Fate of Ergot Alkaloids during Laboratory Scale Durum Processing and Pasta Production
Abstract
:1. Introduction
2. Results
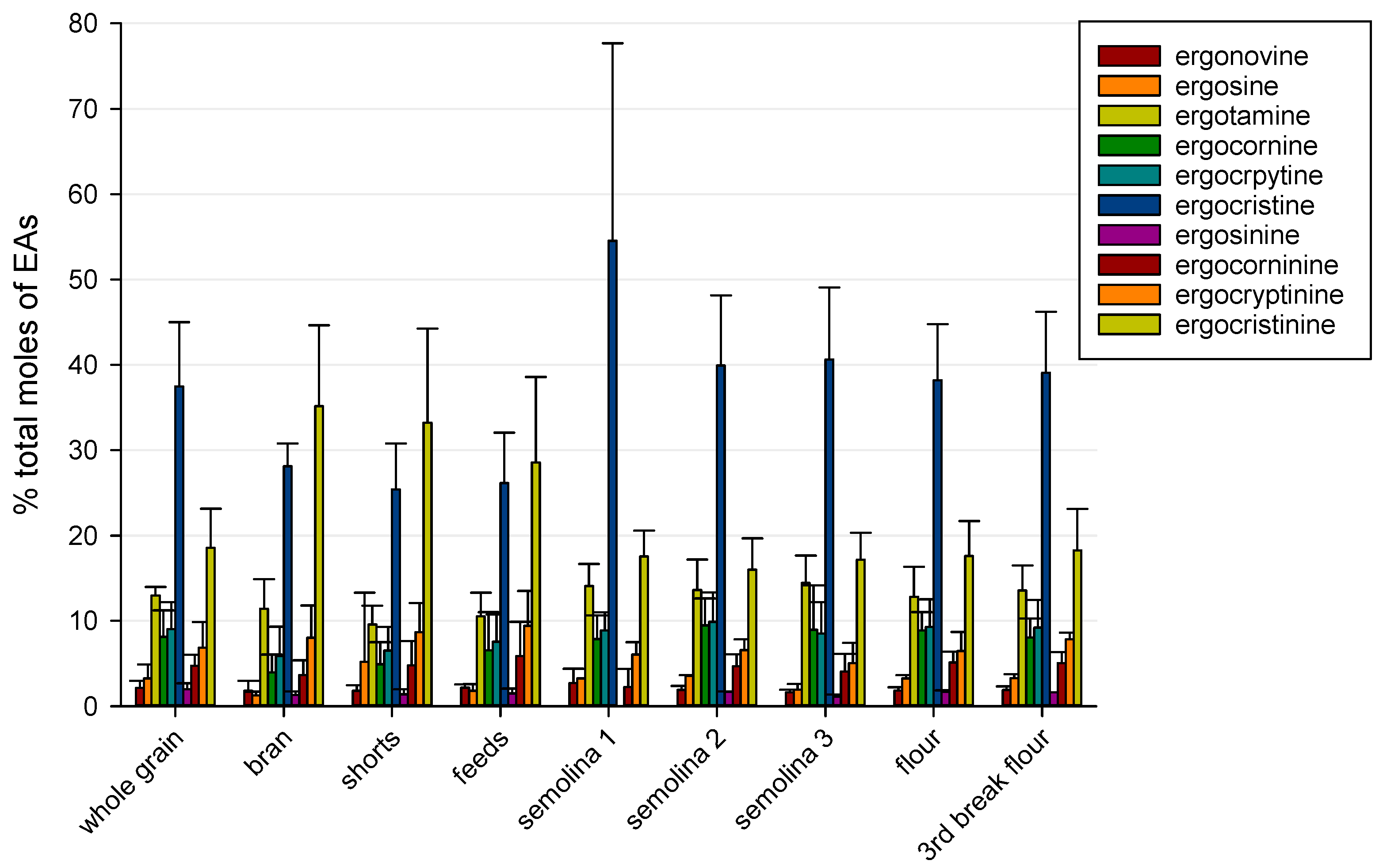
2.1. Ergot Alkaloids in Whole Grain and Milling Products
2.2. Ergot Alkaloids in Spaghetti
3. Discussion
3.1. Fate of Ergot Alkaloids in Milling Products and Spaghetti
3.2. Implications of Ergot Alkaloid Fate in Milling Products and Spaghetti
4. Materials and Methods
4.1. Samples
4.2. Milling and Processing into Spaghetti
4.3. Cooking and Moisture Content Determination
4.4. Determination of Ergot Alkaloids
4.5. Evaluation of Method for Analysis of Cooking Water and Boiled Spaghetti
4.6. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Menzies, J.G.; Turkington, T.K. An overview of the ergot (Claviceps purpurea) issue in western Canada: Challenges and solutions. Can. J. Plant Pathol. 2015, 37, 40–51. [Google Scholar] [CrossRef]
- Appelt, M.; Ellner, F.M. Investigations into the occurrence of alkaloids in ergot and single sclerotia from the 2007 and 2008 harvests. Mycotoxin Res. 2009, 25, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Blaney, B.J.; Molloy, J.B.; Brock, I.J. Alkaloids in australian rye ergot (Claviceps purpurea) sclerotia: Implications for food and stockfeed regulations. Animal Product. Sci. 2009, 49, 975–982. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; Menzies, J.G. The effects of selected factors on measured ergot alkaloid content in Claviceps purpurea-infected hexaploid and durum wheat. World Mycotoxin J. 2016, 9, 555–564. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; McKendry, T. Occurrence of ergot and ergot alkaloids in western Canadian wheat and other cereals. J. Agricult. Food Chem. 2015, 63, 6644–6650. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.; Maumené, C.; Piraux, F. Ergot and ergot alkaloids in French cereals: Occurrence, pattern and agronomic practices for managing the risk. World Mycotoxin J. 2017, 10, 327–338. [Google Scholar] [CrossRef]
