The Toxins of Nemertean Worms
Abstract
:1. Introduction
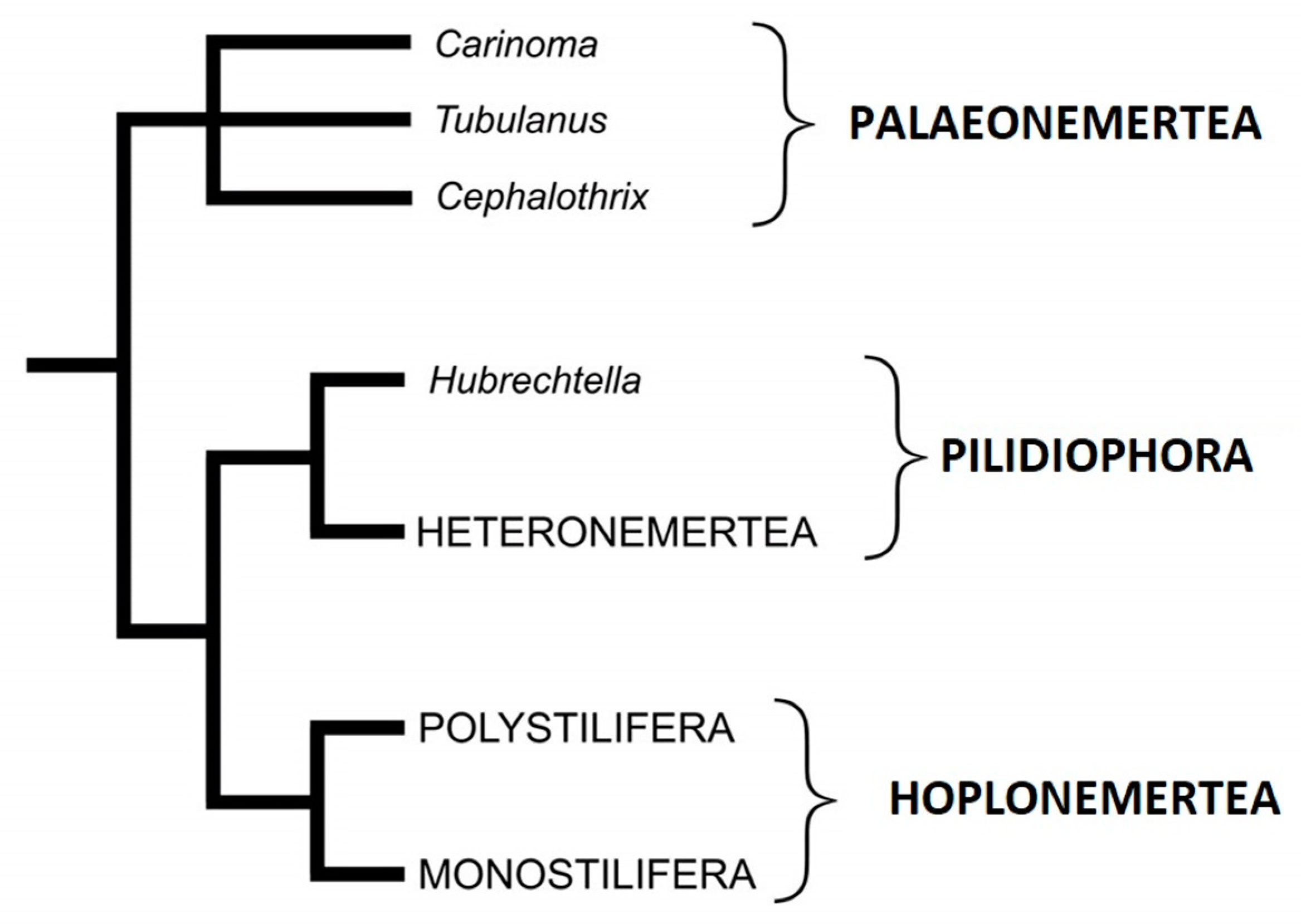
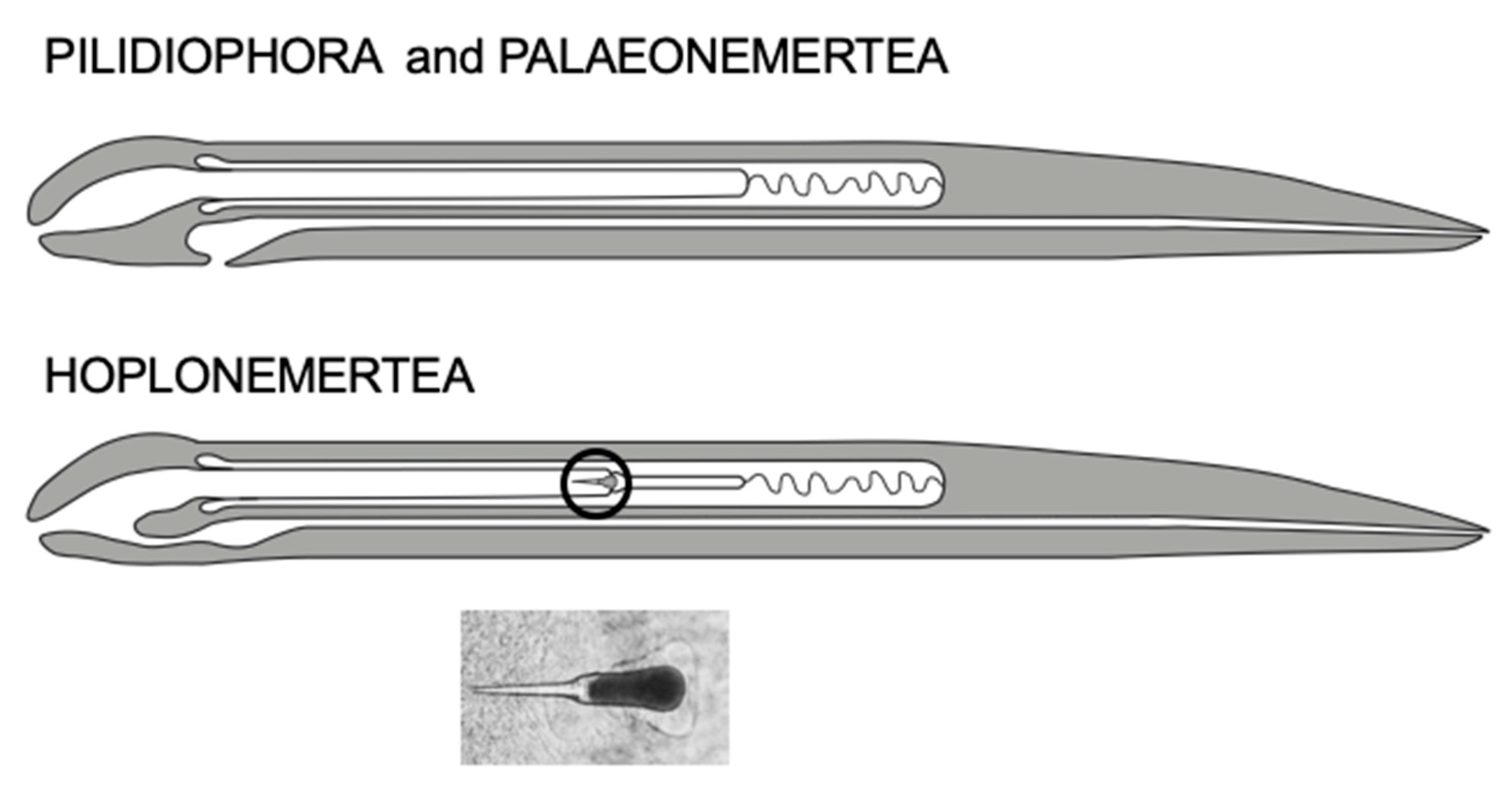
2. Taxonomy and Phylogeny
3. Anatomy
4. Early Descriptions of Ribbon Worms and Their Toxins
5. Ribbon Worm Toxins
5.1. Pyridine Alkaloids
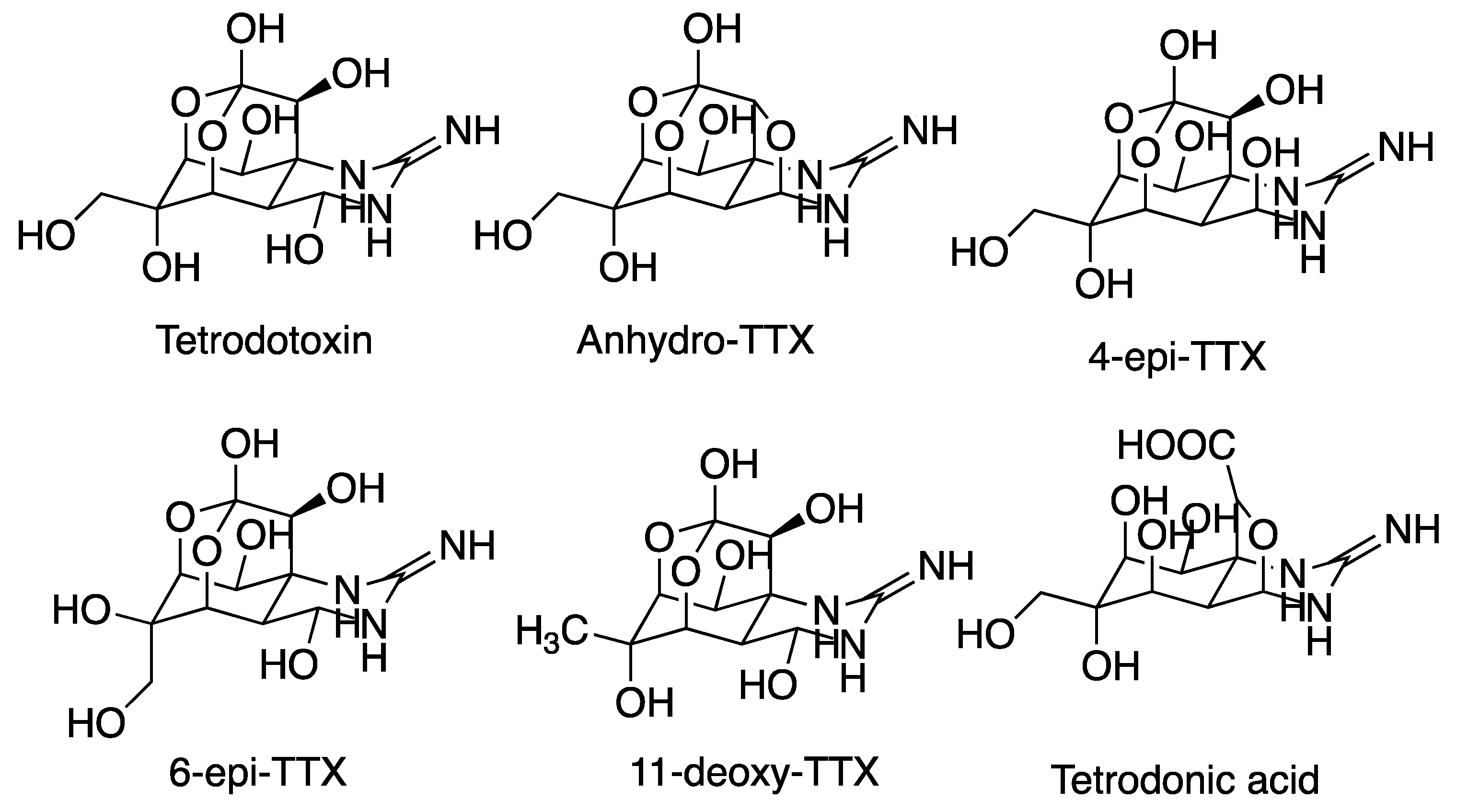
5.2. Tetrodotoxin (TTX)
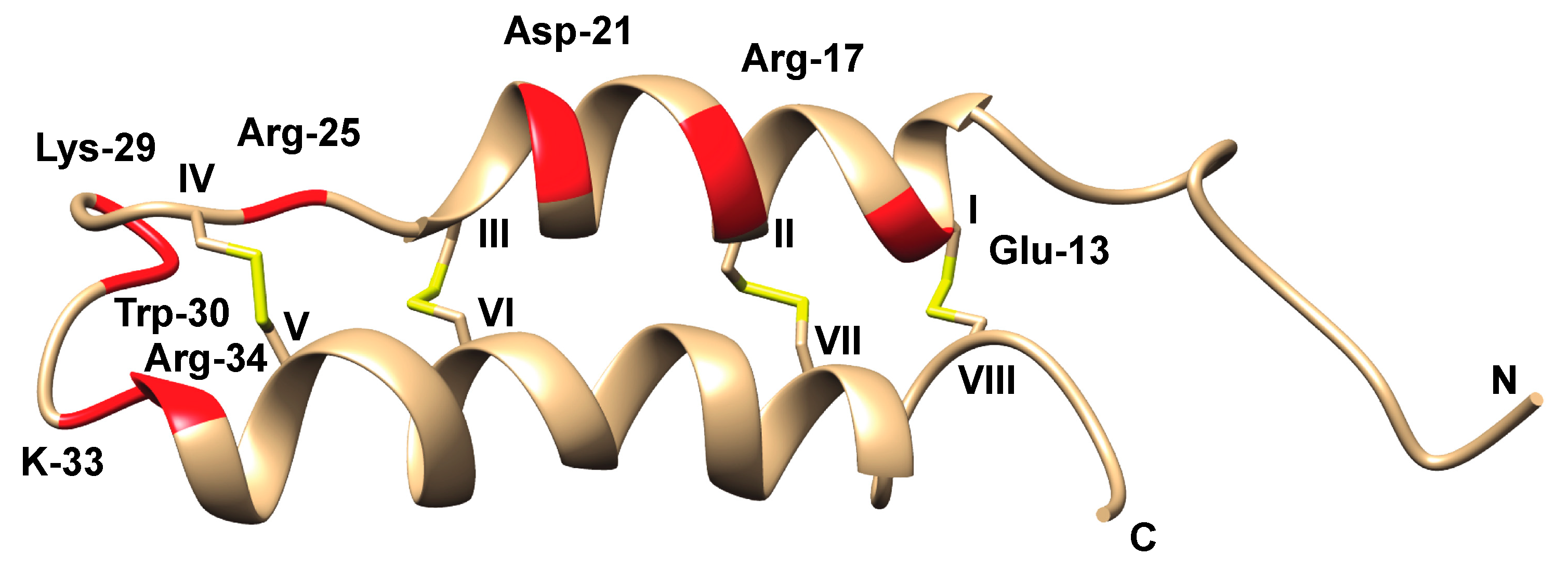
5.3. Peptide Toxins
5.3.1. Cerebratulus Toxins
B-neurotoxins
Cerebratulus A-Toxins
5.3.2. Parborlasia Toxins
5.3.3. Lineus Toxins
5.3.4. Other Peptide and Protein Toxins
6. The Ecological Roles of Nemertean Toxins
6.1. Nemerteans as Preys
6.2. Nemerteans as Predators and Scavengers
6.3. Commensal Nemerteans
7. Conclusions and Future Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Gibson, R. Nemertean genera and species of the world: an annotated checklist of original names and description citations, synonyms, current taxonomic status, habitats and recorded zoogeographic distribution. J. Nat. Hist. 1995, 29, 271–561. [Google Scholar] [CrossRef]
- Kajihara, H.; Chernyshev, A.V.; Sun, S.C.; Sundberg, P.; Crandall, F.B. Checklist of nemertean genera and species published between 1995 and 2007. Spec. Div. 2008, 13, 145–174. [Google Scholar] [CrossRef]
- Moore, J.; Gibson, R.; Jones, H.D. Terrestrial nemerteans thirty years on. Hydrobiologia 2001, 456, 1–6. [Google Scholar] [CrossRef]
- Sundberg, P.; Gibson, R. Global diversity of nemerteans (Nemertea) in freshwater. Hydrobiologia 2008, 595, 61–66. [Google Scholar] [CrossRef]
- Kem, W.R. Biochemistry of nemertine toxins. In Marine Pharmacognosy. Action of Marine Biotoxins at the Cellular Level; Martin, D., Padilla, G., Eds.; Academic Press: New York, NY, USA; pp. 38–85.
- Kem, W.R. Structure and activity of Nemertine Toxins. Am. Zool. 1985, 25, 99–111. [Google Scholar] [CrossRef]
- Kem, W.R. Chapter 57—Worm Venom Peptides. In Handbook of Biologically Active Peptides; Kastin, A.J., Ed.; Academic Press: Burlington, VT, USA, 2006; pp. 397–401. [Google Scholar]
- Kem, W.R. Nemertine Toxins. In Handbook of Neurotoxicology: Volume I; Massaro, E.J., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 573–593. [Google Scholar]
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.; Corbit, A.G.; Hayes, W.K. Poisons, toxungens, and venoms: redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef]
- McDermott, J.J. Status of the Nemertea as prey in marine ecosystems. Hydrobiologia 2001, 456, 7–20. [Google Scholar] [CrossRef]
- Thiel, M.; Kruse, I. Status of the Nemertea as predators in marine ecosystems. Hydrobiologia 2001, 456, 21–32. [Google Scholar] [CrossRef]
- Johnston, G. Miscellanea Zoologica. A description of some planarian worms. Mag. Zool. Bot. 1837, 1, 529–538. [Google Scholar]
- Strand, M.; Norenburg, J.; Alfaya, J.E.; Fernández-Álvarez, F.Á.; Andersson, H.S.; Andrade, S.C.; Bartolomeus, T.; Beckers, P.; Bigatti, G.; Cherneva, I.; et al. Nemertean taxonomy-implementing changes in the higher ranks, dismissing Anopla and Enopla. Zool. Scr. 2019, 48, 118–119. [Google Scholar] [CrossRef]
- Krämer, D.; Schmidt, C.; Podsiadlowski, L.; Beckers, P.; Horn, L.; Döhren, J. Unravelling the Lineus ruber/viridis species complex (Nemertea, Heteronemertea). Zool. Scr. 2017, 46, 111–126. [Google Scholar] [CrossRef]
- Luo, Y.-J.; Kanda, M.; Koyanagi, R.; Hisata, K.; Akiyama, T.; Sakamoto, H.; Sakamoto, T.; Satoh, N. Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads. Nature Ecol. Evol. 2018, 2, 141. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Strand, M.; Schwartz, M.; Chen, H.; Kajihara, H.; von Döhren, J.; Sun, S.; Junoy, J.; Thiel, M.; Norenburg, J.L. Disentangling ribbon worm relationships: multi-locus analysis supports traditional classification of the phylum Nemertea. Cladistics 2012, 28, 141–159. [Google Scholar] [CrossRef]
- Kem, W.R. A study of the occurrence of anabaseine in Paranemertes and other nemertines. Toxicon 1971, 9, 23–32. [Google Scholar] [CrossRef]
