Cyanotoxins and Cyanobacteria Cell Accumulations in Drinking Water Treatment Plants with a Low Risk of Bloom Formation at the Source
Abstract
:1. Introduction
2. Results and Discussion
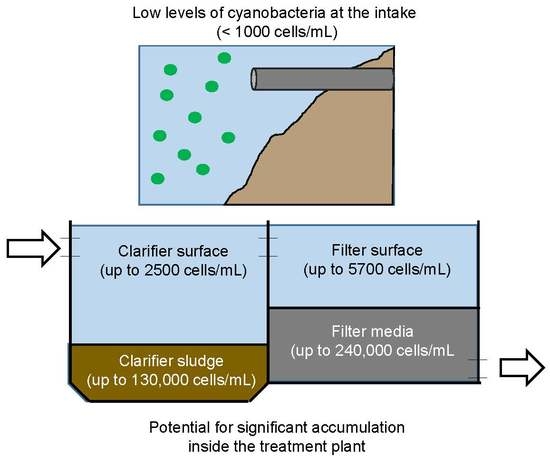
2.1. Cyanobacteria Accumulation across Treatment Trains
2.2. Risk Assessment for the Worst-Case Scenario Toxin Release Event
2.3. Use of A Fluorescence Probe for Improved Cyanobacteria Monitoring
3. Conclusions
4. Materials and Methods
4.1. Cell Accumulation Sites
4.2. Sampling Procedure
4.3. YSI EXO2 Monitoring Probe
4.4. ELISA Analysis
4.5. LC-MS/MS Analysis
4.6. Cell Microscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloglu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, J.G.; Desellas, A.M.; Fletcher, R.; Heintsch, L.; Morley, A.; Nakamoto, L.; Utsumi, K. Algal blooms in Ontario, Canada: Increases in reports since 1994. Lake Reserv. Manag. 2011, 27, 105–112. [Google Scholar] [CrossRef]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Oehrle, S.A.; Southwell, B.; Westrick, J. Detection of various freshwater cyanobacterial toxins using ultra-performance liquid chromatography tandem mass spectrometry. Toxicon 2010, 55, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kommineni, S.; Amante, K.; Karnik, B.; Sommerfeld, M.; Dempster, T. Strategies for Controlling and Mitigating Algal Growth within Water Treatment Plants; Water Research Foundation: Denver, CO, USA, 2009; ISBN 9781605730585. [Google Scholar]
- McQuaid, N.; Zamyadi, A.; Prévost, M.; Bird, D.F.; Dorner, S. Use of in vivophycocyanin fluorescence to monitor potential microcystin-producing cyanobacterial biovolume in a drinkingwater source. J. Environ. Monit. 2011, 13, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Feng, M.; Xu, X.; Liu, F.; Ke, F.; Li, W. Co-occurrence of microcystins and taste-and-odor compounds in drinking water source and their removal in a full-scale drinking water treatment plant. Toxins 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Dorner, S.; Sauvé, S.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Species-dependence of cyanobacteria removal efficiency by different drinking water treatment processes. Water Res. 2013, 47, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Dorner, S.; Ndong, M.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Low-risk cyanobacterial bloom sources: Cell accumulation within full-scale treatment plants. J. Am. Water Works Assoc. 2013, 105, 65–66. [Google Scholar] [CrossRef]
- Zamyadi, A.; MacLeod, S.L.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Fortin, N.; Aranda-Rodriguez, R.; Jing, H.; Pick, F.; Bird, D.; Greer, C.W. Detection of microcystin-producing cyanobacteria in missisquoi bay, Quebec, Canada, using quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 5105–5112. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mishra, D.R. A novel remote sensing algorithm to quantify phycocyanin in cyanobacterial algal blooms. Environ. Res. Lett. 2014, 9. [Google Scholar] [CrossRef]
- Gregor, J.; Maršálek, B.; Šípková, H. Detection and estimation of potentially toxic cyanobacteria in raw water at the drinking water treatment plant by in vivo fluorescence method. Water Res. 2007, 41, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hodges, C. A Validation Study of Phycocyanin Sensors for Monitoring Cyanobacteria in Cultures and Field Samples. Ph.D. Thesis, The University of Waikato, Hamilton, New Zealand, 2016. [Google Scholar]
- Izydorczyk, K.; Tarczynska, M.; Jurczak, T.; Mrowczynski, J.; Zalewski, M. Measurement of phycocyanin fluorescence as an online early warning system for cyanobacteria in reservoir intake water. Environ. Toxicol. 2005, 20, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Lou, I.; Zhang, Y.; Lou, C.U.; Mok, K.M. Using an online phycocyanin fluorescence probe for rapid monitoring of cyanobacteria in Macau freshwater reservoir. Hydrobiologia 2014, 741, 33–49. [Google Scholar] [CrossRef]
- Pazouki, P. Cyanobacteria in Surface and Bank Filtered Drinking Water Sources: Application of Phycocyanin Probes for Monitoring Blooms. Ph.D. Thesis, École Polytechnique De Montréal, Montréal, QC, Canada, 2016. [Google Scholar]
- Zamyadi, A.; Dorner, S.; Ndong, M.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Application of in vivo measurements for the management of cyanobacteria breakthrough into drinking water treatment plants. Environ. Sci. Process. Impacts 2014, 16, 313. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Ho, L.; Newcombe, G.; Bustamante, H.; Prévost, M. Fate of toxic cyanobacterial cells and disinfection by-products formation after chlorination. Water Res. 2012, 46, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; McQuaid, N.; Prévost, M.; Dorner, S. Monitoring of potentially toxic cyanobacteria using an online multi-probe in drinking water sources. J. Environ. Monit. 