Abstract
Aflatoxins (AFs) are secondary metabolites produced by Aspergillus section Flavi during their development, particularly in maize. It is widely accepted that AFB1 is a major contaminant in regions where hot climate conditions favor the development of aflatoxigenic species. Global warming could lead to the appearance of AFs in maize produced in Europe. This was the case in 2015, in France, when the exceptionally hot and dry climatic conditions were favorable for AF production. Our survey revealed AF contamination of 6% (n = 114) of maize field samples and of 15% (n = 81) of maize silo samples analyzed. To understand the origin of the contamination, we characterized the mycoflora in contaminated samples and in samples produced in the same geographic and climatic conditions but with no AFs. A special focus was placed on Aspergillus section Flavi. A total of 67 strains of Aspergillus section Flavi were isolated from the samples. As expected, the strains were observed in all AF+ samples and, remarkably, also in almost 40% of AF− samples, demonstrating the presence of these potent toxin producers in fields in France. A. flavus was the most frequent species of the section Flavi (69% of the strains). But surprisingly, A. parasiticus was also a frequent contaminant (28% of the strains), mostly isolated from AF+ samples. This finding is in agreement with the presence of AFG in most of those samples.
Key Contribution:
This study reports for the first time the contamination of French maize kernels with AFs. It also reveals that AF contamination was not only associated with A. flavus but also with the presence of other species—particularly A. parasiticus—leading to the contamination with both AFs of B and G types.
1. Introduction
Aflatoxins (AFs), and more specifically Aflatoxin B1 (AFB1), are the most dangerous mycotoxins identified to date. Indeed, AFB1 is the most potent natural carcinogenic compound, responsible for hepatocarcinoma in humans and classified by IARC in the group I of carcinogenic molecules for both humans and animals [1,2]. This toxin is also immunosuppressive and has been associated with growth impairment in children [3,4]. In 2010, analysis of several foodborne chemicals by the Chemical and Toxins Disease Task force reported that AFB1 was associated with the highest number of DALYs (death and disability adjusted life years) [5].
AFs are secondary metabolites produced by Aspergillus section Flavi. Aspergillus flavus and A. parasiticus are the main species associated with AF contamination of crops. However, in the last decade, section Flavi has been studied in depth using molecular tools and several new species have been identified. This section currently comprises 33 different species of which 16 are aflatoxigenic [6,7,8,9,10,11]. These species can be distinguished by subtle morphologic characteristics, gene sequences and by their ability to produce different mycotoxins. For example, A. flavus, A. pseudotamarii and A. togoensis, produce AFs of B type whereas others, including A. parasiticus, A. minisclerotigenes, A. korhogoensis, A. mottae, A. nomius, and A. arachidicola, produce both B and G type AFs. Some species can produce other toxic secondary metabolites such as cyclopiazonic acid (CPA) [12].
Most Aspergillus species grow above latitude 25° N & S, with high occurrence between latitudes 26° and 35°, while these species are uncommon in latitudes above 45° [13]. The optimal temperature for growth of A. flavus is close to 33 °C [14]. This explains why these fungal species are frequent contaminants of many crops in tropical and subtropical regions where hydrothermal conditions are favorable to both fungal development and the production of toxins [15]. In these geographic areas, AFs are often reported in different kinds of products, mainly maize, spices, peanuts, pistachio nuts and cottonseeds which may be contaminated both before harvest and during storage [16,17].
In Europe, due to its latitude and associated climate, AF contamination was formerly not considered as a real threat to agricultural products produced within the European Union (E.U.) and attention was focused on products imported from third countries [18]. However, global climate change could modify AF distribution and lead to their appearance in areas usually considered as risk free. In 2007, a survey carried out by the European Food Safety Agency (EFSA) demonstrated that AF contamination of agricultural crops within E.U. borders and particularly in southern Europe was an emerging issue [19]. From this date on, several publications reported the presence of AFs in maize in E.U. countries including Romania [20], Italy [21,22], Spain [23], and Croatia [24,25]. In the neighboring country Serbia, contamination of maize harvested in 2012 and used to feed dairy cattle [26] led to the frequent serious contamination by Aflatoxin M1, the hydroxylated metabolite of AFB1, of milk marketed in 2013 [27]. Indeed, 60 to 80% of milk samples analyzed in Serbia were found to be contaminated with levels exceeding E.U. regulatory levels [28,29,30,31]. Moreover, the presence of aflatoxigenic isolates was also reported in other productions such as grapes [32,33].
The contamination of European maize was usually associated with noticeable drought periods [24,34] and it has been demonstrated that both heat and water stress can strongly influence the production of AFs [35]. Recent works that modeled the spread of AFs in Europe showed that global warming could considerably increase the presence of AFs in southern Europe [36]. As an illustration of this trend, the Rapid Alert System for Food and Feed has reported several cases of European maize contamination with AFs since 2013 [37].
In France, no contamination by AFs had been reported before 2015, when exceptional climate conditions in southern France, characterized by high temperatures during maize flowering together with an unusual rainfall deficit, increased the risk of contamination of maize with AFs, as reported in other European countries at the same period [38].
In this context, the aim of this study was to (i) measure the contamination with AFs in maize produced in southern France in 2015 and to (ii) characterize the nature of Aspergillus section Flavi in these samples. Our results demonstrate that Aspergillus section Flavi is frequently present in maize exposed to heat and drought and that, in the case of favorable environmental conditions, its development can lead to AF contamination. Surprisingly, A. parasiticus appeared to be a frequent contaminant of French maize and its presence leads to the contamination of products with G type AF.
2. Results
2.1. Climatic Data
Summer 2015 was exceptionally hot and dry in France, with two successive heat waves in July, the maize flowering period, which extends from 1 July to 25 July. Temperatures exceeded 33 °C for 10 to 15 days (Figure 1A), with values 1 to 3 °C above normal in the southwest and eastern half of the country, and differences in sum temperatures ranging from 75 to 145 °C in the same regions (Figure 1A). For the whole summer, the average temperature in France was +1.5 °C above normal, making summer 2015 the second warmest summer after 2003 (anomaly of +3.2 °C) and before 2006 (anomaly of + 1.1 °C).
