investigation of GABA(C) receptors. Neurochem. Res. 2010, 35, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Chebib, M.; Johnston, G.A.R. The “ABC” of GABA receptors: A brief review. Clin. Exp. Pharmacol. Physiol. 1999, 26, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Atack, J.R. GABAA receptor subtype-selective modulators. I. α2/α3-selective agonists as non-sedating anxiolytics. Curr. Top. Med. Chem. 2011, 11, 1176–1202. [Google Scholar] [CrossRef] [PubMed]
- Antonaccio, M.J.; Kerwin, L.; Taylor, D.G. Reductions in blood pressure, heart rate and renal sympathetic nerve discharge in cats after the central administration of muscimol, a GABA agonist. Neuropharmacology 1978, 17, 783–791. [Google Scholar] [CrossRef]
- Roberts, E.; Simonsen, D.G. Some properties of cyclic 3′,5′-nucleotide phosphodiesterase of mouse brain: Effects of imidazole-4-acetic acid, chlorpromazine, cyclic 3′,5′-GMP, and other substances. Brain Res. 1970, 24, 91–111. [Google Scholar] [CrossRef]
- Walland, A. cAMP as a second messenger in central blood pressure control. Naunyn Schmiedebergs Arch. Pharmacol. 1975, 290, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Tomi, M.; Terayama, T.; Isobe, T.; Egami, F.; Morito, A.; Kurachi, M.; Ohtsuki, S.; Kang, Y.-S.; Terasaki, T.; Hosoya, K.-I. Function and regulation of taurine transport at the inner blood–retinal barrier. Microvasc. Res. 2007, 73, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Valembois, S.; Krall, J.; Frølund, B.; Steffansen, B. Imidazole-4-acetic acid, a new lead structure for interaction with the taurine transporter in outer blood-retinal barrier cells. Eur. J. Pharm. Sci. 2017, 103, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Holmseth, S.; Guo, C.; Hassel, B.; Höfner, G.; Huitfeldt, H.S.; Wanner, K.T.; Danbolt, N.C. Deletion of the γ-Aminobutyric Acid Transporter 2 (GAT2 and SLC6A13) Gene in Mice Leads to Changes in Liver and Brain Taurine Contents. J. Biol. Chem. 2012, 287, 35733–35746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Lopez-Corcuera, B.; Mandiyan, S. Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected]. J. Biol. Chem. 1993, 268, 2106–2112. [Google Scholar] [PubMed]
- Vinnakota, S.; Qian, X.; Egal, H.; Sarthy, V.; Sarkar, H.K. Molecular Characterization and In Situ Localization of a Mouse Retinal Taurine Transporter. J. Neurochem. 1997, 69, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.B. Tyrosine aminotransferase: A transaminase among others? Cell. Mol. Biol. 1992, 38, 95–114. [Google Scholar] [PubMed]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids—Interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Nucaro, E.; Jodra, M.; Russell, E.; Anderson, L.; Dennison, P.; Dufton, M. Conversion of tyrosine to phenolic derivatives by Taiwan cobra venom. Toxicon 1998, 36, 1173–1187. [Google Scholar] [CrossRef]
- Sheu, S.Y.; Chiang, H. Inhibitory effects of plant growth regulators on xanthine oxidase. Anticancer Res. 1996, 16, 311–315. [Google Scholar] [PubMed]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur. J. Neurosci. 2007. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A.; Kuroki, Y.; Urata, T.; Mori, N.; Fukuwatari, T. Inhibition of Large Neutral Amino Acid Transporters Suppresses Kynurenic Acid Production Via Inhibition of Kynurenine Uptake in Rodent Brain. Neurochem. Res. 2016, 41, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Williams, S.K.; Stojic, A.; Iwantscheff, S.; Sonner, J.K.; Grabitz, C.; Becker, S.; Böhler, L.-I.; Mohapatra, S.R.; Sahm, F.; et al. Tryptophan-2,3-Dioxygenase (TDO) deficiency is associated with subclinical neuroprotection in a mouse model of multiple sclerosis. Sci. Rep. 2017, 7, 41271. [Google Scholar] [CrossRef] [PubMed]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Robinson, H.; Cai, T.; Tagle, D.A.; Li, J. Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K. J. Med. Chem. 2009, 52, 2786–2793. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Karlsson, E. Dendrotoxin from the venom of the green mamba, Dendroaspis angusticeps A neurotoxin that enhances acetylcholine release at neuromuscular junction. Naunyn Schmiedebergs Arch. Pharmacol. 1980, 312, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Anderson, A.J. Dendrotoxins: Snake toxins that block potassium channels and facilitate neurotransmitter release. Pharmacol. Ther. 1985, 31, 33–55. [Google Scholar] [CrossRef]
- Wangai, J.; Thairu, K.; Bharaj, B.S.; Telang, B.V. Identification and isolation of three acetylcholinesterase inactivating fractions in the venom of Dendroaspis angusticeps. Acta Physiol. Acad. Sci. Hung. 1982, 60, 83–88. [Google Scholar] [PubMed]
- Rodríguez-Ithurralde, D.; Silveira, R.; Barbeito, L.; Dajas, F. Fasciculin, a powerful anticholinesterase polypeptide from Dendroaspis angusticeps venom. Neurochem. Int. 1983, 5, 267–274. [Google Scholar] [CrossRef]
- Karlsson, E.; Mbugua, P.M.; Rodriguez-Ithurralde, D. Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angusticeps. J. Physiol. 1984, 79, 232–240. [Google Scholar]
- Wangai, J.; Nganga, J.N.; Njoroge, D.; Dossaji, S.F.; Thairu, K.; Telang, B.V. Identification and assay of acetylcholine-like substance in venom of Dendroaspis angusticeps. Agressologie 1977, 18, 33–37. [Google Scholar]
- Hendriks, M.G.; Pfaffendorf, M.; Van Zwieten, P.A. Characterization of the muscarinic receptor subtype mediating vasodilation in the rat perfused mesenteric vascular bed preparation. J. Auton. Pharmacol. 1992, 12, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Bruning, T.A.; Hendriks, M.G.; Chang, P.C.; Kuypers, E.A.; van Zwieten, P.A. In vivo characterization of vasodilating muscarinic-receptor subtypes in humans. Circ. Res. 1994, 74, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Lamping, K.G.; Wess, J.; Cui, Y.; Nuno, D.W.; Faraci, F.M. Muscarinic (M) receptors in coronary circulation gene-targeted mice define the role of M2 and M3 receptors in response to acetylcholine. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Sniatecki, J.J.; Mayer, V.G.A.; Goloborodko, E.; Patzak, A.; Wess, J.; Pfeiffer, N. Role of M1, M3, and M5 muscarinic acetylcholine receptors in cholinergic dilation of small arteries studied with gene-targeted mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1602–H1608. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Watanabe, Y.; Kawanishi, K.; Hashimoto, Y.; Hayaishi, O. Inhibition of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by β-carboline and indole derivatives. Arch. Biochem. Biophys. 1984, 232, 602–609. [Google Scholar] [CrossRef]