- Stricker, S.A.; Cloney, R.A. The stylet apparatus of the nemertean Paranemertes peregrina: Its ultrastructure and role in prey capture. Zoomorphology 1981, 97, 205–223. [Google Scholar] [CrossRef]
- Stricker, S.A.; Cloney, R.A. The ultrastructure of venom-producing cells in Paranemertes peregrina (Nemertea, Hoplonemertea). J. Morphol. 1983, 177, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, S.; Junoy, J.; Roldán, C.; García-Corrales, P. Ultrastructural study of sensory cells of the proboscidial glandular epithelium of Riseriellus occultus (Nemertea, Heteronemertea). J. Morphol. 1996, 229, 83–96. [Google Scholar] [CrossRef]
- Montalvo, S.; Roldan, C.; Junoy, J.; Garcia-Corrales, P. Ultrastructural study of two glandular systems in the proboscidial glandular epithelium of Riseriellus occultus (Nemertea, Heteronemertea). Zoomorphology 1998, 117, 247–257. [Google Scholar] [CrossRef]
- Junoy, J.; Montalvo, S.; Roldán, C.; García-Corrales, P. Ultrastructural study of the bacillary, granular and mucoid proboscidial gland cells of Riseriellus occultus (Nemertini, Heteronemertini). Acta Zool. 2000, 81, 235–242. [Google Scholar] [CrossRef]
- Turbeville, J.M. Ultrastructure of the pseudocnidae of the palaeonemerteans Cephalothrix cf. rufifrons and Carinomella lactea and an assessment of their phylogenetic utility. J. Nat. Hist. 2006, 40, 967–979. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Chernyshev, A.V.; Turbeville, J.M. Pseudocnidae of archinemerteans (nemertea, palaeonemertea) and their implications for nemertean systematics. J. Morphol. 2018, 279, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.; Hedström, M.; Seth, H.; McEvoy, E.G.; Jacobsson, E.; Goransson, U.; Andersson, H.S.; Sundberg, P. The bacterial (Vibrio alginolyticus) production of tetrodotoxin in the ribbon worm Lineus longissimus—Just a false positive? Mar. Drugs 2016, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Kem, W.R. Structure and membrane actions of a marine worm protein cytolysin, Cerebratulus toxin A-III. Toxicology 1994, 87, 189–203. [Google Scholar] [CrossRef]
- Magnus, O. 1555. Historia de gentibus septentrionalibus. Rome. Translated into Swedish 1909, 1925. [Google Scholar]
- Cedhagen, T.; Sundberg, P. A previously unrecognized report of a nemertean in the literature. Arch. Nat. Hist. 1986, 13, 7–8. [Google Scholar] [CrossRef]
- Borlase, W. The Natural History of Cornwall; Coastal: Wilmington, NC, USA, 1758. [Google Scholar]
- Wilson, C.B. The Habits and Early Development of Cerebratulus lacteus (Verrill); A contribution to physiological morphology; J. and A. Churchill Ltd.: London, UK, 1900. [Google Scholar]
- Reisinger, E. Nemertini. Biol. Tiere Deutchl. 1926, 1, 1–24. [Google Scholar]
- Bacq, Z.M. Les poisons des némertiens. Bull. Cl. Sci., Acad. Roy. Belg. 1936, 22, 1072–1079. [Google Scholar]
- Bacq, Z.M. L’“amphiporine” et la “nemertine”, poisons des vers némertiens. Arch. Int. Physiol. 1937, 44, 109–124. [Google Scholar] [CrossRef]
- King, H. Amphiporine, an active base from the marine worm Amphiporus lactifloreus. J. Chem. Soc. Lond. 1939, 1365. [Google Scholar]
- Kem, W.R.; Abbott, B.C.; Coates, R.M. Isolation and structure of a hoplonemertine toxin. Toxicon 1971, 9, 15–22. [Google Scholar] [CrossRef]
- Kem, W.R.; Scott, K.N.; Duncan, J.H. Hoplonemertine worms—A new source of pyridine neurotoxins. Experientia 1976, 32, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Cruskie, M.P.J.; Zoltewicz, J.A.; Abboud, K.A. Revised structure and convergent synthesis of nemertelline, the neurotoxic quaterpyridine isolated from the hoplonemertine sea worm. J. Org. Chem. 1995, 60, 7491–7495. [Google Scholar] [CrossRef]
- Kem, W.R. Pyridine alkaloid distribution in the hoplonemertines. Hydrobiologia 1988, 156, 145–151. [Google Scholar] [CrossRef]
- Kem, W.R.; Soti, F. Amphiporus alkaloid multiplicity implies functional diversity: initial studies on crustacean pyridyl receptors. Hydrobiologia 2001, 456, 221–231. [Google Scholar] [CrossRef]