- Topi, D.; Jakovac-Strajn, B.; Pavšič-Vrtač, K.; Tavčar-Kalcher, G. Occurrence of ergot alkaloids in wheat from Albania. Food Addit. Contamin A 2017, 34, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Hoffmann, A. The chemistry of the ergot alkaloids. In Chemistry of the Alkaloids; Pelletier, S.W., Ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1970; pp. 267–301. [Google Scholar]
- Krska, R.; Stubbings, G.; Macarthur, R.; Crews, C. Simultaneous determination of six major ergot alkaloids and their epimers in cereals and foodstuffs by LC–MS–MS. Anal. Bioanal. Chem. 2008, 391, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Belser-Ehrlich, S.; Harper, A.; Hussey, J.; Hallock, R. Human and cattle ergotism since 1900: Symptoms, outbreaks, and regulations. Toxicol. Ind. Health 2012, 29, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Canadian Grain Commission. Official Grain Grading Guide. Available online: https://www.grainscanada.gc.ca/oggg-gocg/ggg-gcg-eng.htm (accessed on 27 February 2019).
- Codex Alimentarius Commission. Codex Standard for Wheat and Durum Wheat. Codex Stan 199-1995. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B199-1995%252FCXS_199e.pdf (accessed on 27 February 2019).
- Canadian Food Inspection Agency. Proposal-Contaminant Standards for Aflatoxins, Deoxynivalenol, Fumonisins, Ergot Alkaloids and Salmonella in Livestock Feeds. Available online: http://www.inspection.gc.ca/animals/feeds/consultations/contaminant-standards-for-aflatoxins-deoxynivaleno/eng/1500908795245/1500908795965 (accessed on 27 February 2019).
- Canadian Food Inspection Agency. What We Heard Report-Consultations on Contaminant Standards for Aflatoxins, Deoxynivalenol, Fumonisins, Ergot Alkaloids and Salmonella in Livestock Feeds. Respondent Comments and Cfia Responses. Available online: http://www.inspection.gc.ca/animals/feeds/consultations/contaminant-standards-for-aflatoxins-deoxynivaleno/consultation-summary/eng/1544482075311/1544482140088 (accessed on 27 February 2019).