- Moroni, F. Tryptophan metabolism and brain function: Focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 1999, 375, 87–100. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugimoto, H.; Tanaka, I.; Nishihira, J. Substrate specificity for isomerase activity of macrophage migration inhibitory factor and its inhibition by indole derivatives. J. Biochem. 1997, 122, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Garai, J.; Lorand, T. Macrophage Migration Inhibitory Factor (MIF) Tautomerase Inhibitors as Potential Novel Anti-Inflammatory Agents: Current Developments. Curr. Med. Chem. 2009, 16, 1091–1114. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M. How do Ca2+ and 5-aminolevulinic acid-derived oxyradicals promote injury to isolated mitochondria? Free Radic. Biol. Med. 1995, 19, 381–390. [Google Scholar] [CrossRef]
- Bechara, E.J. Oxidative stress in acute intermittent porphyria and lead poisoning may be triggered by 5-aminolevulinic acid. Braz. J. Med. Biol. Res. 1996, 29, 841–851. [Google Scholar] [PubMed]
- Reddington, M.; Lee, K.S.; Schubert, P. An A1-adenosine receptor, characterized by [3H]cyclohexyladenosine binding, mediates the depression of evoked potentials in a rat hippocampal slice preparation. Neurosci. Lett. 1982, 28, 275–279. [Google Scholar] [CrossRef]
- Prestwich, S.A.; Forda, S.R.; Dolphin, A.C. Adenosine antagonists increase spontaneous and evoked transmitter release from neuronal cells in culture. Brain Res. 1987, 405, 130–139. [Google Scholar] [CrossRef]
- Higgins, M.J.; Hosseinzadeh, H.; MacGregor, D.G.; Ogilvy, H.; Stone, T.W. Release and actions of adenosine in the central nervous system. Pharm. World Sci. 1994, 16, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, D.M.; Choi, S. Activation of adenosine A1 receptors initiates short-term synaptic depression in rat striatum. Neurosci. Lett. 1995, 199, 9–12. [Google Scholar] [CrossRef]
- Di Iorio, P.; Battaglia, G.; Ciccarelli, R.; Ballerini, P.; Giuliani, P.; Poli, A.; Nicoletti, F.; Caciagli, F. Interaction between A1 adenosine and class II metabotropic glutamate receptors in the regulation of purine and glutamate release from rat hippocampal slices. J. Neurochem. 1996, 67, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Burgdorf, C.; Richardt, D.; Kurz, T.; Seyfarth, M.; Jain, D.; Katus, H.A.; Richardt, G. Adenosine inhibits norepinephrine release in the postischemic rat heart: The mechanism of neuronal stunning. Cardiovasc. Res. 2001, 49, 713–720. [Google Scholar] [CrossRef]
- Arrigoni, E.; Chamberlin, N.L.; Saper, C.B.; McCarley, R.W. Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience 2006, 140, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ginsborg, B.L.; Hirst, G.D. Cyclic AMP, transmitter release and the effect of adenosine on neuromuscular transmission. Nat. New Biol. 1971, 232, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Dominguez, M.L. Mechanisms of depression of neuromuscular transmission by ATP and adenosine. J. Physiol. 1978, 74, 491–496. [Google Scholar]
- Silinsky, E.M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J. Physiol. 1984, 346, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.D.; Donnelly, D.F.; Haddad, G.G. Adenosine-induced inhibition of vagal motoneuron excitability: Receptor subtype and mechanisms. Am. J. Physiol. 1993, 264, L124–L132. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Kitamura, A.; Akita, T.; Narita, K.; Kuba, K. Adenosine depresses a Ca2+-independent step in transmitter exocytosis at frog motor nerve terminals. Eur. J. Neurosci. 2002, 15, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Silinsky, E.M. Adenosine decreases both presynaptic calcium currents and neurotransmitter release at the mouse neuromuscular junction. J. Physiol. 2004, 558, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C.; Prestwich, S.A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature 1985, 316, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.A.; Stevens, C.F. Adenosine decreases neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. USA. 1992, 89, 8586–8590. [Google Scholar] [CrossRef] [PubMed]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, A.F.; Flacke, W.E.; Bloor, B.C. Hypotensive effects of adenosine and adenosine triphosphate compared with sodium nitroprusside. Anesth. Analg. 1982, 61, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Barraco, R.A.; Phillis, J.W.; Campbell, W.R.; Marcantonio, D.R.; Salah, R.S. The effects of central injections of adenosine analogs on blood pressure and heart rate in the rat. Neuropharmacology 1986, 25, 675–680. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Sollevi, A. Cardiovascular effects of adenosine. Clin. Physiol. 1986, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.; Barraco, R.A.; Phillis, J.W. A central role for adenosine in the hypotension elicited by hypoxia in anesthetized rats. Brain Res. Bull. 1989, 23, 37–40. [Google Scholar] [CrossRef]
- Belloni, F.L.; Wang, J.; Hintze, T.H. Adenosine causes bradycardia in pacing-induced cardiac failure. Circulation 1992, 85, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Stella, L.; Berrino, L.; Maione, S.; de Novellis, V.; Rossi, F. Cardiovascular effects of adenosine and its analogs in anaesthetized rats. Life Sci. 1993, 53, 755–763. [Google Scholar] [CrossRef]
- Pennanen, M.F.; Bass, B.L.; Dziki, A.J.; Harmon, J.W. Adenosine: Differential effect on blood flow to subregions of the upper gastrointestinal tract. J. Surg. Res. 1994, 56, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Jan, C.R.; Wu, S.N.; Tseng, C.J. Cardiovascular effects of nitric oxide and adenosine in the nucleus tractus solitarii of rats. Hypertension 1998, 32, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.R.; Ngai, A.C.; Winn, H.R. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am. J. Physiol. 1990, 259, H1703–H1708. [Google Scholar] [CrossRef] [PubMed]