- Kem, W.R.; Rocca, J.; Garraffo, H.M.; Spande, T.F.; Daly, J.W.; Soti, F. Synthesis and spectroscopic copmarison of the eight methyl-2,3′-bipyridyls and identification of a hoplonemertine alkaloid as 3-methyl-2,3′-bipyridyl. Heterocycles 2009, 79, 1025–1041. [Google Scholar] [CrossRef]
- Kem, W.R.; Soti, F.; Rittschof, D. Inhibition of barnacle larval settlement and crustacean toxicity of some hoplonemertine pyridyl alkaloids. Biomol. Eng. 2003, 20, 355–361. [Google Scholar] [CrossRef]
- Kem, W.R.; Soti, F.; Rittschof, D. Materials and Methods for Inhibiting Fouling of Surfaces Exposed to Aquatic Environments. U.S. Patent US730717B2, 2006. [Google Scholar]
- Meyer, E.M.; W Arendash, G.; Judkins, J.H.; Ying, L.; Wade, C.; Ken, W.R. Effects of nucleus basalis lesions on the muscarinic and nicotinic modulation of [3H] acetylcholine release in the rat cerebral cortex. J. Neurochem. 1987, 49, 1758–1762. [Google Scholar] [CrossRef]
- Kem, W.R.; Mahnir, V.M.; Papke, R.L.; Lingle, C.J. Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors. J. Pharmacol. Exp. Ther. 1997, 283, 979–992. [Google Scholar]
- Zoltewicz, J.A.; Bloom, L.B.; Kem, W.R. Quantitative determination of the ring-chain hydrolysis equilibrium constant for anabaseine and related tobacco alkaloids. J. Org. Chem. 1989, 54, 4462–4468. [Google Scholar] [CrossRef]
- Kem, W.; Soti, F.; Wildeboer, K.; LeFrancois, S.; MacDougall, K.; Wei, D.-Q.; Chou, K.-C.; Arias, H.R. The nemertine toxin anabaseine and its derivative DMXBA (GTS-21): Chemical and pharmacological properties. Mar. Drugs 2006, 4, 255–273. [Google Scholar] [CrossRef]
- Hunter, B.E.; Christopher, M.; Papke, R.L.; Kem, W.R.; Meyer, E.M. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci. Lett. 1994, 168, 130–134. [Google Scholar] [CrossRef]
- Sahakian, B.; Jones, G.; Levy, R.; Gray, J.; Warburton, D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br. J. Psychiat. 1989, 154, 797–800. [Google Scholar] [CrossRef]
- Woodruff-Pak, D.S.; Li, Y.T.; Kem, W.R. A nicotinic agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Res. 1994, 645, 309–317. [Google Scholar] [CrossRef]
- Meyer, E.M.; Tay, E.T.; Papke, R.L.; Meyers, C.; Huang, G.-L.; de Fiebre, C.M. 3-[2,4-Dimethoxybenzylidene] anabaseine (DMXB) selectively activates rat α7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997, 768, 49–56. [Google Scholar] [CrossRef]
- Zawieja, P.; Kornprobst, J.M.; Métais, P. 3-(2,4-Dimethoxybenzylidene)-anabaseine: A promising candidate drug for Alzheimer’s disease? Geriatr. Gerontol. Int. 2012, 12, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Falkenberg, M. An overview of the marine natural products in clinical trials and on the market. J. Coast. Life Med. 2015, 3, 421–428. [Google Scholar]
- Kline, J.K. Behavioral Responses of the Spiny Lobster (Panulirus argus) to Live Amphiporous Angulatus and Its Bipyridyl Toxins. Biology Senior Project Report, Kalamazoo College. 1986. Available online: http://hdl.handle.net/10920/23073 (accessed on 20 December 2012).
- Horton, T.; Kroh, A.; Bailly, N.; Boury-Esnault, N.; Brandão, S.N.; Costello, M.J.; Gofas, S.; Hernandez, F.; Mees, J.; Paulay, G.; et al. World Register of Marine Species (WoRMS). WoRMS Editorial Board. 2017. Available online: http://www.marinespecies.org (accessed on 20 December 2012).
- Hwang, D.F.; Noguchi, T. Tetrodotoxin Poisoning. In Advances in Food and Nutrition Research; Steve, L.T., Ed.; Academic Press: Amsterdam, The Netherlands, 2007; Volume 52, pp. 141–236. [Google Scholar]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef]
- Blankenship, J. Tetrodotoxin: from poison to powerful tool. Perspect. Biol. Med. 1976, 19, 509–526. [Google Scholar] [CrossRef]
- Nieto, F.R.; Cobos, E.J.; Tejada, M.Á.; Sánchez-Fernández, C.; González-Cano, R.; Cendán, C.M. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar. Drugs 2012, 10, 281–305. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 10 January 2019).