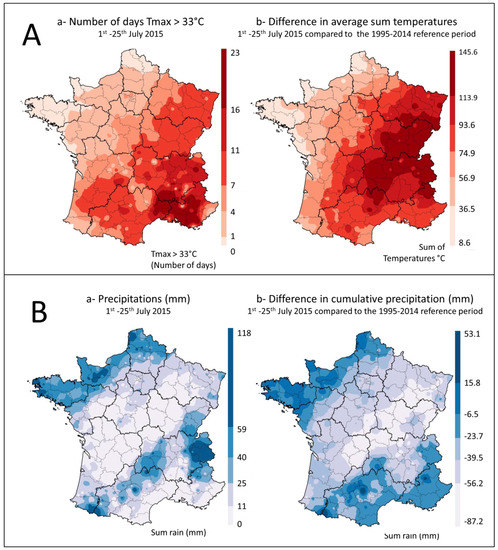
Figure 1.
Temperatures and rainfall during the maize flowering period in France. (A): Temperatures (a) number of days with Tmax > 33 °C between 1 July and 25 July and (b) comparison of the sum of daily temperatures between 1 July and 25 July, with the average for the 1995–2014 period. (B): Precipitation (a) Sum of precipitation between 1 July and 25 July (mm) and (b) difference in cumulative rainfall between 1 July and 25 July 2015 and the average for the same period between 1995 and 2014 years (mm).
During the same period, a rainfall deficit of more than 40% on average was also recorded in a large part of the country that lasted from April to the end of July, causing the drying out of the top soil layer. In areas included in the survey, rainfall mostly ranged between 0 and 25 mm during the maize flowering period (Figure 1B). However, local rainfall was sometimes higher. Compared to the average values recorded in France during the 1995 to 2014 reference period, cumulative rainfall in the warmer regions of the study varied between −6 mm and −87 mm (Figure 1B).
2.2. Occurrence of Aflatoxins in Maize Samples
2.2.1. Number and Location of Contaminated Samples
For analysis of AF contamination, a total of 118 samples were collected from individual farm fields at harvest (field samples) and 81 more samples were collected from silo sites during the harvest period (silo samples). The silo samples were collected after drying but before storage. They thus corresponded to a mixture of kernels from different fields.
Seven field samples (6%) were found to be contaminated with AFs (AF+ field samples). Twelve silo samples (15%) were also contaminated (AF+ silo samples).
All the contaminated samples were collected in the administrative departments most exposed to heat and drought during the maize flowering period (temperatures > 33 °C during more than seven days and rainfall deficit) (Figure 1 and Figure 2).
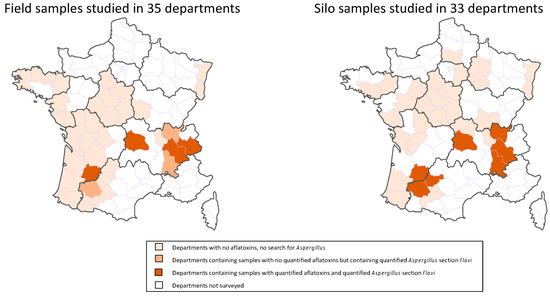
Figure 2.
Geographic distribution of field and silo samples according to their contamination status with AFs and Aspergillus section Flavi.
Logically, a close link was found between the locations of the contaminated field and silo samples (Figure 2). AF+ silo samples came from seven departments. In three of them, some field samples were also contaminated with AFs. In three other departments, field samples were not contaminated with AFs but Aspergillus section Flavi were nevertheless detected. In only one department with AF+ silo samples, neither AF+ nor Flavi + field samples were found, but this department is located close to contaminated zones.
2.2.2. Types and Levels of AF Contamination
All positive field samples were found to be contaminated by both B and G type AFs. In contrast, AFGs were only found in seven silo samples out of the 12 contaminated ones (58%) (Table 1).

Table 1.
AF content of contaminated maize samples.
Based on the concentration of toxins, the levels of AFs in three field samples exceeded the E.U. regulation, set at 5 µg/kg for AFB1 and 10 for total AFs in maize to be subjected to sorting or other physical treatment before human consumption or used as an ingredient in foodstuffs. Two of these samples were highly contaminated, with aflatoxin contents of 50.8 and 70 µg/kg, respectively. The last one displayed 15.3 µg/kg AFs. One out of the 12 contaminated silo samples exceeded regulatory levels. However, to comply with E.U. regulations, 2 µg/kg for AFB1 and 4 µg/kg for total aflatoxins in ready-to-eat maize, five samples would require cleaning before being sold.
2.3. Fungal Flora of Maize Samples
Samples found to be contaminated with AFs (seven AF+ field samples and 12 AF+ silo samples) were further analyzed to determine fungal contamination and the nature of Aspergillus section Flavi that were present. To better understand the factors that may have led to contamination by AFs at field level, we also analyzed 24 aflatoxin-free field samples (AF− field samples), collected in the same geographic areas as the contaminated samples, i.e., cultivated under the same climatic conditions. All the fungal flora found in these different types of samples are listed in Table 2 and the complete results of the mycological analyses are given in Supplementary Table S1.

Table 2.
Total fungal flora found in maize samples.
2.3.1. AF+ Samples
Fusarium was the most frequent genus, in both frequency (100% of samples) and in numbers. Penicillium was also systematically present in the samples but the corresponding counts were lower.
Aspergillus is a frequently observed fungal genus. The high frequency of Aspergillus section Nigri is particularly notable. These species, which are ecologically close to Aspergillus section Flavi, display almost the same pattern as the latter.
Aspergillus section Flavi were found systematically, in accordance with the presence of AFs in samples. They were present at relatively high levels and the three field samples with the highest AF contamination displayed Aspergillus section Flavi counts exceeding 2 × 104 CFU/g.
Other sections were also sometimes observed in some silo samples, particularly Aspergillus section Aspergillus, which are very common contaminants of dried foodstuffs.