- Saito, D.; Mima, T.; Obayashi, N.; Uchida, S.; Maekawa, K.; Sato, T.; Mizuo, K.; Kobayashi, H.; Haraoka, S. Effects of inosine on adenosine-induced coronary vasodilation in the open chest dog. Arzneimittelforschung 1993, 43, 950–953. [Google Scholar] [PubMed]
- Aird, S.D. The Role of Purine and Pyrimidine Nucleosides in Snake Venoms. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 393–419. [Google Scholar]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Toxicovenomics and antivenom profiling of the Eastern green mamba snake (Dendroaspis angusticeps). J. Proteom. 2016, 136, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, N.G.; Fry, B.G.; Ventura, S.; Kini, R.M.; Hodgson, W.C. The in vitro and in vivo pharmacological activity of Boiga dendrophila (mangrove catsnake) venom. Auton. Autacoid Pharmacol. 2004, 24, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Oyama, E.; Takahashi, H. Distribution of low molecular weight platelet aggregation inhibitors from snake venoms. Toxicon 2007, 49, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Fung, S.Y.; Yap, M.K.K.; Leong, P.K.; Liew, J.L.; Tan, N.H. Unveiling the elusive and exotic: Venomics of the Malayan blue coral snake (Calliophis bivirgata flaviceps). J. Proteom. 2016, 132, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernández, J.; Gutiérrez, J.M. Unveiling the nature of black mamba (Dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Cintra-Francischinelli, M.; Caccin, P.; Chiavegato, A.; Pizzo, P.; Carmignoto, G.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Montecucco, C. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc. Natl. Acad. Sci. USA. 2010, 107, 14140–14145. [Google Scholar] [CrossRef] [PubMed]
- Caccin, P.; Pellegatti, P.; Fernandez, J.; Vono, M.; Cintra-Francischinelli, M.; Lomonte, B.; Gutiérrez, J.M.; Di Virgilio, F.; Montecucco, C. Why myotoxin-containing snake venoms possess powerful nucleotidases? Biochem. Biophys. Res. Commun. 2013, 430, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Tonello, F.; Simonato, M.; Aita, A.; Pizzo, P.; Fernández, J.; Lomonte, B.; Gutiérrez, J.M.; Montecucco, C. A Lys49-PLA2 myotoxin of Bothrops asper triggers a rapid death of macrophages that involves autocrine purinergic receptor signaling. Cell Death Dis. 2012, 3, e343. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; Stiles, G.L.; Beaven, M.A.; Ali, H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol. Chem. 1993, 268, 16887–16890. [Google Scholar] [PubMed]
- Hannon, J.P.; Pfannkuche, H.J.; Fozard, J.R. A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br. J. Pharmacol. 1995, 115, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.J.; Jones, C.A.; Sheehan, M.J.; Vardey, C.J.; Whelan, C.J. Adenosine A3 receptors promote degranulation of rat mast cells both in vitro and in vivo. Inflamm. Res. 1997, 46, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shepherd, R.K.; Duling, B.R.; Linden, J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J. Clin. Investig. 1997, 100, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Pereira, J.; Mezzano, D.; Alarcón, M.; Caballero, J.; Palomo, I. Inhibition of platelet activation and thrombus formation by adenosine and inosine: Studies on their relative contribution and molecular modeling. PLoS ONE 2014, 9, e112741. [Google Scholar] [CrossRef] [PubMed]
- Kaster, M.P.; Budni, J.; Gazal, M.; Cunha, M.P.; Santos, A.R.S.; Rodrigues, A.L.S. The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A 2A receptors. Purinergic Signal. 2013, 9, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha Lapa, F.; de Oliveira, A.P.L.; Accetturi, B.G.; de Oliveira Martins, I.; Domingos, H.V.; de Almeida Cabrini, D.; de Lima, W.T.; Santos, A.R.S. Anti-inflammatory effects of inosine in allergic lung inflammation in mice: Evidence for the participation of adenosine A2A and A 3 receptors. Purinergic Signal. 2013, 9, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Welihinda, A.A.; Kaur, M.; Greene, K.; Zhai, Y.; Amento, E.P. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal. 2016, 28, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Yarom, M.; Tang, X.W.; Wu, E.; Carlson, R.G.; Vander Velde, D.; Lee, X.; Wu, J. Identification of inosine as an endogenous modulator for the benzodiazepine binding site of the GABAA receptors. J. Biomed. Sci. 1998, 5, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Teuber, L.; Wätjens, F.; Jensen, L.H. Ligands for the benzodiazepine binding site—A survey. Curr. Pharm. Des. 1999, 5, 317–343. [Google Scholar] [PubMed]
- Dunwiddie, T.V.; Worth, T. Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J. Pharmacol. Exp. Ther. 1982, 220, 70–76. [Google Scholar] [PubMed]
- Moulton, R.; Spector, W.G.; Willoughby, D.A. Histamine release and pain production by xanthosine and related compounds. Br. J. Pharmacol. Chemother. 1957, 12, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, E.; Mori, M.; Yamada, S.; Kumitomo, M. Effects of purine compounds on cholinergic nerves. Specificity of adenosine and related compounds on acetylcholine release in electircally stimulated guinea pig ileum. Eur. J. Pharmacol. 1978, 48, 297–307. [Google Scholar] [CrossRef]
- Imai, S.; Otorii, T.; Takeda, K.; Katano, Y.; Horii, D. Effects of ethyl adenosine-5′-carboxylate on the heart and coronary circulation. Jpn. Heart J. 1975, 16, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Hanski, C.; Kerr, S.J. 1-Methylguanine and 7-methylguanine increase cell agglutinability. Cell Tissue Res. 1985, 241, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.J. Alterations in cell surface properties induced by modified purines. Tumour Biol. 1985, 6, 123–131. [Google Scholar] [PubMed]