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shum, F.H.K. Method of Extracting Tetrodotoxin. U.S. Patent US6552191B1, 22 April 2003. [Google Scholar]
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874. [Google Scholar] [CrossRef]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Noguchi, T.; Ali, A.E.; Arakawa, O.; Miyazawa, K.; Kanoh, S.; Shida, Y.; Nishio, S.; Hashimoto, K. Tetrodonic acid-like substance; A possible precursor of tetrodotoxin. Toxicon 1991, 29, 845–855. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 2000, 38, 763–773. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Ito, K.; Bessho, K.; Yamaguchi, C.; Tsunetsugu, S.; Shida, Y.; Kajihara, H.; Mawatari, S.F.; Noguchi, T.; et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima Bay, Hiroshima Prefecture, Japan and the isolation of tetrodotoxin as a main component of its toxins. Toxicon 2003, 41, 747–753. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef]
- Ueda, H.; Itoi, S.; Sugita, H. TTX-bearing planocerid flatworm (Platyhelminthes: Acotylea) in the Ryukyu Islands, Japan. Mar. Drugs 2018, 16, 37. [Google Scholar] [CrossRef]
- McEvoy, E.G.; Rogers, A.; Gibson, R. Preliminary investigation of Vibrio alginolyticus-like bacteria associated with marine nemerteans. Hydrobiologia 1998, 365, 287–291. [Google Scholar] [CrossRef]
- Carroll, S.; McEvoy, E.G.; Gibson, R. The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria. J. Exp. Mar. Biol. Ecol. 2003, 288, 51–63. [Google Scholar] [CrossRef]
- Strand, M.; Sundberg, P. Sustainable Method for Synthesizing Tetrodotoxin (TTX). U.S. Patent US20110081690A1, 2011. [Google Scholar]
- Matsumura, K. Reexamination of tetrodotoxin production by bacteria. Appl. Environ. Microbiol. 1995, 61, 3468–3470. [Google Scholar] [PubMed]
- Matsumura, K. No ability to produce tetrodotoxin in bacteria. Appl. Environ. Microbiol. 2001, 67, 2393–2394. [Google Scholar] [CrossRef] [PubMed]
- Salvitti, L.; Wood, S.; McNabb, P.; Cary, S. No evidence for a culturable bacterial tetrodotoxin producer in Pleurobranchaea maculata (Gastropoda: Pleurobranchidae) and Stylochoplana sp. (Platyhelminthes: Polycladida). Toxins 2015, 7, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Salvitti, L.; Wood, S.A.; Taylor, D.I.; McNabb, P.; Cary, S.C. First identification of tetrodotoxin (TTX) in the flatworm Stylochoplana sp.; a source of TTX for the sea slug Pleurobranchaea maculata. Toxicon 2015, 95, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Beleneva, I.; Magarlamov, T.Y.; Kukhlevsky, A. Characterization, identification, and screening for tetrodotoxin production by bacteria associated with the ribbon worm (Nemertea) Cephalotrix simula (Ivata, 1952). Microbiology 2014, 83, 220–226. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Beleneva, I.A.; Chernyshev, A.V.; Kuhlevsky, A.D. Tetrodotoxin-producing Bacillus sp. from the ribbon worm (Nemertea) Cephalothrix simula (Iwata, 1952). Toxicon 2014, 85, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.I.; Beleneva, I.A.; Tyunin, A.P.; Magarlamov, T.Y. The taxonomic composition, characteristics, and neurotoxic activities of ribbon worm-associated bacteria from the Sea of Japan. Russ. J. Mar. Biol. 2017, 43, 383–391. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Arakawa, O.; Takatani, T.; Kajihara, H.; Kawatsu, K.; Hamano, Y.; Asakawa, M.; Miyazawa, K.; Noguchi, T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 2004, 44, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Magarlamov, T.Y.; Shokur, O.A.; Chernyshev, A.V. Distribution of tetrodotoxin in the ribbon worm Lineus alborostratus (Takakura, 1898)(Nemertea): Immunoelectron and immunofluorescence studies. Toxicon 2016, 112, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Vlasenko, A.; Velansky, P.; Chernyshev, A.; Kuznetsov, V.; Magarlamov, T.Y. Tetrodotoxin and its analogues profile in nemertean species from the Sea of Japan. Toxicon 2018, 156, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Min, S.K.; Yeon, S.J.; Hwang, J.H.; Hong, J.; Shin, H.S. Assessment of neuronal cell-based cytotoxicity of neurotoxins from an estuarine nemertean in the Han River Estuary. J. Microbiol. Biotechnol. 2017, 27, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Fenwick, D.; Powell, A.; Dhanji-Rapkova, M.; Ford, C.; Hatfield, R.; Santos, A.; Martinez-Urtaza, J.; Bean, T.; Baker-Austin, C. New Invasive Nemertean Species (Cephalothrix Simula) in England with high levels of tetrodotoxin and a microbiome linked to toxin metabolism. Mar. Drugs 2018, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, H.; Sun, S.C.; Chernyshev, A.V.; Chen, H.X.; Ito, K.; Asakawa, M.; Maslakova, S.A.; Norenburg, J.L.; Strand, M.; Sundberg, P.; et al. Taxonomic identity of a tetrodotoxin-accumulating ribbon-worm Cephalothrix simula (Nemertea: Palaeonemertea): A species artificially introduced from the Pacific to Europe. Zool. Sci. 2013, 30, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Lousalet, M.; Campbell, M.E.; Schwartz, M.L. Microdistribution of tetrodotoxin in three species of nemerteans. Abstract. In Proceedings of the 7th International Conference on Nemertean Biology, Santa Barbara, CA, USA, 29 June–3 July 2009. [Google Scholar]
- Campbell, M.E.; Schwartz, M.L. Immunohistological Visualization of Tetrodotoxin in Micrura Verrili and Dushia Atra (Phylum Nemertea). 2008. Available online: https://apps.cur.org/ncur2018/archive/Display_NCUR.aspx?id=10545 (accessed on 20 December 2012).
- Kem, W.R. Purification and characterization of a new family of polypeptide neurotoxins from the heteronemertine Cerebratulus lacteus (Leidy). J. Biol. Chem. 1976, 251, 4184–4192. [Google Scholar] [PubMed]
- Blumenthal, K.M.; Kem, W.R. Structure and action of heteronemertine polypeptide toxins. Primary structure of Cerebratulus lacteus toxin B-IV. J. Biol. Chem. 1976, 251, 6025–6029. [Google Scholar]
- Blumenthal, K.M.; Keim, P.S.; Heinrikson, R.L.; Kem, W.R. Structure and action of heteronemertine polypeptide toxins. Amino acid sequence of Cerebratulus lacteus toxin B-II and revised structure of toxin B-IV. J. Biol. Chem. 1981, 256, 9063–9067. [Google Scholar]
- Blumenthal, K.M.; Kem, W.R. Structure and action of heteronemertine polypeptide toxins: Inactivation of Cerebratulus lacteus toxin B-IV by tyrosine nitration. Arch. Biochem. Biophys. 1980, 203, 816–821. [Google Scholar] [CrossRef]
- Blumenthal, K.M. Structure and action of heteronemertine polypeptide toxins: inactivation of Cerebratulus lacteus toxin B-IV concomitant with tryptophan alkylation. Arch. Biochem. Biophys. 1980, 203, 822–826. [Google Scholar] [CrossRef]
- Blumenthal, K.M. Renaturation of neurotoxin B-IV from the heteronemertine Cerebratulus lacteus. Toxicon 1986, 24, 63–69. [Google Scholar] [CrossRef]
- Toth, G.P.; Blumenthal, K.M. Structure and action of heteronemertine polypeptide toxins: Binding of Cerebratulus lacteus toxin B-IV to axon membrane vesicles. Biochim. Biophys. Acta 1983, 732, 160–169. [Google Scholar] [CrossRef]
- Lieberman, D.L.; Blumenthal, K.M. Structure and action of heteronemertine polypeptide toxins. Specific cross-linking of Cerebratulus lacteus toxin B-IV to lobster axon membrane vesicles. Biochim. Biophys. Acta 1986, 855, 41–48. [Google Scholar] [CrossRef]
- Kem, W.R.; Tu, C.-K.; Williams, R.W.; Toumadje, A.; Johnson, W.C. Circular dichroism and laser Raman spectroscopic analysis of the secondary structure of Cerebratulus lacteus toxin B-IV. J. Protein Chem. 1990, 9, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E.; Kem, W.R.; Bieber, A.L.; Norton, R.S. 1H-NMR study of neurotoxin B-IV from the marine worm Cerebratulus lacteus. Solution properties, sequence-specific resonance assignments, secondary structure and global fold. Eu. J. Biochem. 1992, 210, 231–240. [Google Scholar] [CrossRef]
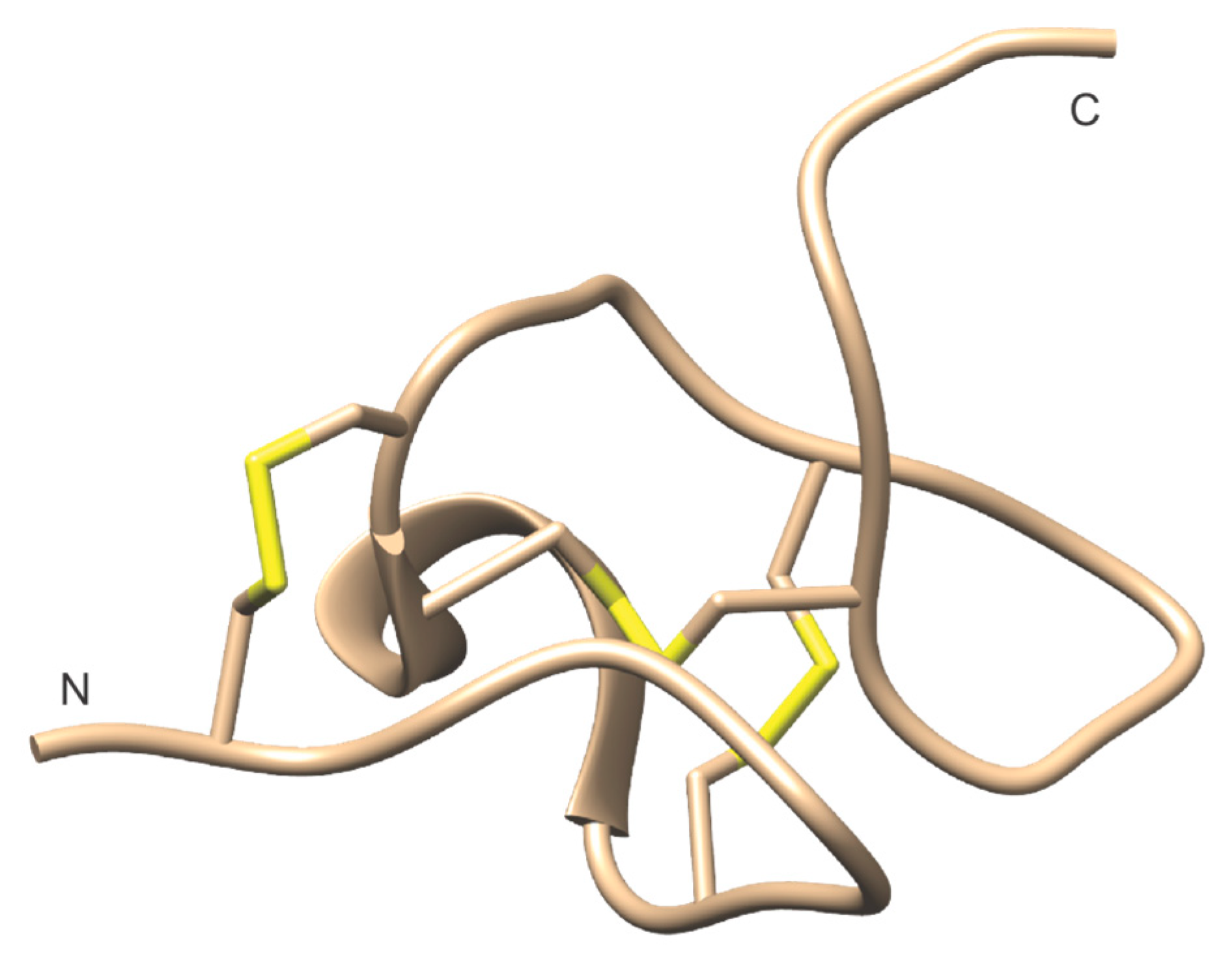
- Barnham, K.J.; Dyke, T.R.; Kem, W.R.; Norton, R.S. Structure of neurotoxin B-IV from the marine worm Cerebratulus lacteus: a helical hairpin cross-linked by disulphide bonding. J. Mol. Biol. 1997, 268, 886–902. [Google Scholar] [CrossRef]
- Howell, M.L.; Blumenthal, K.M. Cloning and expression of a synthetic gene for Cerebratulus lacteus neurotoxin B-IV. J. Biol. Chem. 1989, 264, 15268–15273. [Google Scholar]
- Howell, M.L.; Blumenthal, K.M. Mutagenesis of Cerebratulus lacteus neurotoxin B-IV identifies NH2-terminal sequences important for biological activity. J. Biol. Chem. 1991, 266, 12884–12888. [Google Scholar]
- Wen, P.H.; Blumenthal, K.M. Role of electrostatic interactions in defining the potency of neurotoxin B-IV from Cerebratulus lacteus. J. Biol. Chem. 1996, 271, 29752–29758. [Google Scholar] [CrossRef]
- Wen, P.H.; Blumenthal, K.M. Structure and function of Cerebratulus lacteus neurotoxin B-IV: tryptophan-30 is critical for function while lysines-18, -19, -29, and -33 are not required. Biochemistry 1997, 36, 13435–13440. [Google Scholar] [CrossRef]
- Kem, W.R.; Blumenthal, K.M. Purification and characterization of the cytotoxic Cerebratulus A toxins. J. Biol. Chem. 1978, 253, 5752–5757. [Google Scholar] [PubMed]
- Kuo, J.; Raynor, R.L.; Mazzei, G.J.; Schatzman, R.C.; Turner, R.S.; Kem, W.R. Cobra polypeptide cytotoxin I and marine worm polypeptide cytotoxin A-IV are potent and selective inhibitors of phospholipid-sensitive Ca2+-dependent protein kinase. FEBS Lett. 1983, 153, 183–186. [Google Scholar] [CrossRef]
- Blumenthal, K.M.; Kem, W.R. Structure and action of heteronemertine polypeptide toxins. Primary structure of Cerebratulus lacteus toxin A-III. J. Biol. Chem. 1980, 255, 8266–8272. [Google Scholar] [PubMed]
- Blumenthal, K.M. Structure and action of heteronemertine polypeptide toxins. Disulfide bonds of Cerebratulus lacteus toxin A-III. J. Biol. Chem. 1980, 255, 8273–8274. [Google Scholar] [PubMed]
- Dumont, J.A.; Blumenthal, K.M. Structure and action of heteronemertine polypeptide toxins: importance of amphipathic helix for activity of Cerebratulus lacteus toxin A-III. Arch. Biochem. Biophys. 1985, 236, 167–175. [Google Scholar] [CrossRef]