2012, 14, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bowling, L.C.; Zamyadi, A.; Henderson, R.K. Assessment of in situ fluorometry to measure cyanobacterial presence in water bodies with diverse cyanobacterial populations. Water Res. 2016, 105, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Choo, F.; Newcombe, G.; Stuetz, R.; Henderson, R.K. A review of monitoring technologies for real-time management of cyanobacteria: Recent advances and future direction. TrAC Trends Anal. Chem. 2016, 85, 83–96. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence probes for real-time remote cyanobacteria monitoring: A review of challenges and opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Choo, F.; Zamyadi, A.; Newton, K.; Newcombe, G.; Bowling, L.; Stuetz, R.; Henderson, R.K. Performance evaluation of in situ fluorometers for real-time cyanobacterial monitoring. H2 Open J. 2018, 1–21. [Google Scholar] [CrossRef]
- Genzoli, L.; Kann, J. Evaluation of Phycocyanin Probes as a Monitoring Tool for Toxigenic Cyanobacteria in the Klamath River Below Iron Gate Dam. Prepared by Aquatic Ecosystems LLC for the Klamath Tribal Water Consortium. 2016. Available online: https://www.researchgate.net/profile/Jacob_Kann/publication/309765126_Evaluation_of_phycocyanin_probes_as_a_monitoring_tool_for_toxigenic_cyanobacteria_in_the_Klamath_River_below_Iron_Gate_Dam/links/5822537c08ae7ea5be6af4f3.pdf (accessed on 26 October 2018).
- Chu, Z.; Jin, X.; Yang, B.; Zeng, Q. Buoyancy regulation of Microcystis flos-aquae during phosphorus-limited and nitrogen-limited growth. J. Plankton Res. 2007, 29, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Zhu, T.; Xu, M.; Wang, S.; Xu, X.; Kong, R. pH-dependent gas vesicle formation in Microcystis. FEBS Lett. 2016, 590, 3195–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlag, D.C.; Sinclair, J.L.; Southwell, B.; Westrick, J.A. Cyanobacteria and cyanotoxins occurrence and removal from five high-risk conventional treatment drinking water plants. Toxins 2015, 7, 2198–2220. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Krausfeldt, L.E.; Shao, K.; Lecleir, G.R.; Stough, J.M.A.; Gao, G.; Boyer, G.L.; Zhang, Y.; Paerl, H.W.; Qin, B.; et al. Seasonal Gene Expression and the Ecophysiological Implications of Toxic Microcystis aeruginosa Blooms in Lake Taihu. Environ. Sci. Technol. 2018, 52, 11049–11059. [Google Scholar] [CrossRef] [PubMed]
- Salvador, D.; Churro, C.; Valério, E. Evaluating the in fl uence of light intensity in mcy A gene expression and microcystin production in toxic strains of Planktothrix agardhii and Microcystis aeruginosa. J. Microbiol. Methods 2016, 123, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, F.F.; Madramootoo, C.A.; Jabaji, S. Comparison of cyanobacterial microcystin synthetase (mcy) E gene transcript levels, mcy E gene copies, and biomass as indicators of microcystin risk under laboratory and field conditions. Microbiologyopen 2014, 3, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Vlad, S.; Anderson, W.B.; Peldszus, S.; Huck, P.M. Removal of the cyanotoxin anatoxin-a by drinking water treatment processes: A review. J. Water Health 2014, 12, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masseret, E.; Banack, S.; Boumédiène, F.; Abadie, E.; Brient, L.; Pernet, F.; Juntas-Morales, R.; Pageot, N.; Metcalf, J.; Cox, P.; et al. Dietary BMAA exposure in an amyotrophic lateral sclerosis cluster from southern France. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Clausi, M.T.; Vita, V.; Bruno, M.; Franchino, C.; Trifirò, G.; Palumbo, M.P.; Floridi, F.; De Pace, R. Validation of ELISA methods for search and quantification of β-n-methylamino-l-alanine in water and fish tissue. Int. J. Environ. Anal. Chem. 2016, 96, 1290–1299. [Google Scholar] [CrossRef]
- Faassen, E.J.; Beekman, W.; Lürling, M. Evaluation of a Commercial Enzyme Linked Immunosorbent Assay (ELISA) for the Determination of the Neurotoxin BMAA in Surface Waters. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bláhová, L.; Kohoutek, J.; Kadlecová, E.; Kozáková, L.; Bláha, L. Assessment of non-derivatized β-N-methylamino-L-alanine (BMAA) neurotoxin in free form in urine of patients with nonspecific neurological symptoms. Toxicon 2017, 133, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Bornmann, K.; Schmidt, W. Relevance of intra- and extracellular cyanotoxins for drinking water treatment. Acta Hydrochim. Hydrobiol. 2002, 30, 7–15. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Castro, B.B.; Nunes, M.I.S.; Antunes, S.C.; Pizarro, C.; Coelho, C.; Gonçalves, F.; de Figueiredo, D.R. New insights towards the establishment of phycocyanin concentration thresholds considering species-specific variability of bloom-forming cyanobacteria. Hydrobiologia 2015, 757, 155–165. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R.; Lee, Z.; Tucker, C.S. Quantifying cyanobacterial phycocyanin concentration in turbid productive waters: A quasi-analytical approach. Remote Sens. Environ. 2013, 133, 141–151. [Google Scholar] [CrossRef]
- MyAssays Four Parameter Logistic Curve. Available online: https://www.myassays.com/assay.aspx?id=787 (accessed on 17 October 2018).