- Sun, Q.; Huang, S.; Wang, X.; Zhu, Y.; Chen, Z.; Chen, D. N6-methyladenine functions as a potential epigenetic mark in eukaryotes. Bioessays 2015, 37, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Gustafsson, L.E.; Hedqvist, P.; Sollevi, A. 30. Adenosine in the regulation of neurotransmitter release in the peripheral nervous system. In Regulatory Functions of Adenosine: Proceedings of the International Symposium on Adenosine, Charlottesville, Virginia, 7–11 June 1982; Berne, R.M., Rall, T.W., Rubio, R., Eds.; Martinus Nijoff Publishers: Boston, MA, USA, 1983; pp. 479–498. ISBN 9781461339113. [Google Scholar]
- Mbugua, P.M.; Thairu, K.; Ng’ang’a, J.N.; Telang, B.V. Identification and estimation of a cholinomimetic substance in the venom of Dendroaspis polylepis. Res. Commun. Chem. Pathol. Pharmacol. 1982, 36, 187–198. [Google Scholar] [PubMed]
- Harvey, A.L.; Karlsson, E. Dendrotoxin from the green mamba, Dendroaspis angusticeps: A new type of snake venom neurotoxin. Toxicon 1979, 17, 69. [Google Scholar]
- Aird, S.D.; Villar Briones, A.; Roy, M.C.; and Mikheyev, A.S. Polyamines as Snake Toxins and their Probable Pharmacological Functions in Envenomation. Toxins 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.K.; Pataky, I.; Hajdu, P. A contribution to the effects of creatinine on the central nervous system. II. Anticonvulsant action. Acta Physiol. Acad. Sci. Hung. 1952, 3, 153–164. [Google Scholar] [PubMed]
- Carta, A.; Calvani, M.; Bravi, D.; Ní Bhuachalla, S. Acetyl-l-Carnitine and Alzheimer’s Disease: Pharmacological Considerations beyond the Cholinergic Spherea. Ann. N. Y. Acad. Sci. 1993, 695, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, S.A. Mechanisms of Ischemic Neuroprotection by Acetyl-l-carnitine. Ann. N. Y. Acad. Sci. 2005, 1053, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Scafidi, S.; Racz, J.; Hazelton, J.; McKenna, M.C.; Fiskum, G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev. Neurosci. 2010, 32, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, H.; Zhang, Z.; Wang, T.; Niu, J.; Cui, D.; Xu, S. Neuroprotective effects of pre-treatment with l-carnitine and acetyl-l-carnitine on ischemic injury in vivo and in vitro. Int. J. Mol. Sci. 2012, 13, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; McKenna, M.C. l-Carnitine and Acetyl-l-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.-H.; Zhao, K. Propionyl-l-carnitine induces eNOS activation and nitric oxide synthesis in endothelial cells via PI3 and Akt kinases. Vascul. Pharmacol. 2013, 59, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Giovannini, L.; Galmozzi, G.; Bertelli, A.A. Protective role of propionyl carnitine in vascular disorders experimentally induced by endothelin (ET-1) serotonin and K-carrageenin. Drugs Exp. Clin. Res. 1993, 19, 7–11. [Google Scholar] [PubMed]
- Barak, A.J.; Tuma, D.J. Betaine, metabolic by-product or vital methylating agent? Life Sci. 1983, 32, 771–774. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. The stabilization of proteins by osmolytes. Biophys. J. 1985, 47, 411–414. [Google Scholar] [CrossRef]
- Yancey, P.H.; Burg, M.B. Distribution of major organic osmolytes in rabbit kidneys in diuresis and antidiuresis. Am. J. Physiol. 1989, 257, F602–F607. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Uchida, S.; Kwon, H.M.; Preston, A.S.; Robey, R.B.; Garcia-Perez, A.; Burg, M.B.; Handler, J.S. Cloning of a Na(+)- and Cl(−)-dependent betaine transporter that is regulated by hypertonicity. J. Biol. Chem. 1992, 267, 649–652. [Google Scholar] [PubMed]
- Lopez-Corcuera, B.; Liu, Q.R.; Mandiyan, S.; Nelson, H.; Nelson, N. Expression of a mouse brain cDNA encoding novel gamma-aminobutyric acid transporter. J. Biol. Chem. 1992, 267, 17491–17493. [Google Scholar] [PubMed]
- Schousboe, A.; Larsson, O.M.; Sarup, A.; White, H.S. Role of the betaine/GABA transporter (BGT-1/GAT2) for the control of epilepsy. Eur. J. Pharmacol. 2004, 500, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Borden, L.A.; Smith, K.E.; Gustafson, E.L.; Branchek, T.A.; Weinshank, R.L. Cloning and expression of a betaine/GABA transporter from human brain. J. Neurochem. 1995, 64, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Borden, L.A. GABA transporter heterogeneity: Pharmacology and cellular localization. Neurochem. Int. 1996, 29, 335–356. [Google Scholar] [CrossRef]
- Lever, M.; Sizeland, P.C.; Bason, L.M.; Hayman, C.M.; Chambers, S.T. Glycine betaine and proline betaine in human blood and urine. Biochim. Biophys. Acta 1994, 1200, 259–264. [Google Scholar] [CrossRef]
- Chambers, S.T.; Kunin, C.M. Isolation of glycine betaine and proline betaine from human urine. Assessment of their role as osmoprotective agents for bacteria and the kidney. J. Clin. Investig. 1987, 79, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Matskevitch, I.; Wagner, C.A.; Stegen, C.; Bröer, S.; Noll, B.; Risler, T.; Kwon, H.M.; Handler, J.S.; Waldegger, S.; Busch, A.E.; et al. Functional characterization of the Betaine/gamma-aminobutyric acid transporter BGT-1 expressed in Xenopus oocytes. J. Biol. Chem. 1999, 274, 16709–16716. [Google Scholar] [CrossRef] [PubMed]
- Takanaga, H.; Ohtsuki, S.; Hosoya, K.; Terasaki, T. GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood-brain barrier. J. Cereb. Blood Flow Metab. 2001, 21, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Barakat, L.; Wang, D.; Bordey, A. Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J. Physiol. 2002, 541, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Kakee, A.; Takanaga, H.; Terasaki, T.; Naito, M.; Tsuruo, T.; Sugiyama, Y. Efflux of a suppressive neurotransmitter, GABA, across the blood-brain barrier. J. Neurochem. 2001, 79, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.S.; Hahn, W.C.; Gupta, P.; Weinberg, R.A. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 2000, 12, 705–709. [Google Scholar]
- Balestrieri, M.L.; Balestrieri, A.; Mancini, F.P.; Napoli, C. Understanding the immunoangiostatic CXC chemokine network. Cardiovasc. Res. 2008, 78, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Longobardi, L.; Sirangelo, I.; Giovane, A.; Cautela, D.; Castaldo, D.; Giordano, A.; Balestrieri, M.L. Stachydrine ameliorates high-glucose induced endothelial cell senescence and SIRT1 downregulation. J. Cell. Biochem. 2013, 114, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Mercken, E.M.; Mitchell, S.J.; Martin-Montalvo, A.; Minor, R.K.; Almeida, M.; Gomes, A.P.; Scheibye-Knudsen, M.; Palacios, H.H.; Licata, J.J.; Zhang, Y.; et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 2014, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef] [PubMed]