Acremonium and Cladosporium, both typical field contaminants, were also very frequently observed. Other genera were sporadically observed in some samples, for instance, Verticillium, Alternaria, and Trichoderma, common field contaminants. Both Mucor and Rhizopus were frequently observed in samples but at low counts (Supplementary Table S1).
2.3.2. AF− Samples
From a qualitative point of view, the overall pattern of fungal flora observed in AF− samples closely resembled that found in AF+ samples but with lower counts. Surprisingly, Aspergillus section Flavi were also present in almost 40% of AF− field samples but with moderate counts.
2.3.3. Quantitative Comparison
Quantitative analysis of the mycoflora observed in AF+ vs. AF− field samples pointed to a clear relationship between AF contamination and a global increase in overall fungal contamination, with no change in the nature of fungal genera present (Figure 3).
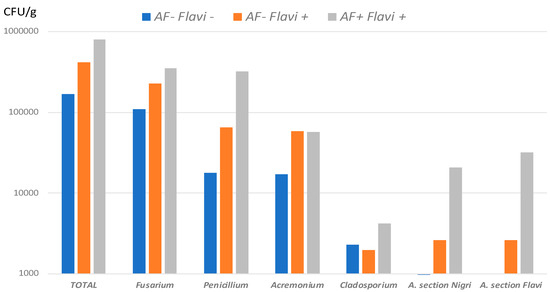
Figure 3.
Fungal flora found in field samples. Mean counts of total mycoflora and of more frequent genera in AF− Flavi− (n = 15), AF− Flavi+ (n = 9) and AF+ Flavi+ (n = 7). Results are expressed in CFU/g.
When considering all AF+ samples (field + silo samples; n = 19) vs. AF− samples (n = 24), a correlation was found between Aspergillus section Flavi counts and AF contamination, counts of more than 104 CFU/g being highly correlated with aflatoxin contamination of samples (Figure 4).
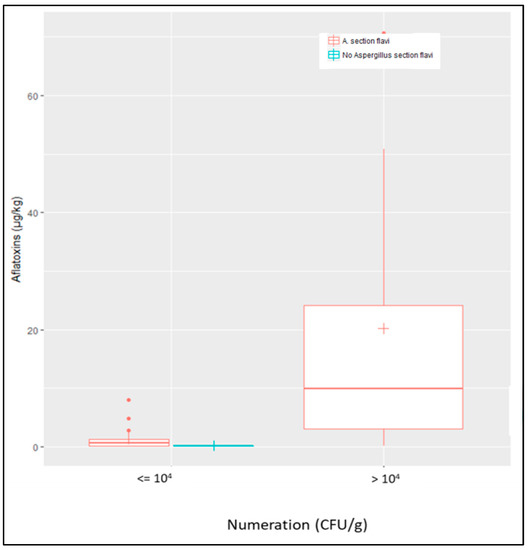
Figure 4.
Correlation between counts of Aspergillus section Flavi and contamination by AFs. Aspergillus section Flavi were systematically found in AF+ samples as shown by red boxplots and no sample was contaminated by AFs in the absence of A. section Flavi (blue boxplot). Counts of A. section Flavi higher than 104 CFU/g were highly correlated with AF contamination of samples, whatever their origin (field or silo).
2.4. Characterization of Aspergillus Section Flavi Strains
A total of 67 strains belonging to the Flavi section were isolated. Fifty-five came from AF+ samples (field + silos). Indeed, many samples were contaminated with several morphologically distinct strains as shown in Figure 5. AF+ samples were most often contaminated by three strains per sample but some silo samples were contaminated by 4 (n =2) and even 6 (n = 2) different strains. In AF− field samples, 12 strains were isolated from the 24 samples analyzed and, in most cases, the samples were contaminated by only one strain.
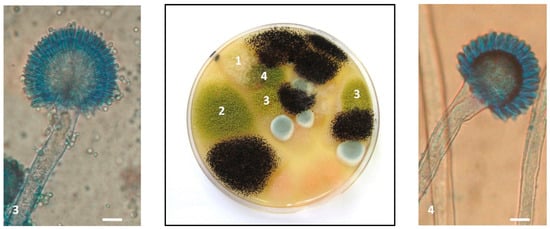
Figure 5.
Presence of four different Aspergillus section Flavi strains in a single sample. The Petri dish in the figure corresponds to the 10−3 dilution of sample M638 and was observed after 7 days of incubation at 25 °C on malt extract agar (MEA). Five colonies corresponding to four morphologically distinct strains of Aspergillus section Flavi were present and were isolated for identification and characterization. 1: A. flavus M638a; 2: A. flavus M638e; 3: A. flavus M638g; and 4: A parasiticus M638b. The three strains of A. flavus displayed very different toxigenic potential. In the left panel, microscope image (×400) of A. flavus M638g characterized by a biseriate conidial head, a globose vesicle, radiate on ¾ and a rough conidiophore. In the right panel, microscope image (×400) of A. parasiticus M638b with uniseriate head, a sub-globose vesicle and a smooth conidiophore. Scale bar: 10 µm.
Identification at the species level using morphological and molecular approaches revealed Aspergillus flavus to be the most frequent strain isolated from maize samples, representing 68% of strains (46/67). However, A. parasiticus was also frequently identified. Indeed, this species corresponded to 4/12 strains from AF− field samples (33%), 5/21 strains from AF+ field samples (24%), and 10/34 strains from silo samples (30%) as illustrated in Figure 6. As can be seen in Figure 7, the molecular identification clearly confirmed the morphological identification and the location of strains in the A. parasiticus clade.
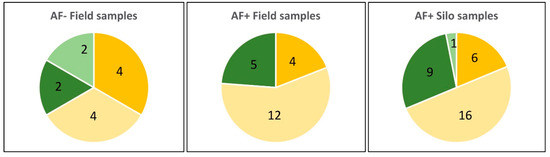
Figure 6.
Number of A. flavus and A. parasiticus strains in samples as a function of their origin and contamination status with AFs. Light green: nontoxigenic A. parasiticus strains; dark green: toxigenic A. parasiticus strains; light yellow: nontoxigenic A. flavus strains; dark yellow: toxigenic A. flavus strains.