- Posner, P.; Kem, W.R. Cardiac effects of toxin A-III from the heteronemertine worm Cerebratulus lacteus (Leidy). Toxicon 1978, 16, 343–349. [Google Scholar] [CrossRef]
- Blumenthal, K.M. Structure and action of heteronemertine polypeptide toxins. Membrane penetration by Cerebratulus lacteus toxin A-III. Biochemistry 1982, 21, 4229–4233. [Google Scholar] [CrossRef]
- Blumenthal, K.M. Release of liposomal markers by Cerebratulus toxin A-III. Biochem. Biophys. Res. Commun. 1984, 121, 14–18. [Google Scholar] [CrossRef]
- Blumenthal, K.M. Binding of Cerebratulus cytolysin A-III to human erythrocyte membranes. Biochim. Biophys. Acta 1985, 812, 127–132. [Google Scholar] [CrossRef]
- Liu, J.W.; Blumenthal, K.M. Membrane damage by Cerebratulus lacteus cytolysin A-III. Effects of monovalent and divalent cations on A-III hemolytic activity. Biochim. Biophys. Acta 1988, 937, 153–160. [Google Scholar] [CrossRef]
- Liu, J.W.; Blumenthal, K.M. Functional interaction between Cerebratulus lacteus cytolysin A-III and phospholipase A2. Implications for the mechanism of cytolysis. J. Biol. Chem. 1988, 263, 6619–6624. [Google Scholar] [PubMed]
- Liu, J.; Blumenthal, K.M. Identification of oleic acid binding sites in cytolysin A-III from the heteronemertine Cerebratulus lacteus. Toxicon 1991, 29, 13–20. [Google Scholar] [CrossRef]
- Heine, J.; McClintock, J.; Slattery, M.; Weston, J. Energetic composition, biomass, and chemical defense in the common Antarctic nemertean Parborlasia corrugatus (McIntosh). J. Exp. Mar. Biol. Ecol. 1991, 153, 15–25. [Google Scholar] [CrossRef]
- McClintock, J.B.; Baker, B.J. A review of the chemical ecology of Antarctic marine invertebrates. Am. Zool. 1997, 37, 329–342. [Google Scholar] [CrossRef]
- Berne, S.; Sepcic, K.; Krizaj, I.; Kem, W.R.; McClintock, J.B.; Turk, T. Isolation and characterisation of a cytolytic protein from mucus secretions of the Antarctic heteronemertine Parborlasia corrugatus. Toxicon 2003, 41, 483–491. [Google Scholar] [CrossRef]
- Butala, M.; Sega, D.; Tomc, B.; Podlesek, Z.; Kem, W.R.; Kupper, F.C.; Turk, T. Recombinant expression and predicted structure of parborlysin, a cytolytic protein from the Antarctic heteronemertine Parborlasia corrugatus. Toxicon 2015, 108, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, E.; Andersson, H.S.; Strand, M.; Peigneur, S.; Eriksson, C.; Lodén, H.; Shariatgorji, M.; Andren, P.E.; Lebbe, E.; Rosengren, K.J.; Tytgat, J.; Göransson, U. Peptide ion channel toxins from the bootlace worm, the longest animal on Earth. Sci. Rep. 2018, 8, 4596. [Google Scholar] [CrossRef]
- Rogers, J.C.; Qu, Y.; Tanada, T.N.; Scheuer, T.; Catterall, W.A. Molecular determinants of high affinity binding of α-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel α subunit. J. Biol. Chem. 1996, 271, 15950–15962. [Google Scholar] [CrossRef]
- Göransson, U.; Rosengren, K.J.; Jacobsson, E.; Andersson, H.S.; Strand, M. Nemertea-Derived Bioactive Compounds. 2018. [Google Scholar]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Whelan, N.V.; Kocot, K.M.; Santos, S.R.; Halanych, K.M. Nemertean toxin genes revealed through transcriptome sequencing. Genome Biol. Evol. 2014, 6, 3314–3325. [Google Scholar] [CrossRef]
- Shiomi, K.; Midorikawa, S.; Ishida, M.; Nagashima, Y.; Nagai, H. Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon 2004, 44, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Toxins and drug discovery. Toxicon 2014, 92, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, A.; Nagai, H.; Ishida, M.; Nagashima, Y.; Shiomi, K. Purification and molecular cloning of SE-cephalotoxin, a novel proteinaceous toxin from the posterior salivary gland of cuttlefish Sepia esculenta. Toxicon 2008, 52, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Liu, X.; Pang, Y.; Yu, T.; Xiao, R.; Jin, M.; Han, Y.; Su, P.; Wang, J.; Lv, L. Characterization, phylogenetic analysis and cDNA cloning of natterin-like gene from the blood of lamprey, Lampetra japonica. Immunol. Lett. 2012, 148, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosado, C.J.; Kondos, S.; Bull, T.E.; Kuiper, M.J.; Law, R.H.; Buckle, A.M.; Voskoboinik, I.; Bird, P.I.; Trapani, J.A.; Whisstock, J.C. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 2008, 10, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Riesgo, A.; Andrade, S.C.; Sharma, P.P.; Novo, M.; Perez-Porro, A.R.; Vahtera, V.; Gonzalez, V.L.; Kawauchi, G.Y.; Giribet, G. Comparative description of ten transcriptomes of newly sequenced invertebrates and efficiency estimation of genomic sampling in non-model taxa. Front. Zool. 2012, 9, 33. [Google Scholar] [CrossRef]
- Romiguier, J.; Gayral, P.; Ballenghien, M.; Bernard, A.; Cahais, V.; Chenuil, A.; Chiari, Y.; Dernat, R.; Duret, L.; Faivre, N.; et al. Comparative population genomics in animals uncovers the determinants of genetic diversity. Nature 2014, 515, 261–263. [Google Scholar] [CrossRef]
- Andrade, S.C.; Montenegro, H.; Strand, M.; Schwartz, M.L.; Kajihara, H.; Norenburg, J.L.; Turbeville, J.M.; Sundberg, P.; Giribet, G. A transcriptomic approach to ribbon worm systematics (nemertea): resolving the Pilidiophora problem. Mol. Biol. Evol. 2014, 31, 3206–3215. [Google Scholar] [CrossRef]
- MacKenzie, C.L., Jr. History of the fisheries of Raritan Bay, New York and New Jersey. Mar. Fish. Rev. 1990, 52, 1–45. [Google Scholar]
- McDermott, J.J.; Roe, P. Food, feeding behavior and feeding ecology of nemerteans. Am. Zool. 1985, 113–125. [Google Scholar] [CrossRef]
- Napier, V.; Turpie, J.; Clark, B. Value and management of the subsistence fishery at Knysna Estuary, South Africa. Afr. J. Mar. Sci. 2009, 31, 297–310. [Google Scholar] [CrossRef]
- Jennings, J.; Gibson, R. Observations on the nutrition of seven species of Rhynchocoelan worms. Biol. Bull. 1969, 136, 405–433. [Google Scholar] [CrossRef]
- Moles, J.; Núñez-Pons, L.; Taboada, S.; Figuerola, B.; Cristobo, J.; Avila, C. Anti-predatory chemical defences in Antarctic benthic fauna. Mar. Biol. 2015, 162, 1813–1821. [Google Scholar] [CrossRef]
- Kruse, I.; Buhs, F. Preying at the edge of the sea: the nemertine Tetrastemma melanocephalum and its amphipod prey on high intertidal sandflats. Hydrobiologia 2000, 426, 43–55. [Google Scholar] [CrossRef]
- Caplins, S.A.; Turbeville, J.M. The occurrence of Ramphogordius sanguineus (Nemertea, Heteronemertea) in the intertidal zone of the Atlantic coast of Virginia and new observations on its feeding behavior. Banisteria 2011, 38, 65–70. [Google Scholar]
- Bourque, D.; Miron, G.; Landry, T. Predator prey relationship between the nemertean Cerebratulus lacteus and the soft-shell clam, Mya arenaria: surface-exploration activity and qualitative observations on feeding behaviour. Can. J. Zool. 2002, 80, 1204–1211. [Google Scholar] [CrossRef]
- Beckers, P.; Bartolomaeus, T.; von Döhren, J. Observations and experiments on the biology and life history of Riseriellus occultus (Heteronemertea: Lineidae). Zool. Sci. 2015, 32, 531–546. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.J. Nemerteans as hosts for symbionts: a review. J. Nat. Hist. 2006, 40, 1007–1020. [Google Scholar] [CrossRef]
- Jensen, K.; Sadeghian, P.S. Nemerteans (ribbon worms). In Marine parasitology; Rohde, K., Ed.; CSIRO publishing: Clayton, Australia, 2015. [Google Scholar]
- Gibson, R. Studies on the biology of the entocommensal rhynchocoelan Malacobdella grossa. J. Mar. Biol. Assoc. UK 1968, 48, 637–656. [Google Scholar] [CrossRef]
- Roe, P. Laboratory studies of feeding and mating in species of Carcinonemertes (Nemertea: Hoplonemertea). Biol. Bull. 1984, 167, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Kuris, A.M.; Wickham, D.E. Effect of nemertean egg predators on crustaceans. Bul. Mar. Sci. 1987, 41, 151–164. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinch, M.S.; Haynesworth, A.; Kinch, S.L.; Hoyer, D. An overview of FDA-approved new molecular entities: 1827–2013. Drug Discov. Today 2014, 19, 1033–1039. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, M.; De Pascale, D.; Andersen, J.H.; Reyes, F.; Crawford, A.D.; Ianora, A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioall. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Moczydlowski, E.G. The molecular mystique of tetrodotoxin. Toxicon 2013, 63, 165–183. [Google Scholar] [CrossRef]





| Compound | Barnacle Larvae Settlement Inhibition IC50 (μM) | Barnacle Larvae Median Lethal Concentration IL50 (μM) | Crayfish Acute Paralytic Dose PD50 (μg) |
|---|---|---|---|
| 2,3′-bipyridyl | 4.1 (3.2–5.3) a | 1.9 (1.0–4.3) | 0.88 (0.71–1.1) |
| Anabaseine | 1.2 (0.91–1.7) | 2 | 3.6 (3.1–4.1) |
| Nemertelline | 3.2 (1.8–6.0) | - | >120 |
| Anabasine | 3.0 (1.5–4.9) | - | 3.9 (3.4–4.5) |
| Species | Origin | Toxin | Sample | Extraction and Analysis | Source |
|---|---|---|---|---|---|
| Class Hoplonemertea | Order Monostilifera | ||||
| Amphiporus angulatus | NH + ME shores, USA | Anabaseine | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Amphiporus angulatus | NH + ME shores, USA | Anabaseine | Whole body | Chromatogr - alkal CHCl3 extr | [36] |
| Amphiporus angulatus | NH + ME shores, USA | Nemertelline | Whole body | Chromatogr - alkal CHCl3 extr | [36] |
| Amphiporus angulatus | NH + ME shores, USA | 2,3′-bipyridyl | Whole body | Chromatogr - alkal CHCl3 extr | [36] |
| Amphiporus angulatus | NH + ME shores, USA | 3-methyl-2,3′-bipyridyl | Whole body | Chromatogr - alkal CHCl3 extr | [36,40] |
| Amphiporus angulatus | Not stated | Bipyridyl toxins | Live animal | Not stated | [53] |
| Amphiporus angulatus | Eastport, MA, USA. | 2,3′-bipyridyl, nemertelline + 4 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus bimaculatus | San Juan Island, Washington, USA. | Unidentified pyridyl | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus cruentatus | Woods hole, MA, USA. | 5 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus formidabilis | San Juan Island, Washington, USA. | 3 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus lactifloreus | Not stated | Low anabaseine activity | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [17] |
