Resistance-Related l-Pyroglutamic Acid Affects the Biosynthesis of Trichothecenes and Phenylpropanoids by F. graminearum Sensu Stricto
Abstract
:1. Introduction
2. Results and Discussion
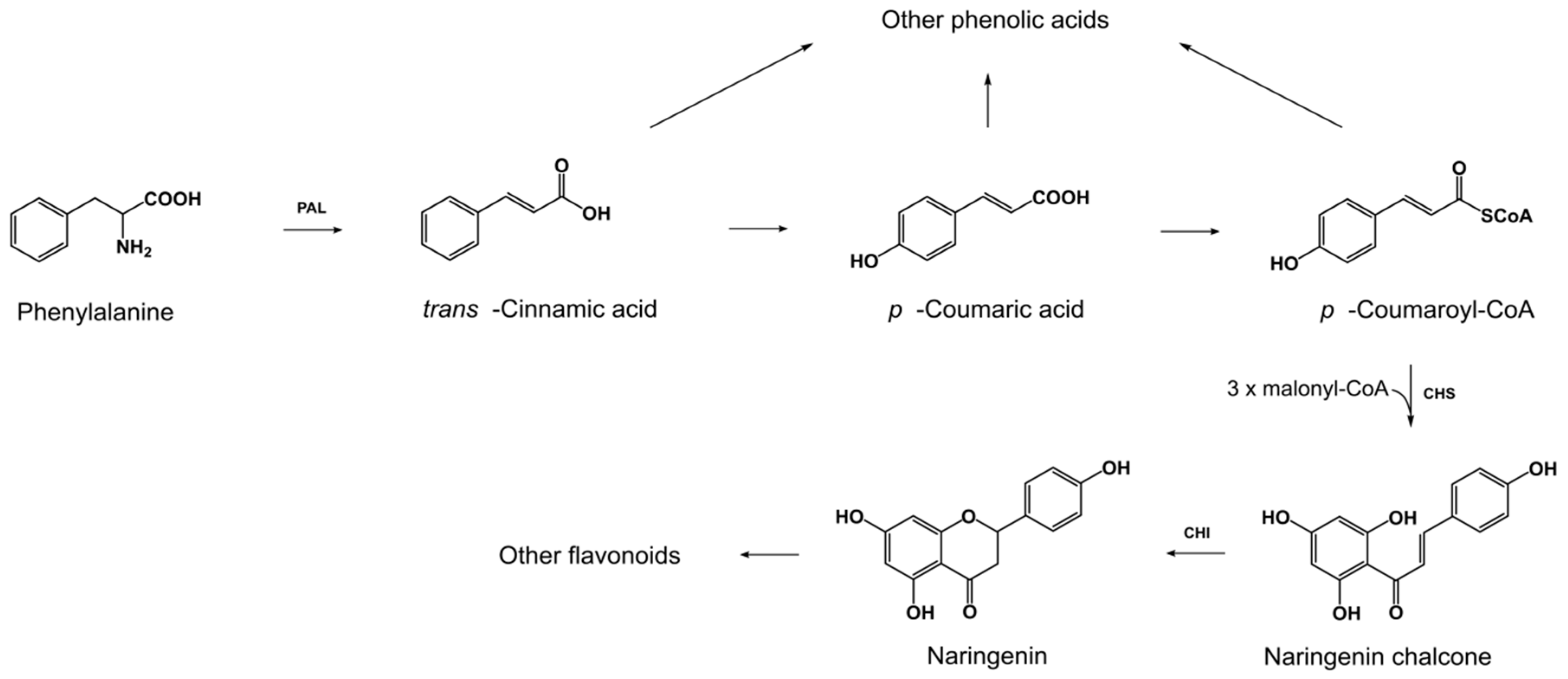
2.1. Exogenous l-Pyroglutamic Acid Affects the Production of Phenolic Acids and Flavonoids by Fungal Strains
2.2. Effect of l-Pyroglutamic Acid on the Accumulation of Trichothecenes in Culture Media
2.3. Effect of l-Pyroglutamic Acid on the Expression of Tri Genes
3. Conclusions
4. Materials and Methods
4.1. Fungal Strains
4.2. Medium and Culture Conditions
4.3. Determination of Contents of Phenolic Acids and Flavonoids in the Culture Medium
4.4. Determination of the Antioxidant Capacity (VCEAC/L) and Radical Scavenging Activity (ABTS+) of l-Pyroglutamic Acid
4.5. Analysis of Trichothecene Concentrations from Fungal Cultures
4.6. Extraction of Total RNA and Preparation of cDNA
4.7. Gene Expression Analysis
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nganje, W.E.; Kaitibie, S.; Wilson, W.W.; Leistritz, F.L.; Bangsund, D.A. Economic impacts of Fusarium Head Blight in wheat and barley: 1993–2001. Agribus. Appl. Econ. Rep. 2004, 538, 1–53. [Google Scholar]
- Ponts, N.; Pinson-Gadais, L.; Boutigny, A.L.; Barreau, C.; Richard-Forget, F. Cinnamic-derived acids significantly affect Fusarium graminearum growth and in vitro synthesis of type B trichothecenes. Phytopathology 2011, 101, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Bonnin-Verdal, M.N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food. Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T. Fungicides application against Fusarium Head Blight in wheat and barley for ensuring food safety. In Fungicides; Carisse, O., Ed.; InTech: Rijeka, Croatia, 2010; pp. 139–156. [Google Scholar]
- Wegulo, S.N. Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Hazel, C.M.; Patel, S. Influence of processing on trichothecene levels. Toxicol. Lett. 2004, 153, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.A.; McMullen, M.P.; Hershman, D.E.; Madden, L.V. Meta-analysis of the effects of triazole-based fungicides on wheat yield and test weight as influenced by Fusarium head blight intensity. Phytopathology 2010, 100, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar] [CrossRef]
- Klix, M.B.; Verreet, J.-A.; Beyer, M. Comparison of the declining triazole sensitivity of Gibberella zeae and increased sensitivity achieved by advances in triazole fungicide development. Crop Prot. 2007, 269, 683–690. [Google Scholar] [CrossRef]