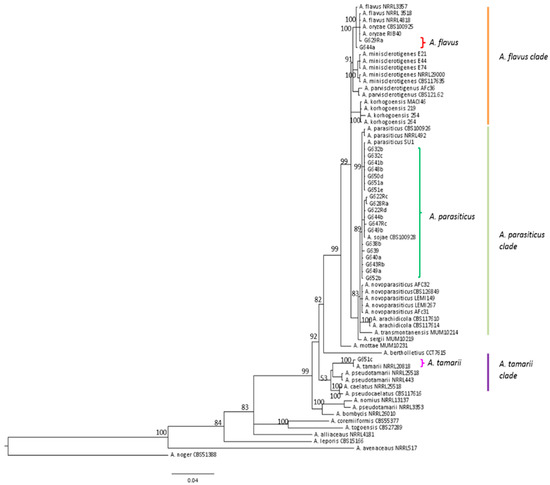
Figure 7.
Phylogenetic trees showing the location of all A. parasiticus, two typical A. flavus and the only A. tamarii strains isolated from French maize samples. benA and cmdA genes were concatenated using Mesquite v3.2. and phylogenic tress were created on FigTree v1.4.2.
Aside from A. flavus and A. parasiticus, one strain of A. minisclerotigenes and one strain of A. tamarii were also identified in silo samples.
The mycotoxin production ability of the strains varied with the species.
Considering strains isolated from AF+ samples, almost 75% of A. flavus strains were non-aflatoxigenic and 66% produced CPA. Nine strains (24%) produced both AFB and CPA. Eighty per cent of A. parasiticus strains were able to produce B and G AFs, in agreement with the systematic contamination of field maize samples with AFG1.
Considering strains isolated from AF− field samples, 50% of both A. flavus and A. parasiticus produced B or B and G type AFs, respectively (Figure 6). However, all strains of A. flavus were also able to produce CPA and four of them produced both AFB and CPA.
As expected, the A. minisclerotigenes strain simultaneously produced AFs B and G and CPA and A. tamarii strains produced no AF, but was able to produce CPA.
The toxigenic potential of all the strains is detailed in Supplementary Table S2.
Since many strains of A. flavus were able to produce CPA, next, this mycotoxin was quantified in the samples. As shown in Supplementary Table S2, 28% and 25% of the AF+ field and AF+ silo samples respectively were contaminated by CPA together with AFs, demonstrating the frequent copresence of these toxins. By contrast, none of the AF− field samples were contaminated by CPA.
3. Discussion
3.1. Impact of Climate on Aflatoxin Emergence in French Maize in 2015
According to Payne [39], the development of Aspergillus species and AF production are favored by hot dry weather conditions during the maize growing season. Temperatures suitable for growth of A. flavus range from 10 to 12.8 °C to 43 to 48.8 °C with an optimum around 33.8 °C [14]. In France in 2015, the summer was particularly hot, especially during the maize flowering stage and the maximum temperatures recorded were very close to those allowing optimum growth of Aspergillus section Flavi. In most of the country, these high temperatures were accompanied by a rainfall deficit of more than 40% compared to the average, which dried out the top soil layer and caused heat stress in maize [40], especially during the flowering and silk tanning periods. These exceptional climatic conditions may therefore have enabled the development of Aspergillus section Flavi and the subsequent AF contamination thus justified a thorough investigation.
The present survey demonstrated that, in 2015, French maize kernels were contaminated by AFs. These toxins were detected in seven out of 118 field samples (6%) and 12 out of 81 silo samples (15%). All contaminated samples came from departments where the weather conditions at maize flowering were abnormally hot and dry in 2015. In half the cases, AF+ silo samples were found in departments where AF+ field samples were also observed. In other cases, AF+ silo samples came from departments in which no AF+ field samples were found but where the Aspergillus section Flavi were present. Therefore, contamination of the silos could be the result of contaminated fields that were not analyzed or the result of postharvest development of toxigenic fungi. Indeed, in maize, the postharvest period may play a major role in mycotoxin contamination, especially during pre-storage of grains that still have relatively high water content (~15–20%) before their final drying stage in storage facilities.
Maximum AFB1 contents of 66 µg/kg were measured in field samples and of 7.2 µg/kg in silo samples. Among the contaminated samples, three field samples and one silo sample exceeded E.U. regulations (5 µg/kg for AFB1 and 10 µg/kg for total AFs in maize to be subjected to sorting or other treatment before consumption) [31]. However, despite dilution during silo filling and the preparation of mixed samples, five silo samples would still require cleaning before sale to reduce AF levels and comply with E.U. regulations (2 µg/kg for AFB1 and 4 µg/kg for total AFs in ready-to-eat maize). Thus, sorting and cleaning of maize kernels appear to be critical points in the maize processing chain. Today, in France, ~80% of maize kernels are cleaned before sale, of which 68% occurs at reception in storage facilities before drying, 20% during storage, and 12% when shipped [41], thereby making it possible to reduce AF content and reach both sanitary and commercial targets. In the future, it would be of great interest to investigate the real impact of this processing step on fungal flora, and if it also reduces A. section Flavi counts, what is more, this step needs to be accomplished as far upstream as possible, i.e., before the kernels are dried to limit the risk of fungal development and subsequent production of toxins during storage.
This is the first report of AF contamination of maize kernels produced in France. Contamination has already been reported in other European countries where average climate conditions are more favorable to Aspergillus section Flavi than conditions in France, including Romania [20] and Italy [42]. More recently, the presence of AFB1 was detected in 57.2% of 180 maize samples collected in 2015 from the main Serbian maize-growing regions, with concentrations ranging from 1.3 to 88.8 μg/kg [38]. Like in France, the authors noted that July 2015 was particularly hot and dry in Serbia with rainfall comparable to that recorded in France and even higher mean temperatures, which may explain why AF contamination was more frequent than in France. These data confirm the emergence of AFs as possible contaminants of European crops and the need to closely monitor the presence of these carcinogenic agents in foods in Europe, which was previously usually considered to be an aflatoxin-free.
