Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS
Abstract
:1. Introduction
2. Results
2.1. Development and Application of an LC-MS/MS Method for the Detection of Toxins from Stachybotrys
2.2. Secondary Metabolite Profiling of Stachybotrys Strains by LC-MS/MS
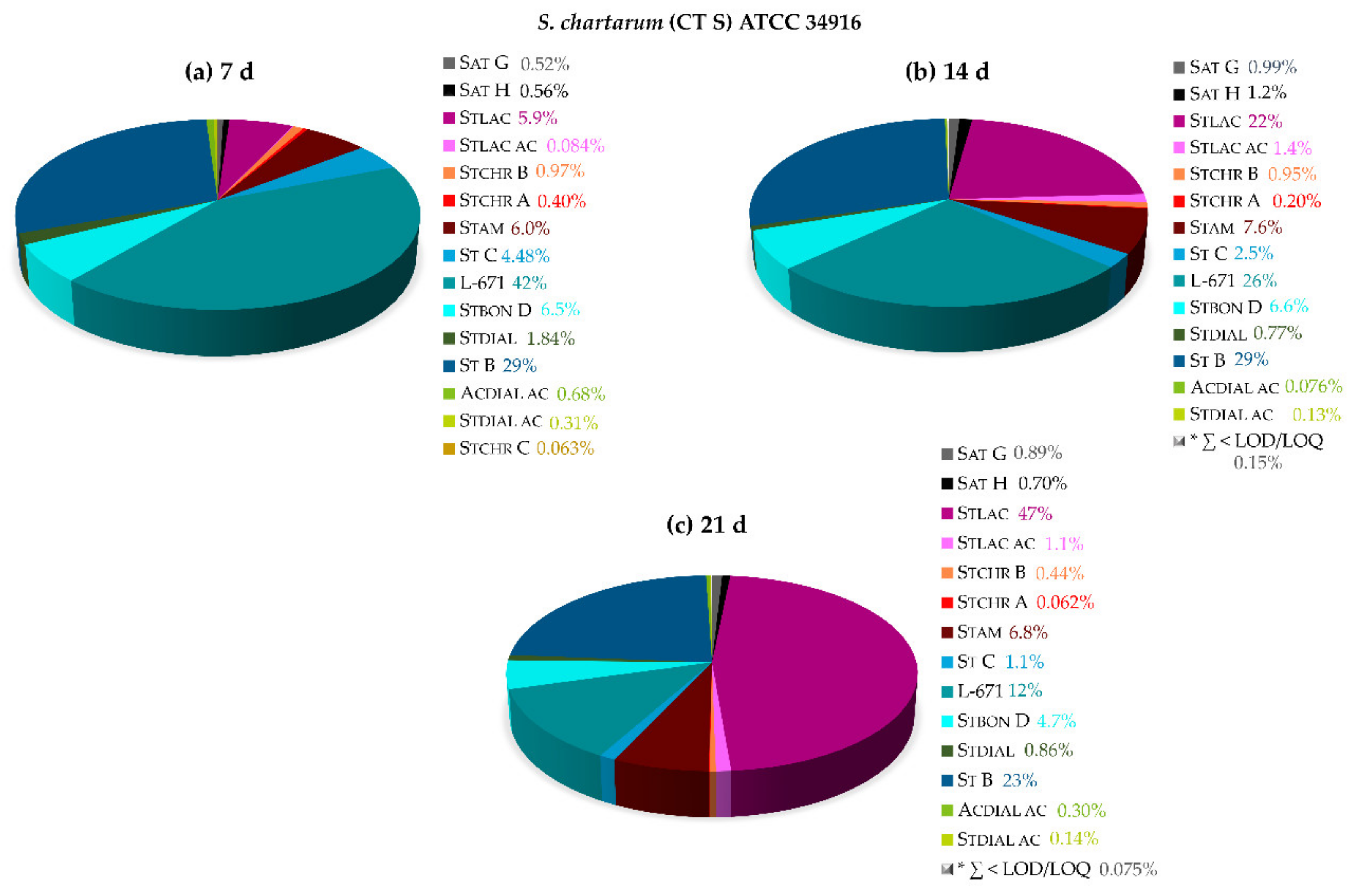
2.2.1. Monitoring of the Biosynthetic Production—S. chlorohalonata vs. S. chartarum CT S
2.2.2. Influence of Nitrogen (N)-Sources and the Formation of Isoindolinone Derivatives
2.2.3. Secondary Metabolite Profiles of Various Stachybotrys Strains on Synthetic-Nutrient-Poor Agar (SNA)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Strains and Media Compositions
5.2. Isolation and Analytical Data of 15 Stachybotrys Toxins
5.2.1. Isolation of Sat G and Sat H
5.2.2. Isolation of St B, St C, L-671, Stbon D, Stdial, Stdial ac, and Acdial ac
- St B: Reprosil-Pur 120 C18-AQ (250 × 10 mm, 5 μm; Dr. Maisch GmbH, Ammerbuch, Germany); 3 mL/min; isocratic elution, water/methanol (15/85; v/v); 254 nm.