- Fajardo, J.E.; Dexter, J.E.; Roscoe, M.M.; Nowicki, T.W. Retention of ergot alkaloids in wheat during processing. Cereal Chem. 1995, 72, 291–298. [Google Scholar]
- Merkel, S.; Dib, B.; Maul, R.; Köppen, R.; Koch, M.; Nehls, I. Degradation and epimerization of ergot alkaloids after baking and in vitro digestion. Anal. Bioanal. Chem. 2012, 404, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S. Toxic effects, metabolism, and carry-over of ergot alkaloids in laying hens, with a special focus on changes of the alkaloid isomeric ratio in feed caused by hydrothermal treatment. Mycotoxin Res. 2016, 32, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, C.; Schröder, J.; Mϋnzing, K.; Wolf, K.; Lindhauer, M.; Humpf, H.-U. Distribution of ergot alkaloids and ricinoleic acid in different milling fractions. Mycotoxin Res. 2011, 27, 13–21. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on ergot alkaloids in food and feed. EFSA J. 2012, 10, 2798–2956. [Google Scholar]
- Prabhasankar, P.; Haridas Rao, P. Effect of different milling methods on chemical composition of whole wheat flour. Eur. Food Res. Technol. 2001, 213, 465–469. [Google Scholar] [CrossRef]
- Dexter, J.E.; Matsuo, R.R.; Kruger, J. The spaghetti-making quality of commercial durum wheat samples with variable α-amylase activity. Cereal Chem. 1990, 67, 405–412. [Google Scholar]
- Fu, B.; Schlicting, L.; Pozniak, C.; Singh, A. Pigment loss from semolina to dough: Rapid measurement and relationship with pasta colour. J. Cereal Sci. 2013, 57, 560–566. [Google Scholar] [CrossRef]
- European Commission. Commission decision implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Com. 2002, 221, 8–36. [Google Scholar]



| Whole Grain | Bran | Shorts | Feeds | Semolina 1 | Semolina 2 | Semolina 3 | Flour | 3rd Break Flour | |
|---|---|---|---|---|---|---|---|---|---|
| Milling yield (%, m/m) | 12.4 ± 0.1 | 5.5 ± 0.1 | 5.3 ± 0.1 | 61.3 ± 0.1 | 5.2 ± 0.1 | 2.39 ± 0.04 | 3.49 ± 0.07 | 4.46 ± 0.05 | |
| No ERG | <2 | <2 | <2 | 12 | 16 | <2 | <2 | <2 | <2 |
| ERG 0.01% | 446 | 1112 | 241 | 946 | 23 | 337 | 406 | 177 | 188 |
| ERG 0.02% | 601 | 1392 | 2267 | 2231 | 173 | 368 | 836 | 376 | 313 |
| ERG 0.03% | 681 | 2996 | 2298 | 3173 | 186 | 461 | 1005 | 506 | 412 |
| ERG 0.04% | 936 | 5715 | 3482 | 4532 | 279 | 1358 | 1300 | 618 | 530 |
| ERG 0.1% | 2147 | 5695 | 8002 | 9955 | 579 | 1603 | 3750 | 1493 | 1165 |
| Cooking Water | Boiled Spaghetti | |
|---|---|---|
| Ergonovine | 102 ± 3 | 143 ± 5 |
| Ergosine | 97 ± 5 | 102 ± 1 |
| Ergotamine | 100 ± 1 | 102 ± 1 |
| Ergocornine | 103 ± 1 | 98 ± 1 |
| Ergocryptine | 103 ± 4 | 100 ± 1 |
| Ergocristine | 113 ± 2 | 143 ± 5 |
| Ergosinine | 98 ± 9 | - |
| Ergocorninine | 93 ± 7 | - |
| Ergocryptinine | 104 ± 9 | - |
| Ergocristinine | 112 ± 11 | - |
| Freshly Extruded Spaghetti 1 | Cooked Spaghetti 2 | Cooking Water 2 | |
|---|---|---|---|
| Moisture content (%, m/m) | 34 | 66 | - |
| No ERG | <2 | <2 | <2 |
| ERG 0.01% | 42.2 ± 0.9 | 26 | <2 |
| ERG 0.02% | 114 ± 12 | 101 | 2 |
| ERG 0.03% | 143 ± 13 | 84 | 2 |
| ERG 0.04% | 175 ± 13 | 124 | 5 |
| ERG 0.1% | 533 ± 13 | 244 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tittlemier, S.A.; Drul, D.; Roscoe, M.; Turnock, D.; Taylor, D.; Fu, B.X. Fate of Ergot Alkaloids during Laboratory Scale Durum Processing and Pasta Production. Toxins 2019, 11, 195. https://doi.org/10.3390/toxins11040195
Tittlemier SA, Drul D, Roscoe M, Turnock D, Taylor D, Fu BX. Fate of Ergot Alkaloids during Laboratory Scale Durum Processing and Pasta Production. Toxins. 2019; 11(4):195. https://doi.org/10.3390/toxins11040195
Chicago/Turabian StyleTittlemier, Sheryl A., Dainna Drul, Mike Roscoe, Dave Turnock, Dale Taylor, and Bin Xiao Fu. 2019. "Fate of Ergot Alkaloids during Laboratory Scale Durum Processing and Pasta Production" Toxins 11, no. 4: 195. https://doi.org/10.3390/toxins11040195
APA StyleTittlemier, S. A., Drul, D., Roscoe, M., Turnock, D., Taylor, D., & Fu, B. X. (2019). Fate of Ergot Alkaloids during Laboratory Scale Durum Processing and Pasta Production. Toxins, 11(4), 195. https://doi.org/10.3390/toxins11040195