| Amphiporus lactifloreus | Not stated | Low anabaseine activity | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Amphiporus lactifloreus | Bangor, Wales, UK | Anabasine | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus ochraceus | Not stated | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Amphiporus ochraceus | Woods hole, MA, USA | 5 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Argonemertes dendyi | Tomales bay, CA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Carcinonemertes errans | Bodega bay, CA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Emplectonema gracile | San Juan Island, WA, USA | Possibly 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Emplectonema gracile | San Juan Island, WA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Emplectonema neesi | Bangor, Wales, UK | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Fasciculonemertes arenicola | Los Molles, Chile | 5 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Geonemertes pelaensis | Miami, FL, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Malacobdella grossa | Not stated | None found | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Nipponnemertes pulchra a | Helsingør, Dk | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Whole body | Al2O3 chrom-alkal CHCl3 extr. | [35] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Body w.o. proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Anterior proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Median proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Posterior proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Periph part body wall | MS + UV - DMAB deriv | [17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Periph part body wall | MS + UV - DMAB deriv | [5] |
| Paranemertes peregrina | San Juan Island, WA, USA | Low anabaseine | Body core tissues | MS + UV - DMAB deriv | [17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Low anabaseine | Body core tissues | MS + UV - DMAB deriv | [5] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Paranemertes peregrina | Bodega Bay, CA, USA | 2 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Prosadenoporus californiensis b | Tomales Bay, CA, USA | 2 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Prostoma graecense c | Not stated | Possibly anabaseine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Tetrastemma candidum | Woods hole, CA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Tetrastemma reticulatum | San Juan Island, WA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Tetrastemma worki | Not stated | Anabaseine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Zygonemertes virescens | Not stated | None found | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Zygonemertes virescens | Woods hole, MA, USA | Anabasine | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Class Palaeonemertea | |||||
| Carinoma sp. | Not stated | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Carinoma tremaphoros d | Woods hole, MA, USA. | Not reported | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Cephalothrix spiralis d,e | Not stated | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Class Pilidiophora | Order Heteronemertea | ||||
| Cerebratulus lacteus | Woods hole, MA, USA. | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Cerebratulus lacteus | Boston, MA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Lineus ruber | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [17] |
| Lineus ruber | NH + ME shores, USA | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Lineus sanguineus f | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Lineus viridis | Not stated | Anabaseine/Nemertine | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [17] |
| Lineus viridis | NH + ME shores, USA | Anabaseine/Nemertine | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Lineus viridis | Woods hole, MA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Micrura leidyi | Not stated | Anabaseine/Nemertine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [17] |
| Micrura leidyi | Woods hole, MA, USA | None found | Whole body Frozen | TLC - alkal CHCl3 extr | [38] |
| Micrura leidyi | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Parvicirrus dubius g | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Siphonenteron bicolour h | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Siphonenteron bicolour h | Woods hole, MA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Species | Origin | Toxin/-s | Sample | Extraction and Analysis | Ref |
|---|---|---|---|---|---|
| Class Hoplonemertea | Order Monostilifera | ||||
| Amphiporus lactifloreus | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Amphiporus sp. | Akkeshi Bay, Hokkaido, Jpn | TTX + analogs | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS-C9 base | [69] |
| Nipponnemertes bimaculata a | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Malacobdella japonica | Akkeshi Bay, Hokkaido, Jpn | TTX + analogs Anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Malacobdella grossa | Peter the Great Bay, Rus/Jpn | Antimicrobial activity. No TTX. | Bacteria - whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
| Nemertellina yamaokai | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Nipponnemertes punctatula | Otsuchi Bay, Iwate, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Paranemertes sp. | Peter the Great Bay, Rus/Jpn | None found | Acidic methanol extract | HPLC-MS/MS | [83] |
| Quasitetrastemma nigrifrons b | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Quasitetrastemma stimpsoni c | Akkeshi Bay, Hokkaido, Jpn | (TTX and analogues) Not analyzed | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Quasitetrastemma stimpsoni c | Peter the Great Bay, Rus/Jpn | Bact. cultures Antimicrob activity. TTX. | Bacteria from whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
| Quasitetrastemma stimpsoni | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic methanol extract | HPLC-MS/MS | [83] |
| Class Palaeonemertea | |||||
| Cephalothrix linearis | Shimoda, Shizuoka, Jpn | TDA-like, TTX, anhydro-, epi-TTX | Proboscis, body | SEC, TLC, el.phoresis, HPLC, GC-C9 base | [65] |
| Cephalothrix linearis | Shimoda, Shizuoka, Jpn | TDA-like | Handling stimulus secretion | SEC, TLC, el.phoresis, HPLC, GC-C9 base | [65,66] |
| Cephalothrix rufifrons | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Cephalotrix rufifrons d | Cornwall, UK | None in extract, but TTX in bacteria isolate | Acidic whole body extract and bacteria isolates | HPLC-MS/MS of extract and isolates | [85] |
| Cephalothrix simula | Hiroshima Bay, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS-C9 base | [69] |
| Cephalothrix simula | Akkeshi Bay, Hokkaido, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS-C9 base | [69] |
| Cephalothrix simula | Otsuchi Bay, Iwate, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS- C9 base | [69] |
| Cephalothrix simula e | Peter the Great Bay, Rus/Jpn | TTX-Bacillus sp. | Bacteria isolates | Immunovisualization | [78,79] |
| Cephalothrix simula | Peter the Great Bay, Rus/Jpn | 7 TTX derivatives | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Cephalotrix simula | Cornwall, UK | TTX and derivatives | Acidic whole body extract and bacteria isolates | HPLC-MS/MS of extract and isolates | [85] |
| Cephalothrix sp. | Hiroshima Bay, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, SEC, IEC. TLC, HPLC, GCMS-C9 base | [67] |
| Cephalothrix sp. | Hiroshima Bay, Jpn | TTX, epi-, anhydro-TTX | Whole body, frozen | Activated charcoal, SEC, IEC, cryst from acidic CH3OH soln, GCMS-C9 base, NMR + MS | [68] |
| Cephalothrix sp. | Hiroshima Bay, Jpn | TTX | Whole body, cross-section | Anti-TTX antibodies | [81] |
| Tubulanus annulatus | Cornwall, UK | None found | Acidic whole body extract and bacteria isolates | HPLC-MS/MS of extract and isolates | [85] |
| Tubulanus polymorphus f | Not stated | TTX | Not stated | Immunostaining | [87] |
| Tubulanus punctatus | Seto Inland sea Hiroshima, Jpn | Anhydro-TTX | Whole body | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [64] |
| Tubulanus punctatus | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Class Pilidiophora | Order Heteronemertea | ||||
| Cerebratulus marginatus | Peter the Great Bay, Rus/Jpn | None found | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Dushia atra f | Not stated | TTX | Not stated | Immunostaining | [87,88] |
| Kulikovia alborostrata | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Kulikovia manchenkoi | Peter the Great Bay, Rus/Jpn | TTX + 3 deriv | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Lineus alborostratus | Akkeshi Bay, Hokkaido, Jpn | TTX, anhydro-, epi-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Lineus alborostratus | Peter the Great Bay, Rus/Jpn | TTX | Whole body | Immunostaining | [82] |
| Lineus alborostratus | Peter the Great Bay, Rus/Jpn | Bact cultured for TTX. Antimicrob activity. | Bacteria from whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
| Lineus bilineatus | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Lineus fuscoviridis | Seto Inland sea, Hiroshima, Jpn | TTX, anhydro-TTX | Whole body | Defatted, SEC, IEC. TLC, HPLC., GC-MS-C9 base | [64] |
| Lineus longissimus | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract and mucus | UV spectroscopy and HPLC | [72] |
| Lineus longissimus | Koster Fiord, Swe, and Millport, Scot, UK | <5 kDa cpd | Mucus + Vibrio cultures | Various purification methods | [25] |