- St C and L-671: Agilent Eclipse XDB-C18 column (250 × 9.4 mm, 5 µm; Waldbronn, Germany); 4 mL/min, isocratic elution water/MeCN (50/50; v/v); 360 nm.
- Stbon D and Stdial ac: Agilent Eclipse XDB-C18 column (250 × 9.4 mm, 5 µm; Waldbronn, Germany); 4 mL/min, gradient elution starting from 50% MeCN in 20 min to 100% MeCN; 254 nm.
- Stdial and Acdial ac: Agilent Eclipse XDB-C18 column (250 × 9.4 mm, 5 µm; Waldbronn, Germany); 4 mL/min, gradient elution starting from 60% MeCN in 15 min to 100% MeCN; 254 nm.
5.2.3. Isolation of Stlac, Stlac ac, and Stam
5.2.4. Isolation of Stchr A-C
5.2.5. Acquirement of Analytical Data of All 15 Reference Standards
5.3. Sample Preparation and Method Performance of 15 Stachybotrys Toxins
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lombard, L.; Houbraken, J.; Decock, C.; Samson, R.A.; Meijer, M.; Réblová, M.; Groenewald, J.Z.; Crous, P.W. Generic hyper-diversity in Stachybotriaceae. Persoonia 2016, 36, 156–246. [Google Scholar] [CrossRef] [PubMed]
- Nikulin, M.; Pasanen, A.L.; Berg, S.; Hintikka, E.L. Stachybotrys atra Growth and Toxin Production in Some Building Materials and Fodder under Different Relative Humidities. Appl. Environ. Microbiol. 1994, 60, 3421–3424. [Google Scholar] [PubMed]
- Andersen, B.; Dosen, I.; Lewinska, A.M.; Nielsen, K.F. Pre-contamination of new gypsum wallboard with potentially harmful fungal species. Indoor Air 2017, 27, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Dearborn, D.G.; Yike, I.; Sorenson, W.G.; Miller, M.J.; Etzel, R.A. Overview of investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ. Health Perspect. 1999, 107, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B.; Sorenson, W.G.; Hintikka, E.L.; Nikulin, M.; Zhou, Y.; Jiang, J.; Wang, S.; Hinkley, S.; Etzel, R.A.; Dearborn, D. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 1998, 64, 3620–3625. [Google Scholar] [PubMed]
- Hossain, M.A.; Ahmed, M.S.; Ghannoum, M.A. Attributes of Stachybotrys chartarum and its association with human disease. J. Allergy Clin. Immunol. 2004, 113, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Johanning, E.; Biagini, R.; Hull, D.; Morey, P.; Jarvis, B.; Landsbergis, P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int. Arch. Occup. Environ. Health 1996, 68, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Page, E.H.; Trout, D.B. The Role of Stachybotrys Mycotoxins in Building-Related Illness. Am. Ind. Hyg. Assoc. J. 2001, 62, 644–648. [Google Scholar] [CrossRef]
- Assouline-Dayan, Y.; Leong, A.; Shoenfeld, Y.; Gershwin, M.E. Studies of Sick Building Syndrome. IV. Mycotoxicosis. J. Asthma 2002, 39, 191–201. [Google Scholar] [CrossRef]
- Miller, J.D.; McMullin, D.R. Fungal secondary metabolites as harmful indoor air contaminants: 10 years on. Appl. Microbiol. Biotechnol. 2014, 98, 9953–9966. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Gareis, M.; Völkel, W.; Gottschalk, C. Overall internal exposure to mycotoxins and their occurrence in occupational and residential settings-An overview. Int. J. Hyg. Environ. Health 2016, 219, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, J. Stachybotryotoxicosis. In Fungal toxins; Kadis, S., Ciegler, A., Ajl, S.J., Eds.; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Harrach, B.; Bata, A.; Bajmócy, E.; Benko, M. Isolation of satratoxins from the bedding straw of a sheep flock with fatal stachybotryotoxicosis. Appl. Environ. Microbiol. 1983, 45, 1419–1422. [Google Scholar] [PubMed]