- Chen, C.-J.; Yu, J.-J.; Bi, C.-W.; Zhang, Y.-N.; Xu, J.-Q.; Wang, J.-X.; Zhou, M.-G. Mutations in a β -tubulin confer resistance of Gibberella zeae to benzimidazole fungicides. Phytopathology 2009, 99, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, X.; Li, B.; Ma, Z. Characterization of sterol demethylation inhibitor-resistant isolates of Fusarium asiaticum and F. graminearum collected from wheat in China. Phytopathology 2009, 99, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Becher, R.; Hettwer, U.; Karlovsky, P.; Deising, H.B.; Wirsel, S.G.R. Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology 2010, 100, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Y.; Zhu, Y.-F.; Liu, Y.-Y.; Deng, Y.-Y.; Li, W.; Zhang, A.-X.; Chen, H.-G. Evaluation of tebuconazole for the management of Fusarium head blight in China. Australasian Plant Pathol. 2014, 43, 631–638. [Google Scholar] [CrossRef]
- Chen, Z.F.; Ying, G.G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal Biofilms: Targets for the development of novel strategies in plant disease management. Front. Microbiol. 2017, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Hamzehzarghani, H.; Paranidharan, V.; Abu-Nada, Y.; Kushalappa, A.C.; Mamer, O.; Somers, D. Metabolic profiling to discriminate wheat near isogenic lines, with quantitative trait loci at chromosome 2DL, varying in resistance to Fusarium Head Blight. Can. J. Plant Sci. 2008, 88, 789–797. [Google Scholar] [CrossRef]
- Paranidharan, V.; Abu-Nada, Y.; Hamzehzarghani, H.; Kushalappa, A.C.; Mamer, O.; Dion, Y.; Rioux, S.; Comeau, A.; Choiniere, L. Resistance-related metabolites in wheat against Fusarium graminearum and the virulence factor deoxynivalenol (DON). Botany 2008, 86, 1168–1179. [Google Scholar] [CrossRef]
- Bollina, V.; Kumaraswamy, G.K.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Faubert, D.; Hamzehzarghani, H. Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against Fusarium head blight. Mol. Plant Pathol. 2010, 11, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, G.K.; Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Mamer, O.; Faubert, D. Metabolomics technology to phenotype resistance in barley against Gibberella zeae. Eur. J. Plant Pathol. 2011, 130, 29–43. [Google Scholar] [CrossRef]
- Kumaraswamy, K.G.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S. Mass spectrometry based metabolomics to identify potential biomarkers for resistance in barley against Fusarium Head Blight (Fusarium graminearum). J. Chem. Ecol. 2011, 37, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Gunnaiah, R.; Kushalappa, A.C. Metabolomics deciphers the host resistance mechanisms in wheat cultivar Sumai-3, against trichothecene producing and non-producing isolates of Fusarium graminearum. Plant. Physiol. Biochem. 2014, 83, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.L.; Atanasova-Pénichon, V.; Benet, M.; Barreau, C.; Richard-Forget, F. Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. Eur. J. Plant Pathol. 