- Gaget, V.; Lau, M.; Sendall, B.; Froscio, S.; Humpage, A.R. Cyanotoxins: Which detection technique for an optimum risk assessment? Water Res. 2017, 118, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Lee, A.K.; Yates, R.S.; Liang, S.; Rochelle, P.A. Analysis of microcystins in drinking water by ELISA and LC/MS/MS. J. Am. Water Works Assoc. 2017, 109, 13–25. [Google Scholar] [CrossRef]
- Qian, S.S.; Chaffin, J.D.; Dufour, M.R.; Sherman, J.J.; Golnick, P.C.; Collier, C.D.; Nummer, S.A.; Margida, M.G. Quantifying and Reducing Uncertainty in Estimated Microcystin Concentrations from the ELISA Method. Environ. Sci. Technol. 2015, 49, 14221–14229. [Google Scholar] [CrossRef] [PubMed]
- McElhiney, J.; Lawton, L.A. Detection of the cyanobacterial hepatotoxins microcystins. Toxicol. Appl. Pharmacol. 2005, 203, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Meriluoto, J.A.; Spoof, L.E. Cyanotoxins: Sampling, sample processing, and toxin uptake. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: Berlin, Germany, 2008; Volume 619, ISBN 9780387758640. [Google Scholar]
- Meissner, S.; Fastner, J.; Dittmann, E. Microcystin production revisited: Conjugate formation makes a major contribution. Environ. Microbiol. 2013, 15, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The Fate of Microcystins in the Environment and Challenges for Monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Stanford, B.D.; Adams, C.; Rosenfeldt, E.J.; Wert, E.C. Varied influence of microcystin structural difference on ELISA cross-reactivity and chlorination efficiency of congener mixtures. Water Res. 2017, 126, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in Northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Sidelev, S.I.; Golokolenova, T.B.; Chernova, E.N.; Russkikh, Y.V. Analysis of phytoplankton in Tsimlyansk Reservoir (Russia) for the presence of cyanobacterial hepato- and neurotoxins. Microbiology 2015, 84, 828–837. [Google Scholar] [CrossRef]
- Vuorio, K.; Lepistö, L.; Holopainen, A.L. Intercalibrations of freshwater phytoplankton analyses. Boreal Environ. Res. 2007, 12, 561–569. [Google Scholar]







| Plant A | Plant B | Plant C | Plant D | |
|---|---|---|---|---|
| Treatment Train | Prechlorination | Prechlorination | ||
| Coagulation/flocculation | Coagulation/flocculation | Coagulation/flocculation | Coagulation/flocculation | |
| Upflow clarification | Sedimentation | Sedimentation | Sedimentation | |
| Anthracite/sand filtration | Anthracite/sand filtration | GAC/sand filtration | Anthracite/sand filtration | |
| Chlorination | Chlorination | Chlorination | Chlorination |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuhtaram, H.; Cui, Y.; Zamyadi, A.; Hofmann, R. Cyanotoxins and Cyanobacteria Cell Accumulations in Drinking Water Treatment Plants with a Low Risk of Bloom Formation at the Source. Toxins 2018, 10, 430. https://doi.org/10.3390/toxins10110430
Almuhtaram H, Cui Y, Zamyadi A, Hofmann R. Cyanotoxins and Cyanobacteria Cell Accumulations in Drinking Water Treatment Plants with a Low Risk of Bloom Formation at the Source. Toxins. 2018; 10(11):430. https://doi.org/10.3390/toxins10110430
Chicago/Turabian StyleAlmuhtaram, Husein, Yijing Cui, Arash Zamyadi, and Ron Hofmann. 2018. "Cyanotoxins and Cyanobacteria Cell Accumulations in Drinking Water Treatment Plants with a Low Risk of Bloom Formation at the Source" Toxins 10, no. 11: 430. https://doi.org/10.3390/toxins10110430
APA StyleAlmuhtaram, H., Cui, Y., Zamyadi, A., & Hofmann, R. (2018). Cyanotoxins and Cyanobacteria Cell Accumulations in Drinking Water Treatment Plants with a Low Risk of Bloom Formation at the Source. Toxins, 10(11), 430. https://doi.org/10.3390/toxins10110430