- Szathmary, C.I.; Mirocha, C.J.; Palyusik, M.; Pathre, S.V. Identification of mycotoxins produced by species of Fusarium and Stachybotrys obtained from Eastern Europe. Appl. Environ. Microbiol. 1976, 32, 579–584. [Google Scholar] [PubMed]
- Biermaier, B.; Gottschalk, C.; Schwaiger, K.; Gareis, M. Occurrence of Stachybotrys chartarum chemotype S in dried culinary herbs. Mycotoxin Res. 2015, 31, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B. Stachybotrys chartarum: A fungus for our time. Phytochemistry 2003, 64, 53–60. [Google Scholar] [CrossRef]
- Andersen, B.; Nielsen, K.F.; Thrane, U.; Szaro, T.; Taylor, J.W.; Jarvis, B.B. Molecular and Phenotypic Descriptions of Stachybotrys chlorohalonata sp. nov. and Two Chemotypes of Stachybotrys chartarum Found in Water-Damaged Buildings. Mycologia 2003, 95, 1227. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R. Biological and Biochemical Actions of Trichothecene Mycotoxins. In Progress in Molecular and Subcellular Biology; Hahn, F.E., Kopecko, D.J., Müller, W.E.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 41–110. [Google Scholar]
- Pestka, J.J.; Forsell, J.H. Inhibition of human lymphocyte transformation by the macrocyclic trichothecenes roridin A and verrucarin A. Toxicol. Letters 1988, 41, 215–222. [Google Scholar] [CrossRef]
- Yang, G.H.; Jarvis, B.B.; Chung, Y.J.; Pestka, J.J. Apoptosis induction by the satratoxins and other trichothecene mycotoxins: Relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000, 164, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Bauer, J.; Meyer, K. Determination of macrocyclic trichothecenes in mouldy indoor materials by LC-MS/MS. Mycotoxin Res. 2006, 22, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Bauer, J.; Meyer, K. Detection of satratoxin g and h in indoor air from a water-damaged building. Mycopathologia 2008, 166, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bloom, E.; Bal, K.; Nyman, E.; Must, A.; Larsson, L. Mass spectrometry-based strategy for direct detection and quantification of some mycotoxins produced by Stachybotrys and Aspergillus spp. in indoor environments. Appl. Environ. Microbiol. 2007, 73, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M.; Gottschalk, C. Stachybotrys spp. and the guttation phenomenon. Mycotoxin Res. 2014, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, B.; Bailly, S.; Draghi, M.; Pestka, J.J.; Oswald, I.P.; Robine, E.; Bailly, J.D.; Lacroix, M.Z. Production of four macrocyclic trichothecenes by Stachybotrys chartarum during its development on different building materials as measured by UPLC-MS/MS. Build. Environ. 2016, 106, 265–273. [Google Scholar] [CrossRef]
- Andersen, B.; Nielsen, K.F.; Jarvis, B.B. Characterization of Stachybotrys from water-damaged buildings based on morphology, growth, and metabolite production. Mycologia 2002, 94, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.F.; Huttunen, K.; Hyvärinen, A.; Andersen, B.; Jarvis, B.B.; Hirvonen, M.