Of note, AFG was observed in most contaminated samples, as also recently reported in Serbia [26,38]. The frequent presence of AFG in maize appears to be a new feature of AF contamination of European crops, since, in available studies, only AFB has previously been reported [21,24,25]. Since A. flavus is not able to produce AFG, this points to the presence of other aflatoxigenic species in maize, and this was subsequently confirmed by mycological analysis. Our study also demonstrated the possible co-contamination of cereals with AFs and CPA. Such co-contamination has already been reported in mixed feed [43] and in peanuts in Argentina [44] but we found no recent data concerning contamination of cereals. An investigation of the possible interaction between AFs and CPA when ingested simultaneously would be needed to assess the risk of co-contamination of foods [45].
3.2. Nature of Aspergillus Section Flavi Responsible for Aflatoxin Contamination of French Maize
Only a few studies describe the mycoflora of European maize but those that do, usually report very similar fungal flora to that we observed, with the frequent presence of Fusarium, Penicillium, and to a lesser extent, genera such as Alternaria and Cladosporium [20,21,23]. In the present study, the high prevalence of Aspergillus section Flavi was also observed but this is not surprising in samples that are being analyzed because of the presence of AFs. Nevertheless, we demonstrated a clear relationship between A. section Flavi counts and the level of AF contamination, and 104 CFU/g is a possible threshold. We also demonstrated the frequent presence of Aspergillus section Flavi in AF− field samples. This finding is of major importance since it demonstrates that A. section Flavi are frequently present in French fields and may therefore be responsible for AF contamination if and when environmental conditions become favorable. This observation is even more important considering that the proportion of toxigenic strains observed in AF− samples was higher than that in AF+ samples. However, counts of Aspergillus section Flavi were lower in AF− samples than in AF+ samples. Since these samples were collected in areas close to contaminated areas, i.e., grown under the same climatic conditions, it would be now of great interest to compare the agricultural practices being used to identify those that interfere with the growth of the toxigenic Aspergillus, as has already been demonstrated for peanuts [46].
The results of our investigation of Aspergillus section Flavi species were surprising since A. parasiticus appeared to be a frequent contaminant of French maize, as confirmed by the presence in many contaminated samples of AFG. Indeed, in most surveys, A. flavus is reported to be the main contaminant of maize particularly in Africa [47,48] and USA [49] but also in Europe [50] even if A. parasiticus was found in few samples [23,51]. This finding is also in agreement with data from Serbia showing the presence of AFG in maize samples [38]. Since A parasiticus requires lower temperatures to colonize substrates, it could be more adapted to the French climate [52].
Among the 67 strains of Aspergillus section Flavi isolated from maize samples, 31 were aflatoxigenic (46%). This proportion is similar to proportions already reported in strains isolated from maize [23,48,50]. When we examined toxigenic potential as a function of the fungal species, the proportion of toxigenic A. parasiticus strains was much higher than the proportion of A. flavus with 84 and 30% of aflatoxigenic strains, respectively. This is extremely important due to the relatively high prevalence of A. parasiticus isolates that could be responsible for AF contamination of French maize if and when environmental conditions allow their development. The relatively high proportion of non-aflatoxigenic strains of A. flavus that are naturally present is also of interest. Indeed, the use of atoxigenic A. flavus strains as a biocontrol agent to compete with toxigenic strains has been shown to be a potent strategy [53]. Thus, some of these strains could be tested for their ability to serve as biocontrol agents. Another possible strategy would be to identify agricultural practices able to favor the implantation of these naturally present atoxigenic strains in fields thus giving them a long-term advantage over aflatoxigenic strains that would subsequently limit the risk of recombination [54]. However, many of these non-aflatoxigenic strains produce CPA. Indeed, almost 74% of A. flavus strains produced CPA, in some cases leading to the contamination of maize with that mycotoxin. In most cases, we found a high correlation between the counts and toxigenic potential of A. section Flavi strains isolated from samples and mycotoxin contamination of maize. However, in two cases, we were unable to identify toxigenic strains from AF+ samples despite several platings and isolation of the strains. Alborch et al. [23] already reported similar differences which could be related to the sample drying procedure that led to the inactivation of spores before fungal analysis.
4. Conclusions
This work reports, for the first time, the contamination of French maize kernels with AFs. Six percent of analyzed samples taken in fields and 15% of those taken in silos where found to be contaminated, sometimes at high levels, largely exceeding E.U. regulations. This survey also demonstrated that contamination may differ in samples produced under the same climatic conditions but using different agricultural practices. Finally, it also highlighted the fact that AFB1 contamination was not only associated with A. flavus but with the presence of other species, particularly A. parasiticus, leading to contamination with both B and G type AFs. All these data confirm the emergence of aflatoxins in France and the need for close monitoring of these contaminants in susceptible crops throughout the territory.
5. Materials and Methods
5.1. Meteorological Data
The weather variables used to qualify the atypical 2015 growing season are based on two daily parameters: temperature and rainfall. These variables were calculated from spatialized climatic data from nearly 700 weather stations distributed throughout metropolitan France [55].
5.2. Sampling
5.2.1. Sampling Strategy
All the samples were collected during the 2015 maize growing season, at harvest.
In the first round, 118 field samples and 81 samples collected from silos were analyzed for AF contamination. The field samples were collected in 35 French administrative departments and the silo samples in 33 departments (Figure 2). Following this first round of analysis, samples found to be contaminated with aflatoxins (7 field samples and 12 silo samples) were further analyzed to identify fungal contamination and the nature of Aspergillus section Flavi present. To better understand the factors that may have led to aflatoxin contamination of the samples, the second round of analyses was undertaken using also 24 uncontaminated field samples collected in the same departments as contaminated ones, i.e., in areas where climatic conditions represented a high risk of aflatoxin synthesis. All the samples in the second-round of analyses were also analyzed for cyclopiazonic acid contamination.
5.2.2. Sample Collection
Field Samples
A total of 118 farm fields planted with maize were sampled: at harvest, the farmer was asked to prepare samples respecting the following recommendations. (a) Avoid sampling the margins of the field, (b) avoid static sampling of grain, and (c) sample moving grains during three different periods of emptying of the combine harvester. In this way, three different subsamples, each weighing at least 1 kg, were manually collected from the moving grains during harvest. These three subsamples were then combined to obtain a 3 kg final sample from each farm field.