- Pasantes-Morales, H.; Bona Venture, N.; Wioland, N.; Mandel, P. Effect of Intravitreal Injections of Taurine and GABA on Chicken Electroretinogram. Int. J. Neurosci. 1973, 5, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, N.; Wioland, N.; Mandel, P. Antagonists of the putative inhibitory transmitter effects of taurine and GABA in the retina. Brain Res. 1974, 80, 281–289. [Google Scholar] [CrossRef]
- Curtis, D.R.; Hösli, L.; Johnston, G.A.R.; Johnston, I.H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp. Brain Res. 1968, 5. [Google Scholar] [CrossRef]
- Curtis, D.R.; Hösli, L.; Johnston, G.A.R. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp. Brain Res. 1968, 6. [Google Scholar] [CrossRef]
- Okamoto, K.; Kimura, H.; Sakai, Y. Evidence for taurine as an inhibitory neurotransmitter in cerebellar stellate interneurons: Selective antagonism by TAG (6-aminomethyl-3-methyl-4H,1,2,4-benzothiadiazine-1,1-dioxide). Brain Res. 1983, 265, 163–168. [Google Scholar] [CrossRef]
- Lin, C.T.; Song, G.X.; Wu, J.Y. Is taurine a neurotransmitter in rabbit retina? Brain Res. 1985, 337, 293–298. [Google Scholar] [CrossRef]
- Kamisaki, K.; Wada, K.; Nakamoto, K.; Itoh, T. Release of taurine and its effects on release of neurotransmitter amino acids in rat cerebral cortex. In Taurine 2 Basic and Clinical Aspects; Advances in Experimental Medicine and Biology; Huxtable, R.J., Azuma, J., Kuriyama, K., Nakagawa, M., Baba, A., Eds.; Springer Science and Business Media, LLC: New York, NY, USA, 1996; Volume 403, pp. 445–454. [Google Scholar]
- Lombardini, J.B. Effects of taurine on calcium ion uptake and protein phosphorylation in rat retinal membrane preparations. J. Neurochem. 1985, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Foos, T.M.; Wu, J.Y. The Role of Taurine in the Central Nervous System and the Modulation of Intracellular Calcium Homeostasis. Neurochem. Res. 2002, 27, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jin, Y.; Wei, J.; Jin, H.; Sha, D.; Wu, J.-Y. Mode of action of taurine as a neuroprotector. Brain Res. 2005, 1038, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.; Wu, H.; Jin, Y.; Wei, J.; Buddhala, C.; Prentice, H.; Wu, J.-Y. Protective function of taurine in glutamate-induced apoptosis in cultured neurons. J. Neurosci. Res. 2009, 87, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Hussy, N.; Deleuze, C.; Pantaloni, A.; Desarménien, M.G.; Moos, F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: Possible role in osmoregulation. J. Physiol. 1997, 502 Pt 3, 609–621. [Google Scholar] [CrossRef]
- Dutertre, S.; Becker, C.-M.; Betz, H. Inhibitory glycine receptors: An update. J. Biol. Chem. 2012, 287, 40216–40223. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, J.P.; Park, S.J.; Chun, S.W.; Cho, D.H.; Han, S.K. Activation of synaptic and extrasynaptic glycine receptors by taurine in preoptic hypothalamic neurons. Neurosci. Lett. 2015, 608, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, A.E.; El Idrissi, A.; Okeke, E.; Yan, X.; Sidime, F.; Neuwirth, L.S. Taurine Regulation of Blood Pressure and Vasoactivity. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; pp. 407–425. [Google Scholar]
- Damerau, B.; Lege, L.; Oldigs, H.D.; Vogt, W. Histamine release, formation of prostaglandin-like activity (SRS-C) and mast cell degranulation by the direct lytic factor (DLF) and phospholipase A of cobra venom. Naunyn Schmiedebergs Arch. Pharmacol. 1975, 287, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Chiu, H.F.; Huang, H.T.; Teng, C.M. Edema formation and degranulation of mast cells by Trimeresurus mucrosquamatus snake venom. Toxicon 1984, 22, 17–28. [Google Scholar] [CrossRef]
- Antunes, E.; Rodrigues-Simioni, L.; Prado-Franceschi, J. Cross-neutralization on the histamine-releasing activity of snake venoms. Acta Physiol. Pharmacol. Latinoam. 1989, 39, 431–438. [Google Scholar] [PubMed]
- Niijima, A.; Okui, T.; Matsumura, Y.; Yamano, T.; Tsuruoka, N.; Kiso, Y.; Nagai, K. Effects of L-carnosine on renal sympathetic nerve activity and DOCA-salt hypertension in rats. Auton. Neurosci. 2002, 97, 99–102. [Google Scholar] [CrossRef]
- Nagai, K.; Tanida, M.; Niijima, A.; Tsuruoka, N.; Kiso, Y.; Horii, Y.; Shen, J.; Okumura, N. Role of l-carnosine in the control of blood glucose, blood pressure, thermogenesis, and lipolysis by autonomic nerves in rats: Involvement of the circadian clock and histamine. Amino Acids 2012, 43, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Niijima, A.; Fukuda, Y.; Sawai, H.; Tsuruoka, N.; Shen, J.; Yamada, S.; Kiso, Y.; Nagai, K. Dose-dependent effects of l-carnosine on the renal sympathetic nerve and blood pressure in urethane-anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R447–R455. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Kaneko, H.; Shen, J.; Nagai, K. Involvement of the histaminergic system in renal sympathetic and cardiovascular responses to leptin and ghrelin. Neurosci. Lett. 2007, 413, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, W.; Kellaway, C.H. Liberation of histamine and its role in the symptomatology of bee venom poisoning. Aust. J. Exp. Biol. Med. Sci. 1937, 15, 461–489. [Google Scholar] [CrossRef]
- Dutta, N.K.; Narayanan, K.G. Release of histamine from skeletal muscle by snake venoms. Br. J. Pharmacol. Chemother. 1954, 9, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Mancin, A.C.; Soares, A.M.; Giglio, C.A.; Andrião-Escarso, S.H.; Vieira, C.A.; Giglio, J.R. The histamine releasers crotamine, protamine and compound 48/80 activate specific proteases and phospholipases A2. Biochem. Mol. Biol. Int. 1997, 42, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-F.; Wei, X.-L.; Mo, Y.-Z.; He, S.-H. Induction of microvascular leakage and histamine release by promutoxin, an Arg49 phospholipase A2. Toxicon 2010, 55, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Chen, I.J.; Teng, C.M. Edema formation and degranulation of mast cells by a basic phospholipase A2 purified from Trimeresurus mucrosquamatus snake venom. Toxicon 1989, 27, 115–125. [Google Scholar] [CrossRef]