-R. Metabolite profiles of Stachybotrys isolates from water-damaged buildings and their induction of inflammatory mediators and cytotoxicity in macrophages. Mycopathologia 2002, 154, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Rand, T.G.; Flemming, J.; David Miller, J.; Womiloju, T.O. Comparison of inflammatory responses in mouse lungs exposed to atranones A and C from Stachybotrys chartarum. J. Toxicol. Environ. Health. Part A 2006, 69, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Vesper, S.J. Stachylysin May Be a Cause of Hemorrhaging in Humans Exposed to Stachybotrys chartarum. Infect. Immun. 2002, 70, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Abe, I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2016, 33, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xu, Y.; Gao, Y.; Huang, Q.; Luo, X.; An, H.; Dong, J. Chemical and bioactive diversities of the genera Stachybotrys and Memnoniella secondary metabolites. Phytochem. Rev. 2015, 14, 623–655. [Google Scholar] [CrossRef]
- Miyazaki, W.; Tamaoka, H.; Shinohara, M.; Kaise, H.; Izawa, T.; Nakano, Y.; Kinoshita, T.; Hong, K.; Inoue, K. A Complement Inhibitor Produced by Stachybotrys complementi, nov. sp. K-76, a New Species of Fungi Imperfecti. Microbiol. Immunol. 1980, 24, 1091–1108. [Google Scholar] [CrossRef] [PubMed]
- Došen, I.; Andersen, B.; Phippen, C.B.W.; Clausen, G.; Nielsen, K.F. Stachybotrys mycotoxins: From culture extracts to dust samples. Anal. Bioanal. Chem. 2016, 408, 5513–5526. [Google Scholar] [CrossRef] [PubMed]
- Jagels, A.; Hövelmann, Y.; Zielinski, A.; Esselen, M.; Köhler, J.; Hübner, F.; Humpf, H.-U. Stachybotrychromenes A-C: Novel cytotoxic meroterpenoids from Stachybotrys sp. Mycotoxin Res. 2018, 34, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bowden, K.; El-Kaissi, F.A.; Ranson, R.J. Intramolecular catalysis. Part 5. The intramolecular Cannizzaro reaction of o-phthalaldehyde and [α,α′-2H2]-o-phthalaldehyde. J. Chem. Soc. Perkin Trans. 2 1990, 57, 2089–2092. [Google Scholar] [CrossRef]
- Smedsgaard, J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chrom. A 1997, 760, 264–270. [Google Scholar] [CrossRef]
- Jarvis, B.B.; Salemme, J.; Morals, A. stachybotrys toxins. 1. Nat. Toxins 1995, 3, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, J.; Tan, Z.; Liu, J.; Zhao, J.; Chen, R.; Xie, K.; Zhang, D.; Li, Y.; Yu, L.; et al. Stachybotrysins A-G, Phenylspirodrimane Derivatives from the Fungus Stachybotrys chartarum. J. Nat. Prod. 2017, 80, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Jia, C.; Lang, J.; Niaz, S.-I.; Li, J.; Yuan, J.; Yu, J.; Chen, S.; Liu, L. Antiviral and anti-inflammatory meroterpenoids: Stachybonoids A–F from the crinoid-derived fungus Stachybotrys chartarum 952. RSC Adv. 2017, 7, 49910–49916. [Google Scholar] [CrossRef]
- Ma, X.-H.; Zheng, W.-M.; Sun, K.-H.; Gu, X.-F.; Zeng, X.-M.; Zhang, H.-T.; Zhong, T.-H.; Shao, Z.-Z.; Zhang, Y.-H. Two new phenylspirodrimanes from the deep-sea derived fungus Stachybotrys sp. MCCC 3A00409. Nat. Prod. Res. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bisby, G.R. Stachybotrys. Trans. Br. Mycol. Soc. 1943, 26, 133–143. [Google Scholar] [CrossRef]
- Kaneto, R.; Dobashi, K.; Kojima, I.; Sakai, K.; Shibamoto, N.; Yoshioka, T.; Nishida, H.; Okamoto, R.; Akagawa, H.; Mizuno, S. Mer-NF5003B, E and F, novel sesquiterpenoids as avian myeloblastosis virus protease inhibitors produced by Stachybotrys sp. J. Antibiot. 1994, 47, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Falck, J.R.; Reddy, K.K.; Chandrasekhar, S. Synthesis and structure revision of the myo-inositol monophosphatase inhibitor L-671,776. Tetrahedron Lett. 1997, 38, 5245–5248. [Google Scholar] [CrossRef]