Silo Samples
A total of 225 samples were collected in silos belonging to storage companies, cooperatives and private merchants located in 33 French administrative departments. At harvest time, three elementary dried samples were taken before storage at different sampling dates (beginning, middle, and end of harvest period). The resulting 225 samples were therefore representative of the different silos. The elementary samples were then mixed to prepare 81 mixed samples representative of each department, each weighing at least 3 kg. AF contents were measured in these 81 mixed samples.
5.2.3. Sample Preparation for Analysis
All the grain samples were cleaned with a laboratory cleaner and separator (MINI-PETKUS 100 and 200, PETKUS Technologie GmbH, Rohr, France) to remove all impurities from the kernels. Then, 1.5 kg of cleaned and homogeneous sample was taken for analysis and ground in a laboratory hammer mill fitted with a 1 mm screen (TITAN 2000, F.A.O., Vitré, France).
5.3. Aflatoxin and Cyclopiazonic Acid Quantification
AFs B1, B2, G1, and G2 were analyzed by liquid chromatography-tandem mass spectrometry in a French accredited laboratory. The limits of detection for AFB1, B2, G1, and G2 were 0.1, 0.1, 0.12, and 0.25 µg/kg, respectively, the corresponding limits of quantification were 0.25, 0.25, 0.25, and 0.5 µg/kg. For CPA, the limit of detection was 5.0 µg/kg, and the corresponding limit of quantification was 10.0 µg/kg.
5.4. Fungal Count and Identification
Fungal counts were made according to ISO 7954 norm [56]. In brief, 20 g of ground sample was mixed with 180 ml of 0.05% Tween 80 for 2 min in a Waring blender and then placed on a horizontal shaking table at 220 rpm for one hour. Decimal dilutions were prepared in 0.05% Tween 80 and 100 µl of each dilution were plated on both MEA medium and salted MEA (MEA + 6% NaCl). The latter medium was used to identify xerophilic species and to limit the development of mucorales which can prevent correct counting and identification of species with low growth rates. Fungal colonies were counted after three days of culture at 25 °C and confirmed after five days. The limit of detection for the fungal count was 10 CFU/g of sample. The colonies were then identified according to Pitt and Hocking [14] and Samson et al. [57]. Aspergillus section Flavi strains were isolated from plates by several platings on MEA and salted MEA.
5.5. Characterization of Aspergillus Section Flavi
5.5.1. Morphological Identification
Aspergillus section Flavi strains were identified at the species level through macroscopic and microscopic examination after five and seven days of culture at 25 °C on MEA and salted MEA according to Pitt et al. [58] and Varga et al. [7].
5.5.2. Molecular Identification
The molecular identification was performed on all Aspergillus section Flavi strains which did not display typical A. flavus morphological features and on two A. flavus strains as control. Identification was performed by internal transcribed spacer (ITS) beta-tubulin (benA) and calmodulin (cmdA) gene amplification and sequencing. The strains were cultured in yeast extract sucrose (YES) broth and placed on a shaking incubator at 160 rpm at 27 °C for three days. Genomic DNA was isolated from mycelia as previously described [59]. Primers used for molecular identification are listed in Table 3. PCR reactions were carried out in a GeneAmp PCR 2700 thermocycler (Applied Biosystems, Foster City, CA, USA). PCR products were purified with a GenElute PCR Clean-Up Kit (Sigma-Aldrich, Saint-Quentin Fallavier, France) and sequenced on an ABI3130XL sequencer (Applied Biosystems, Foster City, CA, USA) using the dye terminator technology. PCR products were sequenced in both directions.

Table 3.
Sequence of the primers used for molecular identification of Aspergillus section Flavi isolates.
New sequences were blasted against NCBI database, and deposited in GenBank. Accession numbers of A. parasiticus strains are listed in Table 4.

Table 4.
Accession numbers deposited in GenBank of A. parasiticus isolated from French maize samples.
The species identification was confirmed by phylogenetic analyses. Data were assembled, aligned and trimmed using ClustalW in BioEdit v7.0.5 [60], and checked. In order to reduce indels and to optimize nucleotide identities, regions with multiple gaps were aligned. The ITS gene was analyzed independently, whereas, benA and cmdA genes were concatenated using Mesquite v3.2. (Mesquite Software, Austin, TX, USA) [61]. The best-fit nucleotide substitution model for ITS was calculated using jModelTest, and resulted on TIM2 + I + G substitution model. For concatenated data, the best-fit nucleotide substitution model and partition scheme were calculated using PartitionFinder v2.0.0 (Australian National University, Canberra, Australia) [62]; one partition was suggested under K80+G substitution model.
Bayesian analyses were performed using MrBayes v3.2 [63]; four independent runs, each one with four chains, were carried out for 107 generations. Sampling was performed every 103 generations. For each analysis, we checked that the average standard deviation of split frequencies among chains were near ≤0.01, and the potential scale reduction factor (PSFR) to 1. From the total number of trees per run, 25% were used as burn-in; and the remaining trees were used to calculate the posterior probabilities (PP), based on the 50% majority rule consensus tree by Tracer v1.6 [64]. Visualization and edition of phylogenetic trees were performed on FigTree v1.4.2 (Institute of Evolutionary Biology, University of Edinburgh, Edinburgh, Scotland) [65].
5.5.3. Mycotoxigenic Potential of Isolates
To assess the toxigenic potential of Aspergillus section Flavi strains, a spore suspension was prepared from a 7-day culture and 100 spores were centrally inoculated on MEA and cultured for seven days at 25 °C. After this incubation period, highly sporulating cultures were analyzed for toxin production as already described [66,67].
Supplementary Materials
The following tables are available online at http://www.mdpi.com/2072-6651/10/12/525/s1, Table S1: Complete fungal flora of maize samples, Table S2: Identification, toxigenic potential of Aspergillus section Flavi strains, numeration and contamination level in corresponding samples.