2010, 127, 275–286. [Google Scholar] [CrossRef]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahanshiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessi, A.; et al. Natural and natural-like phenolic inhibitors of type b trichothecene in vitro production by the wheat (Triticum sp.) pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Buśko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Sinapic acid affects phenolic and trichothecene profiles of F. culmorum and F. graminearum sensu stricto. Toxins 2017, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Buśko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. trans-Cinnamic and chlorogenic acids affect the secondary metabolic profiles and ergosterol biosynthesis by Fusarium culmorum and F. graminearum sensu stricto. Toxins 2017, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in phenylpropanoid and trichothecene production by Fusarium culmorum and F. graminearum sensu stricto via exposure to flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S. Identification of metabolites related to mechanisms of resistance in barley against Fusarium graminearum, based on mass spectrometry. Plant Mol. Biol. 2011, 77, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bachhawat, A. Pyroglutamic acid: throwing light on a lightly studied metabolite. Curr. Sci. 2012, 102, 288–297. [Google Scholar]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttunen, E.; Noro, K.; Yang, Z. Purification and identification of antimicrobial substances produced by two Lactobacillus casei strains. Int. Dairy J. 1995, 5, 503–513. [Google Scholar] [CrossRef]
- Zhu, F.; Yuan, C.; Gang, F.; Yang, C.; Wu, W.; Zhang, J. Bioassay—guided isolation of antifungal compounds from Disporopsis aspersa (Hua) Engl. ex Diels against Pseudoperonospora cubensis and Phytophthora infestans. Chem. Biodivers. 2018, 15, e1800090. [Google Scholar] [CrossRef] [PubMed]
- Van der Werf, P.; Orlowski, M.; Meister, A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the γ-glutamyl cycle. Proc. Natl. Acad. Sci. USA 1971, 68, 2982–2985. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Y.; Wu, H.; Fang, W.; Liang, Q.; Zheng, Y.; Olsson, S.; Zhang, D.; Zhou, J.; Wang, Z.; et al. The 5-oxoprolinase is required for conidiation, sexual reproduction, virulence and deoxynivalenol production of Fusarium graminearum. Curr. Genet. 2018, 64, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: potential involvement in resistance to Fusarium and mycotoxin accumulation. Front Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P.; Desjardins, A.E. Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl. Environ. Microbiol. 1995, 61, 1923–1930. [Google Scholar] [PubMed]
- Hohn, T.M.; Krishna, R.; Proctor, R.H. Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet. Biol. 1999, 26, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Tag, A.G.; Garifullina, G.F.; Peplow, A.W.; Ake, C., Jr.; Phillips, T.D.; Hohn, T.M.; Beremand, M.N. A novel regulatory gene, Tri10, controls trichothecene toxin production and gene expression. Appl. Environ. Microbiol. 2001, 67, 5294–5302. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Abarenkov, K.; Buśko, M.; Bilska, K.; van Diepeningen, A.D.; Ostrowska-Kołodziejczak, A.; Krawczyk, K.; Brankovics, B.; Stenglein, S.; Sawicki, J.; Perkowski, J. ToxGen: An improved reference database for the identification of type B-trichothecene genotypes in Fusarium. PeerJ 2017, 5, e2992. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellergini, N.; Proteggente, A.; Pannala, A.S.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Perkowski, J.; Kiecana, I.; Kaczmarek, Z. Natural occurrence and distribution of Fusarium toxins in 15 naturally-contaminated barley cultivars. Eur. J. Plant. Pathol. 2003, 109, 331–339. [Google Scholar] [CrossRef]
- Kulik, T.; Łojko, M.; Jestoi, M.; Perkowski, J. Sublethal concentrations of azoles induce Tri transcript levels and trichothecene production in Fusarium graminearum. FEMS Microbiol. Lett. 2012, 335, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Buśko, M.; Pszczółkowska, A.; Perkowski, J.; Okorski, A. Plant lignans inhibit growth and trichothecene biosynthesis in Fusarium graminearum. Lett. Appl. Microbiol. 2014, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hohn, T.M.; Desjardins, A.E. Isolation and gene disruption of the Tox5 gene encoding trichodiene synthase in Gibberella pulicaris. Mol. Plant Microbe Interact. 1992, 5, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Hohn, T.M.; Desjardins, A.E.; McCormick, S.P. Analysis of Tox5 gene expression in Gibberella pulicaris strains with different trichothecene production phenotypes. Appl. Environ. Microbiol. 1993, 59, 2359–2363. [Google Scholar] [PubMed]
- Hohn, T.M.; Desjardins, A.E.; McCormick, S.P. The Tri4 gene of Fusarium sporotrichioides encodes a cytochrome P450 monooxygenase involved in trichothecene biosynthesis. Mol. Gen. Genet. 1995, 248, 95–102. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Alexander, N.J.; Proctor, R.H. Fusarium Tri4 encodes a multifunctional oxygenase required for trichothecene biosynthesis. Can. J. Microbiol. 2006, 52, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Tokai, T.; Koshino, H.; Takahashi-Ando, N.; Sato, M.; Fujimura, M.; Kimura, M. Fusarium Tri4 encodes a key multifunctional cytochrome P450 monooxygenase for four consecutive oxygenation steps in trichothecene biosynthesis. Biochem. Biophys. Res. Commun. 2007, 353, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
| l-Pyroglutamic Acid Concentration | Strain | Tri Genotype | Trichothecene Levels (mg/kg) (n = 3 in Each Condition) | RQ (n = 6 in Each Condition) | Relative Radial Growth (n = 3 in Each Condition) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DON | 3-AcDON | 15-AcDON | NIV | Total | Tri4 | Tri5 | Tri10 | ||||
| YES + Fungal Controls | CBS 138561 | 15ADON | 1.43 ± 0.7 | 1.47 ± 0.85 | 2.9 | 100 | |||||
| CBS 119173 | 3ADON | 49.55 ± 3.57 | 12.24 ± 0.76 | 61.79 | 100 | ||||||
| MUCL 53455 | NIV | 4.41 ± 0.18 | 4.41 | 100 | |||||||