- Thiele, J.; Schneider, J. Ueber Condensationsproducte des o-Phtalaldehyds. Justus Liebigs Ann. Chem. 1909, 369, 287–299. [Google Scholar] [CrossRef]
- Do Minh, T.; Johnson, A.L.; Jones, J.E.; Senise, P.P. Reactions of phthalaldehyde with ammonia and amines. J. Org. Chem. 1977, 42, 4217–4221. [Google Scholar] [CrossRef]
- Takahashi, I.; Nishiuchi, K.; Miyamoto, R.; Hatanaka, M.; Uchida, H.; Isa, K.; Sakushima, A.; Hosoi, S. Reaction Systems Peripheral to the 1: 2 Mannich Condensation Reaction between o-Phthalaldehyde and Primary Amine. Lett. Org. Chem. 2005, 2, 40–43. [Google Scholar] [CrossRef]
- Zuman, P. Reactions of orthophthalaldehyde with nucleophiles. Chem. Rev. 2004, 104, 3217–3238. [Google Scholar] [CrossRef] [PubMed]
- Donkeng Dazie, J.; Liška, A.; Ludvík, J.; Fábry, J.; Dušek, M. A study of the planarity of the pyrrolone fragment in 2-isopropyl-2,3-dihydro-1H-isoindol-1-one. Acta Crystallogr. C Struct. Chem. 2016, 72, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Alajarín, M.; Sánchez-Andrada, P.; López-Leonardo, C.; Alvarez, A. On the mechanism of phthalimidine formation via o-phthalaldehyde monoimines. New 1,5-H sigmatropic rearrangements in molecules with the 5-aza-2,4-pentadienal skeleton. J. Org. Chem. 2005, 70, 7617–7623. [Google Scholar] [CrossRef] [PubMed]
- D’Hollander, A.C.A.; Westwood, N.J. Assessment of the regioselectivity in the condensation reaction of unsymmetrical o-phthaldialdehydes with alanine. Tetrahedron 2018, 74, 224–239. [Google Scholar] [CrossRef]
- Vértesy, L.; Kogler, H.; Markus, A.; Schiell, M.; Vogel, M.; Wink, J. Memnopeptide A, a Novel Terpene Peptide from Memnoniella with an Activating Effect on SERCA2. J. Antibiot. 2001, 54, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Cen, S.; Proksch, P.; Lin, W. Isoindolinone-type alkaloids from the sponge-derived fungus Stachybotrys chartarum. Tetrahedron 2014, 70, 7010–7015. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Chartarlactams A-P, phenylspirodrimanes from the sponge-associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Shen, Y.; Tan, Z.; Zhang, M.; Chen, R.; Zhao, J.; Zhang, D.; Yu, L.; Dai, J. Stachybotrysams A–E, prenylated isoindolinone derivatives with anti-HIV activity from the fungus Stachybotrys chartarum. Phytochem. Lett. 2017, 20, 289–294. [Google Scholar] [CrossRef]
- Nishimura, Y.; Suzuki, E.; Hasegawa, K.; Nishimura, N.; Kitano, Y.; Hasumi, K. Pre-SMTP, a key precursor for the biosynthesis of the SMTP plasminogen modulators. J. Antibiot. 2012, 65, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Fu, Q.; Wu, W.; Cai, M.; Zhou, X.; Zhang, Y. Producing Novel Fibrinolytic Isoindolinone Derivatives in Marine Fungus Stachybotrys longispora FG216 by the Rational Supply of Amino Compounds According to Its Biosynthesis Pathway. Mar. Drugs 2017, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Lösel, D.M. Lipids in the Structure and Function of Fungal Membranes. In Biochemistry of Cell Walls and Membranes in Fungi; Kuhn, P.J., Trinci, A.P.J., Jung, M.J., Goosey, M.W., Copping, L.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 119–133. [Google Scholar] [CrossRef]