Author Contributions
Conceptualization S.B., B.O., and J.-D.B; Methodology S.B. and B.O.; Validation, S.B., B.O., O.P., and J.-D.B; Investigation, S.B., A.E.M., and A.C.-C.; Resources, B.O.; Data Curation, S.B.; Writing—Original draft Preparation, S.B., B.O., and J.-D.B.; Writing—Review and editing, S.B., B.O., O.P., S.L., I.P.O., and J.-D.B.; Visualization, S.B. and B.O; Supervision, J.-D.B.; Funding Acquisition, B.O. and J.-D.B.
Funding
This research received no external funding.
Acknowledgments
The authors thank FranceAgriMer for sampling the silos and all the farmers involved in the study for their investment. The doctoral studies of Amaranta Carvajal-Campos were funded by the Secretaría de Educación Superior, Ciencia, Tecnología e Inovación (SENESCYT), Ecuador. The authors also warmly thank Joseph and Pierrette Le Bars for passing on their expertise in mycology to S.B., indispensable for this type of study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- IARC Monographs on the evaluation of carcinogenic risks to humans. Aflatoxins 2012, 100F, 225–244.
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food. Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Khlangwiset, P.; Shephard, G.S.; Wu, F. Aflatoxins and growth impairment: A review. Crit. Rev. Toxicol. 2011, 41, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Gibb, H.; Devleesschauwer, B.; Bolger, B.M.; Wu, F.; Ezenman, J.; Cliff, J.; Zeilmaker, M.; Verger, P.; Pitt, J.; Baines, J. World Health Organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: A data synthesis. F1000Research 2015, 4, 1393. [Google Scholar] [CrossRef] [PubMed]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008, 58, 725–735. [Google Scholar] [CrossRef]
- Varga, J.; Frisvad, J.C.; Samson, R.A. Two new aflatoxin producing species and an overview of Aspergillus section. Flavi. Stud. Mycol. 2011, 69, 57–80. [Google Scholar] [CrossRef]
- Soares, C.; Rodrigues, P.; Peterson, S.W.; Lima, N.; Venancio, A. Three new species of Aspergillus section Flavi isolated from almonds and maize in Portugal. Mycologia 2012, 104, 682–697. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Iamanaka, B.T.; Sartori, D.; Copetti, M.V.; Balajee, A.; Fungaro, M.H.; Frisvad, J.C. Aspergillus bertholletius sp. nov. from Brazil nuts. PLoS ONE 2012, 7, e42480. [Google Scholar] [CrossRef]
- Carvajal-Campos, A.; Manizan, A.L.; Tadrist, S.; Koffi-Akaki, D.; Koffi-Nevry, R.; Moore, G.G.; Fapohunda, S.O.; Bailly, S.; Montet, D.; Oswald, I.P.; et al. Aspergillus korhogoensis, a novel aflatoxin producing species from the Côte d’Ivoire. Toxins 2017, 9, 353. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.B.; Novakova, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenar, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Uka, V.; Moore, G.G.; Aroyo-Manzanares, N.; Nebija, D.; De Saeger, S.; Di Mavungu, J.D. Unravelling the diversity of the cyclopiazonic acid family of mycotoxins in Aspergillus flavus by UHPLC triple TOF HRMS. Toxins 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant. Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: London, UK, 2009; p. 519. ISBN 978-0-387-92206-5. [Google Scholar]
- Santin, E. Mould growth and mycotoxin production. In Mycotoxin Blue Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; pp. 225–234. ISBN 978-1-904761-19-8. [Google Scholar]
- Rustom, I.Y. Aflatoxin in food and feed: Occurrence, legislation and inactivation by physical methods. Food Chem. 1997, 59, 57–67. [Google Scholar] [CrossRef]
- Sharma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implication on health. Ind. J. Biochem. 2017, 32, 124–133. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 39, 1–27. [Google Scholar]
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA J. 2007, 446, 1–127. [Google Scholar]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and mycotoxin content of cereals in southeastern Romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Salvi, S. Infection by mycotoxigenic fungal species and mycotoxin contamination of maize grain in Umbria, central Italy. Food Chem. Toxicol. 2011, 49, 2365–2369. [Google Scholar] [CrossRef]
- Amorini, S.; Altafini, A.; Zaghini, A.; Roncada, P. Occurrence of aflatoxin B1 in conventional and organic flour in Italy and the role of sampling. Food Control. 2015, 50, 858–863. [Google Scholar] [CrossRef]
- Alborch, L.; Bragulat, M.R.; Castella, G.; Abarca, M.L.; Cabanes, F.J. Mycobiota and mycotoxin contamination of maize flours and popcorn kernels for human consumption commercialized in Spain. Food Microbiol. 2012, 32, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Vulic, A.; Persi, N.; Skrivanko, M.; Capek, B.; Cvetnic, Z. Aflatoxin B1 occurrence in maize sampled from Croatian farms and feed factories during 2013. Food Control. 2014, 40, 286–291. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulic, A.; Persi, N.; Skrivanko, M.; Capek, B.; Cvetnic, Z. Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control. 2015, 47, 221–225. [Google Scholar] [CrossRef]
- Kos, J.; Skrinjar, M.M.; Mandic, A.I.; Misan, A.C.; Bursic, V.P.; Saric, B.M.; Janic-Hajnal, E.P. Presence of aflatoxins in cereals from Serbia. Food Feed. Res. 2014, 41, 31–38. [Google Scholar] [CrossRef]
- Ketney, O.; Santini, A.; Oancea, S. Recent aflatoxin survey data in milk products: A review. Int. J. Dairy Technol. 2017, 70, 320–331. [Google Scholar] [CrossRef]
- Kos, J.; Levic, J.; Duragic, O.; Kokic, B.; Miladinovic, I. Occurrence and estimation of aflatoxin M1 exposure un milk in Serbia. Food Control. 2014, 38, 41–46. [Google Scholar] [CrossRef]
- Skrbic, B.; Zivancev, J.; Antic, I.; Godula, M. Levels of aflatoxin M1 in different types of milk collected in Serbia: Assessment of human and animal exposure. Food Control. 2014, 40, 113–119. [Google Scholar] [CrossRef]
- Tomasevic, I.; Petrovic, J.; Jovetic, M.; Raicevic, S.; Milojevic, M.; Miocinovic, J. Two year survey on the occurrence and seasonal variation of aflatoxin M1 in milk and milk products in Serbia. Food Control. 2015, 56, 64–70. [Google Scholar] [CrossRef]
- European Union. Commission regulation 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Mikusova, P.; Sulyok, M.; Santini, A.; Srobarova, A. Aspergillus spp and their secondary metabolite production in grape berries from Slovakia. Phytopathol. Mediterr. 2014, 53, 109–114. [Google Scholar] [CrossRef]
- Mikusova, P.; Ritieni, A.; Santini, A.; Juhasova, G.; Srobarova, A. Contamination by moulds of grape berries in Slovakia. Food Addit. Contam. 2010, 27, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Udovicki, B.; Audenaert, K.; De Saeger, S.; Rajkovic, A. Overview on the mycotoxin incidence in Serbia in the period 2004-2016. Toxins 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Carmado Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- European Commission. RAFF—Food and Feed Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 6 December 2018).