- Chai, O.H.; Kim, E.K.; Lee, Y.H.; Kim, J.G.; Baik, B.J.; Lee, M.S.; Han, E.H.; Kim, H.T.; Song, C.H. Histamine release induced by dendroaspis natriuretic peptide from rat mast cells. Peptides 2001, 22, 1421–1426. [Google Scholar] [CrossRef]
- Chacur, M.; Longo, I.; Picolo, G.; Gutiérrez, J.M.; Lomonte, B.; Guerra, J.L.; Teixeira, C.F.P.; Cury, Y. Hyperalgesia induced by Asp49 and Lys49 phospholipases A2 from Bothrops asper snake venom: Pharmacological mediation and molecular determinants. Toxicon 2003, 41, 667–678. [Google Scholar] [CrossRef]
- Tilmisany, A.K.; Mustafa, A.A.; Abdel Aziz, A.; Osman, O.H. Evidence for the presence of histamine in Gaboon viper (Bitis gabonica) venom. Toxicon 1986, 24, 1159–1161. [Google Scholar] [CrossRef]
- Mamede, C.C.N.; de Sousa, B.B.; da Pereira, D.F.C.; Matias, M.S.; de Queiroz, M.R.; de Morais, N.C.G.; Vieira, S.A.P.B.; Stanziola, L.; de Oliveira, F. Comparative analysis of local effects caused by Bothrops alternatus and Bothrops moojeni snake venoms: Enzymatic contributions and inflammatory modulations. Toxicon 2016, 117, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y. Elevation of histamine levels in rat and mouse tissues by the deacetylation of administered N-acetylhistamine. Eur. J. Pharmacol. 1979, 60, 299–305. [Google Scholar] [CrossRef]
- Evans, C.S.; Bell, E.A.; Johnson, E.S. N-methyltyramine, a biologically active amine in Acacia seeds. Phytochemistry 1979, 18, 2022–2023. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Where does N(ε)-trimethyllysine for the carnitine biosynthesis in mammals come from? PLoS ONE 2014, 9, e84589. [Google Scholar] [CrossRef] [PubMed]
- Birrell, G.W.; Earl, S.; Masci, P.P.; de Jersey, J.; Wallis, T.P.; Gorman, J.J.; Lavin, M.F. Molecular diversity in venom from the Australian Brown snake, Pseudonaja textilis. Mol. Cell. Proteom. 2006, 5, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Zelanis, A.; Serrano, S.M.T.; Reinhold, V.N. N-glycome profiling of Bothrops jararaca newborn and adult venoms. J. Proteom. 2012, 75, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Osipov, A.V.; Astapova, M.V.; Tsetlin, V.I.; Utkin, Y.N. The first representative of glycosylated three-fingered toxins. Cytotoxin from the Naja kaouthia cobra venom. Eur. J. Biochem. 2004, 271, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, J.-M.; Wang, Y.-M.; Wu, S.-W.; Tsai, I.-H.; Khoo, K.-H. Terminal disialylated multiantennary complex-type N-glycans carried on acutobin define the glycosylation characteristics of the DeinAgkistrodon acutus venom. Glycobiology 2011, 21, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Quinton, L.; Gilles, N.; Smargiasso, N.; Kiehne, A.; De Pauw, E. An unusual family of glycosylated peptides isolated from Dendroaspis angusticeps venom and characterized by combination of collision induced and electron transfer dissociation. J. Am. Soc. Mass Spectrom. 2011, 22, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Gowda, D.C.; Davidson, E.A. Structural features of carbohydrate moieties in snake venom glycoproteins. Biochem. Biophys. Res. Commun. 1992, 182, 294–301. [Google Scholar] [CrossRef]
- Lunow, D.; Kaiser, S.; Rückriemen, J.; Pohl, C.; Henle, T. Tryptophan-containing dipeptides are C-domain selective inhibitors of angiotensin converting enzyme. Food Chem. 2015, 166, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Bakhle, Y.S. Conversion of angiotensin I to angiotensin II by cell-free extracts of dog lung. Nature 1968, 220, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Bakhle, Y.S.; Reynard, A.M.; Vane, J.R. Metabolism of the angiotensins in isolated perfused tissues. Nature 1969, 222, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.J.; Camargo, A.C.; Krieger, E.M.; Stewart, J.M.; Ferreira, S.H. Inhibition of the conversion of angiotensin I to II and potentiation of bradykinin by small peptides present in Bothrops jararaca venom. Circ. Res. 1972, 31 (Suppl. 2), 62–71. [Google Scholar]
- Wang, Z.; Zhang, S.; Jin, H.; Wang, W.; Huo, J.; Zhou, L.; Wang, Y.; Feng, F.; Zhang, L. Angiotensin-I-converting enzyme inhibitory peptides: Chemical feature based pharmacophore generation. Eur. J. Med. Chem. 2011, 46, 3428–3433. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Gottfried, E.; Roelse, J.; Grommé, M.; Obst, R.; Hämmerling, G.J.; Momburg, F. Analysis of the fine specificity of rat, mouse and human TAP peptide transporters. Eur. J. Immunol. 1995, 25, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; De Luca, L.; Marconi, C.; Regazzoni, L.; Aldini, G.; Vistoli, G. Fragmental modeling of hPepT2 and analysis of its binding features by docking studies and pharmacophore mapping. Bioorg. Med. Chem. 2011, 19, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Keep, R.F.; Smith, D.E. Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab. Pharmacokinet. 2008, 23, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Lu, K. Substrates of the human oligopeptide transporter hPEPT2. Biosci. Trends 2015, 9, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Huo, J.-L.; Xu, W.; Xiong, J.; Li, Y.-M.; Wu, W.-T. A novel anti-platelet aggregation tripeptide from Agkistrodon acutus venom: Isolation and characterization. Toxicon 2009, 54, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Nonaka, A.; Matsushita, A.; Uchida, N.; Ohki, K.; Asakura, M.; Kitakaze, M. Milk casein-derived tripeptides, VPP and IPP induced NO production in cultured endothelial cells and endothelium-dependent relaxation of isolated aortic rings. Heart Vessels 2011, 26, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Menin, L.; Perchuć, A.; Favreau, P.; Perret, F.; Michalet, S.; Schöni, R.; Wilmer, M.; Stöcklin, R. High throughput screening of bradykinin-potentiating peptides in Bothrops moojeni snake venom using precursor ion mass spectrometry. Toxicon 2008, 51, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Iwanaga, S.; Suzuki, T. The isolation and amino acid sequences of new pyroglutamylpeptides from snake venoms. Experientia 1966, 22, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.; Kaiser, I.I. Inhibition of metalloproteinases in Bothrops asper venom by endogenous peptides. Toxicon 1993, 31, 889–899. [Google Scholar] [CrossRef]