| Lineus ruber | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Lineus sanguineus g | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Lineus torquatus | Akkeshi Bay, Hokkaido, Jpn | TTX, anhydro-, epi-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Lineus viridis | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Micrura akkeshiensis | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Micrura bella | Peter the Great Bay, Rus/Jpn | None found | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Micrura verrilli f | Not stated | TTX | Not stated | Immunostaining | [87,88] |
| Nipponomicrura uchidai | Peter the Great Bay, Rus/Jpn | None found | Acidic methanol extract | HPLC-MS/MS | [83] |
| Riseriellus occultus | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Yininemertes pratensis | Haengjunaru, Han river Estuary, South Korea | TTX + analogs, derivatives. Tox cpd mass 291.1 | EtOH extract | Dry homogenate lysis in pure EtOH, hydrophobic HPLC, MALDI-TOF | [84] |
| Class Pilidiophora | Genus Hubrechtella | ||||
| Hubrechtella juliae | Peter the Great Bay, Rus/Jpn | Bact cultured for TTX. Antimicrob activity. | Bacteria from whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
| Residue | AA | Modification | Effect | Source |
|---|---|---|---|---|
| 1 | Ala | Extra Met-1 | 35-40% of native | [100] |
| 3 | Ala | Ser (+8 Ser) | x2 activity | [101] |
| Gly (+8 Gly) | Slightly less active | [101] | ||
| 5 | Trp | HNB a w Trp 30, 1 eq. | Slightly less active | [92] |
| HNB w Trp 30, 2 eq. | Inactive | [93] | ||
| 8 | Ala | Ser (+8 Ser) | x2 activity | [101] |
| Gly (+8 Gly) | Slightly less active | [101] | ||
| 9 | Tyr | Nitration | Inactive | [92] |
| 10 | Hyp | Pro | Active | [101] |
| 12 | Cys | Reduction (all Cys) | Inactive | [94] |
| 13 | Glu | Gly | Active | [102] |
| Ala | Active | [102] | ||
| Gln | Active | [102] | ||
| 16 | Cys | Reduction (all Cys) | Inactive | [94] |
| 17 | Arg | Gln | Inactive | [102] |
| Ala | Inactive | [102] | ||
| Lys | Inactive | [102] | ||
| 18 | Lys | Gln | Active | [103] |
| Gln + Gln 19 | Slightly less active | [103] | ||
| 19 | Lys | Gln + Gln 18 | Slightly less active | [103] |
| 21 | Asp | Ala | Active | [102] |
| Asn | Active | [102] | ||
| Pro | 75% helix reduction. 10-fold reduction in activity | [102] | ||
| 22 | Leu | Asp | Active | [103] |
| 23 | Cys | Reduction (all Cys) | Inactive | [94] |
| 25 | Arg | Gln | 400-fold reduction | [102] |
| Lys | Slightly less active | [102] | ||
| 26 | Cys | Reduction (all Cys) | Inactive | [94] |
| 29 | Lys | Asn | Active | [103] |
| 30 | Trp | HNB | Inactive | [93] |
| Ser | 40-fold reduction | [103] | ||
| Tyr | Active | [103] | ||
| Phe | Active | [103] | ||
| HNB with Trp 5, 1 eq. | Slightly less active | [93] | ||
| HNB with Trp 5, 2 eq. | Inactive | [93] | ||
| 33 | Lys | Asn | Active | [103] |
| 34 | Arg | Gln | 20-fold reduction. Structure destabilized. | [102] |
| Ala | 80-fold reduction | [102] | ||
| 37 | Cys | Reduction (all Cys) | Inactive | [94] |
| 41 | Cys | Reduction (all Cys) | Inactive | [94] |
| 48 | Cys | Reduction (all Cys) | Inactive | [94] |
| 52 | Cys | Reduction (all Cys) | Inactive | [94] |
| 53 | Lys | Truncation | Active | [102] |
| 54 | Lys | Truncation | Active | [102] |
| 55 | Glu | Truncation | Active | [102] |
| ID | Toxin | Species | Geographic Origin | Function | Ref |
|---|---|---|---|---|---|
| Pore-forming/hemolytic | |||||
| - | Cytotoxin A-I a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [5,104] |
| - | Cytotoxin A-II a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [5,104] |
| P01527 | Cytotoxin A-III a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [5,104] |
| - | Cytotoxin A-IV a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [104] |
| 6ENA b | Nemertide α-1 | Lineus longissimus | Koster Fiord, Swe | VGSC activator, paralytic | [120] |
| - | Nemertide α-2 | Lineus longissimus | Koster Fiord, Swe | Likely VGSC activator, paralytic | [120] |
| - | Nemertide β-1 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog. Likely paralytic. | [120] |
| - | ”Nemertine” | Lineus ruber | Possible nemertide | [5] | |
| - | ”Nemertine” | Tenuilineus bicolor f | Not stated | Possible nemertide | [5] |
| - | ”Nemertine” | Lineus viridis | Not stated | Possible nemertide | [5] |
| - | <5 kDa component | Lineus longissimus | Koster Fiord, Swe, and Millport Scot, UK | Paralytic. Likely α-nemertide | [25] |
| - | Neurotoxin B-I c | Cerebratulus lacteus | Long Island Sound, NY, USA | Likely paralytic | [89] |
| P01526 | Neurotoxin B-II c | Cerebratulus lacteus | Long Island Sound, NY, USA | Paralytic | [89] |
| P01526 | Neurotoxin B-II | Cerebratulus lacteus | Woods hole, MA, USA | Paralytic | [104] |
| - | Neurotoxin B-III c | Cerebratulus lacteus | Long Island Sound, NY, USA | Likely paralytic | [89] |
| P01525 | Neurotoxin B-IV | Cerebratulus lacteus | Woods hole, MA, USA | Paralytic | [104] |
| ID | Toxin Gene Homolog | Species | Geographic Origin | Proposed Function | Ref |
|---|---|---|---|---|---|
| Pore-forming/hemolytic | |||||
| P01527 | Cytotoxin A-III | Cerebratulus marginatus | San Juan Island, WA, USA [130] | Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Lineus longissimus | Erdeven + Roscoff, Fra [131] | Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Lineus ruber | Roscoff, Wimereux, Fra [131] | Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Pore-forming; hemolytic | [15] |
| Q54316 | Hemolysin B | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hemolytic | [15] |
| P54176 | Hemolysin-3 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hemolytic | [15] |
| A0A0N7HUN6 | Parborlysin-4 | Parborlasia corrugatus | Adelaide Island, Antarctica | Likely pore-forming, hemolytic | [119] |
| A0A0P0CC97 | Parborlysin-5 | Parborlasia corrugatus | Adelaide Island, Antarctica | Likely pore-forming, hemolytic | [119] |
| A0A0P0BUQ6 | Parborlysin-6 | Parborlasia corrugatus | Adelaide Island, Antarctica | Liekely pore-forming, hemolytic | [119] |
| A0A0P0CHY3 | Parborlysin-7 | Parborlasia corrugatus | Adelaide Island, Antarctica | Likely pore-forming, hemolytic | [119] |
| - | Parborlysin/cytotoxin homolog 1 (Locus_9778) | Cerebratulus marginatus | San Juan Island, WA, USA [124,130,132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 2 ( Locus_9778) | Cerebratulus marginatus | San Juan Island, WA, USA [124,130,132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 3 (Locus_40830) | Hubrechtella ijimai | Hamanko, Honshu, Jpn [132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 4 (comp17199) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 5 (comp55821) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 6 (Contig1463) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 7 (Comp16298) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 8 (Comp9226) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 9 (contig46055) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 10 (Comp45258) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 11 (comp48) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 12 (comp21702) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 13 (contig49129) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 14 (comp17823/17-134) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 15 (contig31748) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 16 (comp17823/17-147) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 17 (comp17823/17-145) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 17 (contig56815) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 18 (comp52392) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 19 (contig63996) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 20 (contig3234) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 21 (comp40150/seq2) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 22 (comp40150/seq2) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 23 (contig21527) | Lineus sanguineus | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 24 (contig61445) | Lineus sanguineus | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 25 (contig2073) | Ramphogordius pseudolacteus b | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 26 (contig6541) | Ramphogordius pseudolacteus b | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 27 (Locus_8475) | Riseriellus occultus | Liverpool, UK [132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 28 (Locus_39410) | Riseriellus occultus | Liverpool, UK [132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 29 (Locus_13571) | Riseriellus occultus | Liverpool, UK [132] | Likely pore-forming | [120] |
| Q76CA2 | Echotoxin-2 | Cephalothrix hongkongiensis | Akkeshi, Hokkaido, Jpn [130] | Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Cephalothrix linearis | Not stated | Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Cerebratulus marginatus | San Juan Island, WA, USA [130] | Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Tubulanus polymorphus | San Juan Island, WA, USA | Pore forming, hemolytic | [124] |
| Q66SO3 | Galactose-specific lectin nattectin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Ca2+-dependent hemagglutination activity | [15] |
| A0ZSK3 | Neoverrucotoxin subunit alpha | Cerebratulus marginatus | San Juan Island, WA, USA [130] | SNTX/VTX toxin; hemolytic activity | [124] |
| A0ZSK3 | Neoverrucotoxin subunit alpha | Paranemertes peregrina | San Juan Island, WA, USA | SNTX/VTX toxin; hemolytic activity | [124] |
| A0ZSK4 | Neoverrucotoxin subunit beta c | Lineus longissimus | Erdeven + Roscoff, Fra [131] | SNTX/VTX toxin; hemolytic activity | [124] |
| A0ZSK4 | Neoverrucotoxin subunit beta | Malacobdella grossa | Rhode Island, USA | SNTX/VTX toxin; hemolytic activity | [124] |
| Q98989 | Stonustoxin | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | SNTX/VTX toxin; pore-forming, hemolytic | [124] |
| Q98989 | Stonustoxin | Lineus ruber | Roscoff, Wimereux, Fra [131] | SNTX/VTX toxin; pore-forming, hemolytic | [124] |