- Janic Hajnal, E.; Kos, J.; Krulj, J.; Krstovic, S.; Jajic, V.; Pezo, L.; Saric, B.; Nedeljkovic, N. Aflatoxins contamination of maize in Serbia: The impact of weather conditions in 2015. Food Addit. Contam. Part A 2017, 34, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A. Process of contamination by aflatoxin-producing fungi and their impact on crops. In Mycotoxins in Agriculture and Food Safety; Sinha, K.K., Bhatnagar, D., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1998; pp. 279–300. [Google Scholar]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate change on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, A.; Niquet, G. Le nettoyage des grains devrait prendre du poids. Persp. Agric. 2006, 327, 22–24. [Google Scholar]
- Battilani, P.; Barbano, C.; Piva, G. Aflatoxin B1 contamination in maize related to the aridity index in North Italy. World Mycotoxin J. 2008, 1, 449–456. [Google Scholar] [CrossRef]
- Martins, M.L.; Martins, H.M. Natural and in vitro coproduction of cyclopiazonic acid and aflatoxins. J. Food Protect. 1999, 62, 292–294. [Google Scholar] [CrossRef]
- Fernandez Pinto, V.; Patriarca, A.; Locani, O.; Vaamonde, G. Natural co-occurrence of aflatoxin and cyclopiazonic acid in peanuts grown in Argentina. Food Addit. Contam. 2001, 18, 1017–1020. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Marin, D.; Taranu, I.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. 2017, 16, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Diao, E.; Dong, H.; Hou, H.; Zhang, Z.; Ji, N.; Ma, W. Factors influencing aflatoxin contamination in before and after harvest peanuts: A review. J. Food Res. 2015, 4, 148–154. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam. 2008, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Haidukowski, M.; Stea, G.; Epifani, F.; Bandyopadhyay, R.; Leslie, J.F.; Logrieco, A. Population structure and aflatoxin production by Aspergillus sect. Flavi from maize in Nigeria and Ghana. Food Microbiol. 2014, 41, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W. Ecology and population biology of aflatoxigenic fungi in soil. In Aflatoxin and Food Safety; Abbas, H.K., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 95–116. [Google Scholar]
- Mauro, A.; Battilani, P.; Callicott, A.; Giorni, P.; Pietri, A.; Cotty, P. Structure pf an Aspergillus flavus population from maize kernels in northern Italy. Int. J. Food Microbiol. 2013, 162, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W. Biodiversity of Aspergillus section Flavi in the United states: A review. Food Addit. Contam. 2007, 24, 1088–1101. [Google Scholar] [CrossRef]
- Sarrocco, S.; Vannacci, G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop Protect. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitation. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef]
- Deudon, O.; Le Bris, X.; Piraux, F. Interest and implementation of a spatialization method of meteorological data used in Agricultural Decision Support Tools. In Proceedings of the 2017 EFITA Congress, Montpellier, France, 2–6 July 2017. [Google Scholar]
- International Organisation for Standardization. ISO 7954:1987. Microbiology, general guidance for enumeration of yeasts and moulds—Colony count technique at 25 °C; ISO, Ed.; International Organisation for Standardization: Geneva, Switzerland, 1987. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS Knaw: Uthrecht, The Netherlands, 2010. [Google Scholar]
- Pitt, J.I.; Hocking, A.D.; Glenn, D.R. An improved medium for detection of Aspergillus flavus and A. Parasiticus. J. Appl. Bacteriol. 1983, 54, 109–114. [Google Scholar] [CrossRef]
- Adjovi, Y.; Bailly, S.; Gnonlonfin, B.J.G.; Tadrist, S.; Querin, A.; Sanni, A.; Oswald, I.P.; Puel, O.; Bailly, J.D. Contrast between natural occurrence of toxigenic Aspergillii of the Flavi section and Aflatoxin B1 in cassava: Possible explanation. Food Microbiol. 2014, 38, 151–159. [Google Scholar] [CrossRef] [PubMed]
- BioEdit v7.0.5. Available online: http://www.mbio.ncsu.edu/BioEdit/page2.html (accessed on 15 November 2018).
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.2. 2017. Available online: http://mesquiteproject.org (accessed on 15 November 2018).
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1.6. 2014. Available online: http://tree.bio.ed.ac.uk/software/tracer (accessed on 15 November 2018).
- Rambaut, A. FigTree v1.4.2, A Graphical Viewer of Phylogenetic Trees. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 November 2018).
- El Mahgubi, A.; Puel, O.; Bailly, S.; Tadrist, S.; Querin, A.; Ouadia, A.; Oswald, I.P.; Bailly, J.D. Distribution and toxigenicity of Aspergillus section Flavi in spices marketed in Morocco. Food Cont. 2013, 32, 143–148. [Google Scholar] [CrossRef]
- Caceres, I.; El Khoury, R.; Medina, A.; Lippi, Y.; Naylis, C.; Atoui, A.; El Khoury, A.; Oswald, I.P.; Bailly, J.D.; Puel, O. Deciphering the anti-aflatoxinogenic properties of eugenol using a large scale q-PCR approach. Toxins 2016, 8, 123. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).