- Lo, T.B. Chemical studies of Formosan snake venoms. J. Chin. Biochem. Soc. 1972, 1, 39–46. [Google Scholar]
- Okada, K.; Nagai, S.; Kato, H. A new pyroglutamylpeptide (Pyr-Lys-Ser) isolated from the venom of Agkistrodon halys blomhoffii. Experientia 1974, 30, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Hou, J.; Liang, X.; Chen, J.; Qiu, P.; Liu, Y.; Li, M.; Rao, Z.; Yan, G. Crystal structure of a non-hemorrhagic fibrin(ogen)olytic metalloproteinase complexed with a novel natural tri-peptide inhibitor from venom of Agkistrodon acutus. J. Struct. Biol. 2005, 152, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.; Pitts, M.; Tongyoo, P.; Rojnuckarin, P.; Wilkinson, M. Snake Venom Metalloproteinases and Their Peptide Inhibitors from Myanmar Russell’s Viper Venom. Toxins 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, S.C.; Favreau, P.; Cheneval, O.; Laing, G.D.; Wilkinson, M.C.; Miller, R.L.; Stöcklin, R.; Harrison, R.A. Molecular characterisation of endogenous snake venom metalloproteinase inhibitors. Biochem. Biophys. Res. Commun. 2008, 365, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Hung, C.C.; Wu, S.H.; Chiou, S.H. Characterization of three endogenous peptide inhibitors for multiple metalloproteinases with fibrinogenolytic activity from the venom of Taiwan habu (Trimeresurus mucrosquamatus). Biochem. Biophys. Res. Commun. 1998, 248, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Marques-Porto, R.; Lebrun, I.; Pimenta, D.C. Self-proteolysis regulation in the Bothrops jararaca venom: The metallopeptidases and their intrinsic peptidic inhibitor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Sciani, J.M.; Pimenta, D.C. The modular nature of bradykinin-potentiating peptides isolated from snake venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Munekiyo, S.M.; Mackessy, S.P. Presence of peptide inhibitors in rattlesnake venoms and their effects on endogenous metalloproteases. Toxicon 2005, 45, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Robeva, A.; Politi, V.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Synthetic and endogenous inhibitors of snake venom metalloproteinases. Biomed. Biochim. Acta 1991, 50, 769–773. [Google Scholar] [PubMed]
- Bai, L.; Fang, W.-R.; Kong, Y.; Li, Y.-M. Inhibitory effects and mechanisms of snake venom tripeptide pENW on platelet adhesion. Yao Xue Xue Bao 2015, 50, 1107–1115. [Google Scholar] [PubMed]
- Orosz, G.; Rónai, A.Z.; Bajusz, S.; Medzihradszky, K. N-terminally protected penta- and tetrapeptide opioid antagonists based on a pentapeptide sequence found in the venom of Philippine cobra. Biochem. Biophys. Res. Commun. 1994, 202, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Murayama, N.; Saguchi, K.-I.; Ohi, H.; Fujita, Y.; da Silva, N.J., Jr.; de Siqueira, R.J.B.; Lahlou, S.; Aird, S.D. A novel peptide from the ACEI/BPP-CNP precursor in the venom of Crotalus durissus collilineatus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Picolo, G.; Gutierrez, V.P.; Brigatte, P.; Zambelli, V.O.; Camargo, A.C.M.; Cury, Y. Crotalphine, a novel potent analgesic peptide from the venom of the South American rattlesnake Crotalus durissus terrificus. Peptides 2008, 29, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, V.P.; Konno, K.; Chacur, M.; Sampaio, S.C.; Picolo, G.; Brigatte, P.; Zambelli, V.O.; Cury, Y. Crotalphine induces potent antinociception in neuropathic pain by acting at peripheral opioid receptors. Eur. J. Pharmacol. 2008, 594, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.L.; Konno, K.; Conceição, I.M.; Ianzer, D.; Yamanouye, N.; Prezoto, B.C.; Assakura, M.T.; Rádis-Baptista, G.; Yamane, T.; Santos, R.A.; et al. Identification of novel bradykinin-potentiating peptides (BPPs) in the venom gland of a rattlesnake allowed the evaluation of the structure—Function relationship of BPPs. Biochem. Pharmacol. 2007, 74, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Ianzer, D.; Santos, R.A.S.; Etelvino, G.M.; Xavier, C.H.; de Almeida Santos, J.; Mendes, E.P.; Machado, L.T.; Prezoto, B.C.; Dive, V.; de Camargo, A.C.M. Do the cardiovascular effects of angiotensin-converting enzyme (ACE) I involve ACE-independent mechanisms? New insights from proline-rich peptides of Bothrops jararaca. J. Pharmacol. Exp. Ther. 2007, 322, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.A.; Portaro, F.C.V.; Fernandes, B.L.; Ianzer, D.A.; Guerreiro, J.R.; Gomes, C.L.; Konno, K.; Serrano, S.M.T.; Nascimento, N.; Camargo, A.C.M. Tissue distribution in mice of BPP 10c, a potent proline-rich anti-hypertensive peptide of Bothrops jararaca. Toxicon 2008, 51, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.; Hayashi, M.A.F.; Cuniasse, P.; Vazeux, G.; Ianzer, D.; De Camargo, A.C.M.; Dive, V. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry 2002, 41, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Morais, K.L.P.; Ianzer, D.; Miranda, J.R.R.; Melo, R.L.; Guerreiro, J.R.; Santos, R.A.S.; Ulrich, H.; Lameu, C. Proline rich-oligopeptides: Diverse mechanisms for antihypertensive action. Peptides 2013, 48, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Gothe, R.; Siems, W.-D.; Vietinghoff, G.; Paegelow, I.; Reissmann, S. Potentiation of bradykinin actions by analogues of the bradykinin potentiating nonapeptide BPP9alpha. Peptides 2005, 26, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Ianzer, D.; Xavier, C.H.; Fraga, F.C.; Lautner, R.Q.; Guerreiro, J.R.; Machado, L.T.; Mendes, E.P.; de Camargo, A.C.M.; Santos, R.A.S. BPP-5a produces a potent and long-lasting NO-dependent antihypertensive effect. Ther. Adv. Cardiovasc. Dis. 2011, 5, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Lameu, C.; Hayashi, M.A.F.; Guerreiro, J.R.; Oliveira, E.F.; Lebrun, I.; Pontieri, V.; Morais, K.L.P.; Camargo, A.C.M.; Ulrich, H. The central nervous system as target for antihypertensive actions of a proline-rich peptide from Bothrops jararaca venom. Cytometry A 2010, 77, 220–230. [Google Scholar] [PubMed]