| 100 μg/g | CBS 138561 | 15ADON | 0.47 ± 0.02 (b) | 0.03 ± 0.001 (b) | 0.5 | 0.76 (0.635–0.905) | NS | NS | 106.28 ± 5.02 (NS) | ||
| CBS 119173 | 3ADON | 5.05 ± 0.3 (d) | 1.54 ± 0.03 (d) | 6.59 | 0.349 (0.271–0.458) | NS | NS | 95.63 ± 0.44 (NS) | |||
| MUCL 53455 | NIV | 0.49 ± 0.04 (c) | 0.49 | NS | NS | NS | 89.22 ± 0.43 (b) | ||||
| 400 μg/g | CBS 138561 | 15ADON | 0.17 ± 0.007 (b) | 0.02 ± 0.001 (b) | 0.19 | 0.035 (0.028–0.044) | 0.34 (0.272–0.429) | 0.71 (0.615–0.83) | 98.33 ±2.93 (NS) | ||
| CBS 119173 | 3ADON | 21.14 ± 0.85 (b) | 5.95 ± 0.36 (b) | 27.09 | 0.117 (0.088–0.148) | 0.421 (0.28–0.637) | 0.123 (0.098–0.1) | 107.42 ± 2.62 (NS) | |||
| MUCL 53455 | NIV | 0.6 ± 0.05 (bc) | 0.6 | 0.558 (0.420–0.777) | 0.645 (0.531–0.786) | NS | 92.24 ± 2.59 (ab) | ||||
| 800 μg/g | CBS 138561 | 15ADON | 0.08 ± 0.003 (b) | 0.02 ± 0.001 (b) | 0.1 | 0.065 (0.055–0.08) | 0.25 (0.213–0.3) | 0.427 (0.368–0.495) | 94.14 ± 6.28 (NS) | ||
| CBS 119173 | 3ADON | 15.00 ± 0.3 (c) | 4.4 ± 0.09 (c) | 19.4 | 0.072 (0.003–0.456) | 0.215 (0.143–0.325) | 0.04 (0.008–0.097) | 108.73 ± 11.79 (NS) | |||
| MUCL 53455 | NIV | 0.79 ± 0.08 (b) | 0.79 | 0.451 (0.362–0.563) | 0.23 (0.161–0.342) | NS | 82.76 ± 2.59 (b) | ||||
| Degree of mycotoxin inhibition: | <25% | 25–50% | 50–75% | >75% | |||||||
| Acid | VCEAC/L | ABTS (μmol TROLOX/100 g d.m.) |
|---|---|---|
| l-Pyroglutamic Acid | 421.2 | 568.1 |
| trans-Cinnamic Acid * | 812.3 | 314.9 |
| Chlorogenic Acid * | 12.2 | 57.1 |
| Sinapic Acid ** | 121 | 194.5 |
| Species | Strain | Trichothecene Genotype | Origin, Host, and Year of Isolation |
|---|---|---|---|
| F. graminearum s.s. | CBS 138561 | 15ADON | Poland, wheat, 2010 |
| CBS 119173, NRRL 38369 | 3ADON | USA, Louisiana, wheat, 2005 | |
| MUCL 53455 | NIV | Belgium, corn, 2007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Perkowski, J. Resistance-Related l-Pyroglutamic Acid Affects the Biosynthesis of Trichothecenes and Phenylpropanoids by F. graminearum Sensu Stricto. Toxins 2018, 10, 492. https://doi.org/10.3390/toxins10120492
Bilska K, Stuper-Szablewska K, Kulik T, Buśko M, Załuski D, Perkowski J. Resistance-Related l-Pyroglutamic Acid Affects the Biosynthesis of Trichothecenes and Phenylpropanoids by F. graminearum Sensu Stricto. Toxins. 2018; 10(12):492. https://doi.org/10.3390/toxins10120492
Chicago/Turabian StyleBilska, Katarzyna, Kinga Stuper-Szablewska, Tomasz Kulik, Maciej Buśko, Dariusz Załuski, and Juliusz Perkowski. 2018. "Resistance-Related l-Pyroglutamic Acid Affects the Biosynthesis of Trichothecenes and Phenylpropanoids by F. graminearum Sensu Stricto" Toxins 10, no. 12: 492. https://doi.org/10.3390/toxins10120492
APA StyleBilska, K., Stuper-Szablewska, K., Kulik, T., Buśko, M., Załuski, D., & Perkowski, J. (2018). Resistance-Related l-Pyroglutamic Acid Affects the Biosynthesis of Trichothecenes and Phenylpropanoids by F. graminearum Sensu Stricto. Toxins, 10(12), 492. https://doi.org/10.3390/toxins10120492