| Q91453 | Stonustoxin subunit beta | Tubulanus polymorphus | San Juan Island, WA, USA | SNTX/VTX toxin; pore-forming, hemolytic | [124] |
| P58912 | Toxin PsTX-60B | Cephalothrix hongkongiensis | Akkeshi, Hokkaido, Jpn [130] | MACPF toxin domain; hemolytic | [124] |
| P58912 | Toxin PsTX-60B | Cephalothrix linearis | Not stated | MACPF toxin domain; hemolytic | [124] |
| Q76DT2 | Toxin AvTX-60A | Cephalothrix linearis | Not stated | MACPF toxin domain; hemolytic | [124] |
| Neurotoxins/Acting on ion-channels | |||||
| Q92035 | Acetylcholinesterase | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Synaptic cleft hydrolysis of acetylcholine | [15] |
| P05486 | Conophysin-conopressin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Targets vasopressin-oxytocin related receptors | [15] |
| 6ENA d | Nemertide α-1 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | VGSC activator, paralytic | [120] |
| 6ENA d | Nemertide α-1 | Lineus longissimus | Koster Fiord, Swe | VGSC activator, paralytic | [120] |
| 6ENA d | Nemertide α-1 | Lineus ruber | Roscoff, Wimereux, Fra [131] | VGSC activator, paralytic | [120] |
| - | Nemertide α-2 | Lineus longissimus | Koster Fiord, Swe | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-2 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-3 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-3 | Ramphogordius pseudolacteus b | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-4 | Lineus sanguineus | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-5 | Ramphogordius pseudolacteus b | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-6 | Lineus sanguineus | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-7 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-8 | Riseriellus occultus | Liverpool, UK [132] | Likely VGSC activator, paralytic; incomplete sequence | [120] |
| - | Nemertide β-1 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-1 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-2 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-2 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-3 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-4 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-5 | Ramphogordius pseudolacteus b | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-6 | Ramphogordius pseudolacteus b | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-7 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-8 | Lineus sanguineus | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-9 | Lineus sanguineus | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| Q09GJ9 | Cysteine-rich venom protein | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Ca2+-channel impairment; paralytic | [15] |
| Q3SB03 | Cysteine-rich venom protein pseudechetoxin-like | Malacobdella grossa | Rhode Island, USA | Possible Shk-toxin | [124] |
| Q3SB03 | Cysteine-rich venom protein pseudechetoxin-like | Paranemertes peregrina | San Juan Island, WA, USA | Possible Shk-toxin | [124] |
| Q3SB05 | Cys-rich venom protein pseudechetoxin-like | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Possible Shk-toxin | [15] |
| P69929 | Delta-actitoxin-Amc1a | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | May inhibit VGSC | [15] |
| D2Y1Y2 | Mu-theraphotoxin-Hhn2a 4 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Blocks TTX-sensitive VGSC | [15] |
| P0C8G6 | Perivitellin-2 67 kDa subunit | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Neurotoxin present in eggs, acts on enterocytes | [15] |
| P0DKT2 | Turripeptide Gsg9.2 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Ion-channel inhibitor | [15] |
| Coagulation | |||||
| Q7T3S7 | Acidic phospholipase A2 EC-I | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibits platelet aggregation | [15] |
| Q3C2C1 | Phospholipase A2 AP-PLA2-II | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Capillary permeability-increasing and hemorrhagic activities | [15] |
| Q0ZZJ6 | A. superbus venom factor 1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Complement-activating | [15] |
| P0CH47 | Probable phospholipase A1 magnifin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Causes platelet aggreagation, phospholipase activity | [15] |
| E0Y418 | Serine protease VLSP-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | May act in prey hemostasis system | [15] |
| Q6X5S5 | Snaclec 27 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Interferes with platelet aggregation | [15] |
| Q9DEA1 | Snaclec agkicetin-C subunit beta | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Antithrombotic action | [15] |
| P0C929 | Snaclec bothroinsularin subunit alpha | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibits platelet aggregation | [15] |
| I7ICN3 | Snaclec bothroinsularin subunit beta | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Integrin antagonist | [15] |
| Q38L02 | Snaclec dabocetin subunit alpha | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibits platelet aggregation | [15] |
| Q58L91 | Venom prothrombin activator oscutarin-C non-catalytic subunit | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Prothrombin activator; attacks hemostatic system | [15] |
| J3SBP3 | Venom phosphodiesterase 2 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Nuclease activity and platelet aggregator | [15] |
| Other proposed activities | |||||
| A0FKN6 | Astacin-like metalloprotease toxin 1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Degrades fibronectin, fibrinogen and gelatin in vitro | [15] |
| P0DN10 | U-actitoxin-Avd3i | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Serine protease inhibitor of kallikreins | [15] |
| P81382 | L-amino acid oxidase | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Oxidative deamination of hydrophobic and aromatic residues | [15] |
| B2DCR8 | SE-cephalotoxin | Cephalothrix linearis | Not stated | Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Cerebratulus marginatus | San Juan Island, WA, USA [130] | Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Lineus longissimus | Erdeven + Roscoff, Fra [131] | Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Lineus ruber | Roscoff, Wimereux, Fra [131] | Toxic function unknown | [124] |
| P0A968 | Cold shock-like protein CspD | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibitor of DNA replication | [15] |
| P26831 | Hyaluronoglycosaminidase | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Likely to act on connective tissue | [15] |
| P00984 | Kunitz-type serine protease inhibitor dendrotoxin E | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibitor of trypsin and chymotrypsin | [15] |
| Q9KS12 | Multifunctional-autoprocessing repeats-in-toxin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Destroys actin cytoskeleton | [15] |
| Q66S25 | Natterin-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Edema and nociception | [15] |
| Q66S13 | Natterin-4 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Edema and nociception | [124] |
| Q66S13 | Natterin-4 | Tubulanus polymorphus | San Juan Island, WA, USA | Edema and nociception | [124] |
| Q66S13 | Natterin-4 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Edema and nociception | [15] |
| - | Natterin-4 homolog (Cm6) | Cerebratulus marginatus | San Juan Island, WA, USA [130] | Inferred from reciprocal BLAST, likely edema and nociception | [124] |
| K7Z9Q9 | Nematocyst expressed protein 6 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Metalloendopeptidase activity | [15] |
| A4USB4 | Phospholipase D LiSicTox-AlphaVI | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hydrolysis of sphingomyelin; causes dermonecrosis and inflammation | [15] |
| Q1W694 | Phosopholipase D LiSicTox-betaID1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hydrolysis of sphingomyelin; causes dermonecrosis and inflammation | [15] |
| Q75WF2 | Plancitoxin-1 | Cephalothrix hongkongiensis | Akkeshi, Hokkaido, Jpn [130] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hepatotoxin | [15] |
| Q75WF2 | Plancitoxin-1 | Cephalothrix linearis | Not stated | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Cerebratulus marginatus | San Juan Island, WA, USA [130] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Lineus longissimus | Erdeven + Roscoff, Fra [131] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Lineus ruber | Roscoff, Bretagne, Wimereux, France [131] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Malacobdella grossa | Rhode Island, USA | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Paranemertes peregrina | San Juan Island, WA, USA | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Tubulanus polymorphus | San Juan Island, WA, USA | Hepatotoxin | [124] |
| C1IC50 | Protease inhibitor-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Serine protease inhibitor | [15] |
| A8YPR9 | Snake venom metalloprotease inhibitor 02A10 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Metalloproteinase inhibitor during glandular storage | [15] |
| P86548 | Soybean toxin 17 kDa chain | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Involved in plant defense | [15] |
| B5U2W0 | Venom serine protease Bi-VSP | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Serine protease, induces melanization in target insects | [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göransson, U.; Jacobsson, E.; Strand, M.; Andersson, H.S. The Toxins of Nemertean Worms. Toxins 2019, 11, 120. https://doi.org/10.3390/toxins11020120
Göransson U, Jacobsson E, Strand M, Andersson HS. The Toxins of Nemertean Worms. Toxins. 2019; 11(2):120. https://doi.org/10.3390/toxins11020120
Chicago/Turabian StyleGöransson, Ulf, Erik Jacobsson, Malin Strand, and Håkan S. Andersson. 2019. "The Toxins of Nemertean Worms" Toxins 11, no. 2: 120. https://doi.org/10.3390/toxins11020120
APA StyleGöransson, U., Jacobsson, E., Strand, M., & Andersson, H. S. (2019). The Toxins of Nemertean Worms. Toxins, 11(2), 120. https://doi.org/10.3390/toxins11020120