- Harrach, B.; Bata, A.; Sándor, G.; Ványi, A. Isolation of macrocyclic and non-macrocyclic trichothecenes (stachybotrys and fusarium toxins) from the Environment of 200 III Sport Horses. Mycotoxin Res. 1987, 3, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Hohn, T.M. Metabolic Pathway Gene Clusters in Filamentous Fungi. Fungal Genet. Biol. 1997, 21, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kaise, H.; Shinohara, M.; Miyazaki, W.; Izawa, T.; Nakano, Y.; Sugawara, M.; Sugiura, K.; Sasaki, K. Structure of K-76, a complement inhibitor produced by Stachybotrys complementi nov. Sp. K-76. J. Chem. Soc. Chem. Commun. 1979, 726. [Google Scholar] [CrossRef]
- Sakai, K.; Watanabe, K.; Masuda, K.; Tsuji, M.; Hasumi, K.; Endo, A. Isolation, characterization and biological activities of novel triprenyl phenols as pancreatic cholesterol esterase inhibitors produced by Stachybotrys sp. F-1839. J. Antibiot. 1995, 48, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Sawadjoon, S.; Kittakoop, P.; Isaka, M.; Kirtikara, K.; Madla, S.; Thebtaranonth, Y. Antiviral and antiplasmodial spirodihydrobenzofuran terpenes from the fungus Stachybotrys nephrospora. Planta Med. 2004, 70, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-W.; Chang, W.-W.; Chen, C.-C.; Tsai, Y.-C. Stachybotrydial, a potent inhibitor of fucosyltransferase and sialyltransferase. Biochem. Biophys. Res. Commun. 2005, 331, 953–957. [Google Scholar] [CrossRef] [PubMed]









| Species | Origin/Substrate | Collection |
|---|---|---|
| S. chlorohalonataa | Denmark/building material | CBS 109283 c |
| S. chartarumb CT A | California/building material | IBT 40288 d |
| S. chartarumb CT S | California/building material | IBT 40293 d |
| S. chartarumb CT A | Nepal/soil | DSM 63425 e |
| S. chartarumb CT S | Hungary/oats | ATCC 34916 f |
| S. chlorohalonata CBS 109283 | S. chartarum CT S IBT 40293 | |
|---|---|---|
| Day | Toxin Level [mg/cm2] | Toxin Level [mg/cm2] |
| 3 | 0.0063 | 0.063 |
| 4 | 0.12 | 0.088 |
| 5 | 0.27 | 0.52 |
| 6 | 0.67 | 1.1 |
| 7 | 0.73 | 1.0 |
| 14 | 1.9 | 1.4 |
| 21 | 3.0 | 1.9 |
| 14 Days | S. chlorohalonata CBS 109283 | S. chartarum CT A DSM 63425 | S. chartarum CT A IBT 40288 | S. chartarum CT S IBT 40293 | S. chartarum CT S ATCC 34916 |
|---|---|---|---|---|---|
| Toxin level [mg/cm2] | 0.17 | 0.17 | 0.065 | 0.053 | 0.089 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagels, A.; Lindemann, V.; Ulrich, S.; Gottschalk, C.; Cramer, B.; Hübner, F.; Gareis, M.; Humpf, H.-U. Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS. Toxins 2019, 11, 133. https://doi.org/10.3390/toxins11030133
Jagels A, Lindemann V, Ulrich S, Gottschalk C, Cramer B, Hübner F, Gareis M, Humpf H-U. Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS. Toxins. 2019; 11(3):133. https://doi.org/10.3390/toxins11030133
Chicago/Turabian StyleJagels, Annika, Viktoria Lindemann, Sebastian Ulrich, Christoph Gottschalk, Benedikt Cramer, Florian Hübner, Manfred Gareis, and Hans-Ulrich Humpf. 2019. "Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS" Toxins 11, no. 3: 133. https://doi.org/10.3390/toxins11030133
APA StyleJagels, A., Lindemann, V., Ulrich, S., Gottschalk, C., Cramer, B., Hübner, F., Gareis, M., & Humpf, H.-U. (2019). Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS. Toxins, 11(3), 133. https://doi.org/10.3390/toxins11030133