- Yanoshita, R.; Kasuga, A.; Inoue, S.; Ikeda, K.; Samejima, Y. Blomhotin: A novel peptide with smooth muscle contractile activity identified in the venom of Agkistrodon halys blomhoffii. Toxicon 1999, 37, 1761–1770. [Google Scholar] [CrossRef]
- Higuchi, S.; Murayama, N.; Saguchi, K.; Ohi, H.; Fujita, Y.; Camargo, A.C.; Ogawa, T.; Deshimaru, M.; Ohno, M. Bradykinin-potentiating peptides and C-type natriuretic peptides from snake venom. Immunopharmacology 1999, 44, 129–135. [Google Scholar] [CrossRef]
- Higuchi, S.; Murayama, N.; Saguchi, K.; Ohi, H.; Fujita, Y.; Silva, N.J., Jr.; Aird, S.D. A novel peptide from the ACEI/BPP-CNP precursor in the venom of Crotalus durissus collilineatus. In Proceedings of the 14th World Congress of the International Society on Toxinology; International Society on Toxinology: Adelaide, Australia, 2003; p. 58. [Google Scholar]
- Wang, S.-S.; Zeng, Y.-L.; Zhang, W.-W.; Dong, S.-L. A snake venom peptide with the contrary effects on rat stomach fundus and guinea pig ileum. Pharmazie 2010, 65, 384–386. [Google Scholar] [PubMed]
- Soares, M.R.; Oliveira-Carvalho, A.L.; Wermelinger, L.S.; Zingali, R.B.; Ho, P.L.; Junqueira-de-Azevedo, I.L.M.; Diniz, M.R.V. Identification of novel bradykinin-potentiating peptides and C-type natriuretic peptide from Lachesis muta venom. Toxicon 2005, 46, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pahari, S.; Mackessy, S.P.; Kini, R.M. The venom gland transcriptome of the Desert Massasauga rattlesnake (Sistrurus catenatus edwardsii): Towards an understanding of venom composition among advanced snakes (Superfamily Colubroidea). BMC Mol. Biol. 2007, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- James Graham, R.L.; Graham, R.L.J.; Graham, C.; McClean, S.; Chen, T.; O’Rourke, M.; Hirst, D.; Theakston, D.; Shaw, C. Identification and functional analysis of a novel bradykinin inhibitory peptide in the venoms of New World Crotalinae pit vipers. Biochem. Biophys. Res. Commun. 2005, 338, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H. A bradykinin-potentiating factor (BPF) present in the venom of Bothrops jararaca. Br. J. Pharmacol. 1965, 24, 163–169. [Google Scholar] [CrossRef]
- Kato, H.; Suzuki, T. Bradykinin-potentiating peptides from the venom of Agkistrodon halys blomhoffi. Isolation of five bradykinin potentiators and the amino acid sequences of two of them, potentiators B and C. Biochemistry 1971, 10, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.A. Hypotensin: A hypotensive peptide isolated from the venom of Crotalus atrox; purification, amino acid composition and terminal amino acid residues. FEBS Lett. 1976, 68, 297–302. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Henriques, O.B.; Lebrun, I.; Batista, M.B.; Prezoto, B.C.; Andreoni, A.S.; Zelnik, R.; Habermehl, G. Biologically active peptides from Bothrops jararacussu venom. Agents Actions Suppl. 1992, 36, 209–214. [Google Scholar] [PubMed]
- Jorge, M.T.; Sano-Martins, I.S.; Tomy, S.C.; Castro, S.C.; Ferrari, R.A.; Ribeiro, L.A.; Warrell, D.A. Snakebite by the bushmaster (Lachesis muta) in Brazil: Case report and review of the literature. Toxicon 1997, 35, 545–554. [Google Scholar] [CrossRef]
- Aird, S.D.; Arora, J.; Barua, A.; Qiu, L.; Terada, K.; Mikheyev, A.S. Population Genomic Analysis of a Pitviper Reveals Microevolutionary Forces Underlying Venom Chemistry. Genome Biol. Evol. 2017, 9, 2640–2649. [Google Scholar] [CrossRef] [PubMed]
- Wermelinger, L.S.; Dutra, D.L.S.; Oliveira-Carvalho, A.L.; Soares, M.R.; Bloch, C.; Zingali, R.B. Fast analysis of low molecular mass compounds present in snake venom: Identification of ten new pyroglutamate-containing peptides. Rapid Commun. Mass Spectrom. 2005, 19, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Tashima, A.K.; Zelanis, A.; Kitano, E.S.; Ianzer, D.; Melo, R.L.; Rioli, V.; Sant’anna, S.S.; Schenberg, A.C.G.; Camargo, A.C.M.; Serrano, S.M.T. Peptidomics of three Bothrops snake venoms: Insights into the molecular diversification of proteomes and peptidomes. Mol. Cell. Proteom. 2012, 11, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; Kaiser, I.I. Comparative studies on three rattlesnake toxins. Toxicon 1985, 23, 361–374. [Google Scholar] [CrossRef]
- Pluskal, T.; Yanagida, M. Measurement of Metabolome Samples Using Liquid Chromatography-Mass Spectrometry, Data Acquisition, and Processing. Cold Spring Harb. Protocols 2016, 2016, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]














| Acidic Amino Acid | Excitation | α-Decarboxylation Product | Inhibition |
|---|---|---|---|
| Aspartic Acid | +++ | β-Alanine | − − − |
| Glutamic Acid | +++ | GABA | − − − |
| Cysteic Acid | +++ | Taurine | − − − |
| β-Hydroxyglutamic Acid | ++ | γ-Amino-β-hydroxy-n-butyric Acid | − − |
| N-Methylaspartic Acid | ++ | N-Methyl-β-Alanine | − − |
| Aminomalonic Acid | + | Glycine | − − |
| α-Aminoadipic Acid | + | δ-Aminoadipic Acid | − − |
| α-Aminopimelic Acid | + | ε-Aminocaproic Acid | − |
| N,N-Dimethylaspartic Acid | + | N,N-Dimethyl-β-Alanine | 0 |
| N-Methylglutamic Acid | 0 | N-Methyl-γ-Amino-n-butyric Acid | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villar-Briones, A.; Aird, S.D. Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined. Toxins 2018, 10, 392. https://doi.org/10.3390/toxins10100392
Villar-Briones A, Aird SD. Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined. Toxins. 2018; 10(10):392. https://doi.org/10.3390/toxins10100392
Chicago/Turabian StyleVillar-Briones, Alejandro, and Steven D. Aird. 2018. "Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined" Toxins 10, no. 10: 392. https://doi.org/10.3390/toxins10100392
APA StyleVillar-Briones, A., & Aird, S. D. (2018). Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined. Toxins, 10(10), 392. https://doi.org/10.3390/toxins10100392