Hydrophobized Reversed-Phase Adsorbent for Protection of Dairy Cattle against Lipophilic Toxins from Diet. Efficiensy In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
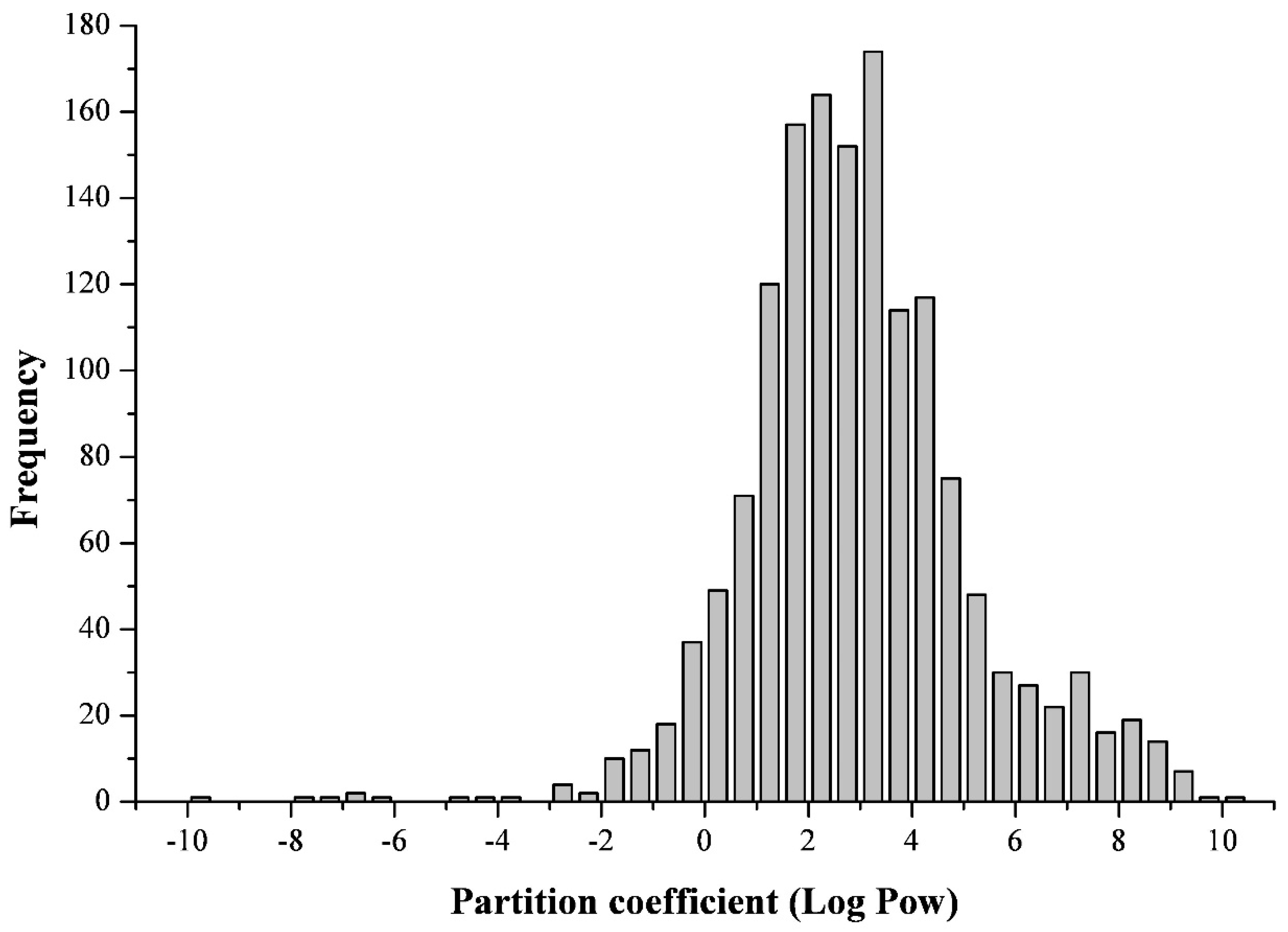
2.1. On the Polarity of Mycotoxins
2.2. On the Polarity of Polyaromatic Hydrocarbons and Persistent Organic Pollutants
2.3. Comparative Sorption Capacity of Adsorbents in Relation to Lipophilic Sorbates
2.4. Effects of POPSH on the Transfer of Chlorinated Pesticides into Milk
3. Discussion
3.1. On the Polarity of Mycotoxins in Feed
3.2. Other Non-Polar Cattle Feed Contaminants
3.3. The Use of Adsorbents to Reduce the Toxic Load of Feeds
3.4. The Use of Non-Polar Adsorbents to Reduce the Toxic Load of Feed
3.5. Selection of the “Right” Adsorbents for the Protection of the Dairy Cattle Digestive Tract
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Adsorbent Binding
5.3. Chromatographic Analysis
5.4. Determination of Pesticides in Raw Milk
5.5. Tables of Partition Coefficients for Mycotoxins, Polyaromatic Hydrocarbons and Persistent Organic Pollutants
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dohoo, I.R.; Meek, A.H.; Martin, S.W. Somatic cell counts in bovine milk: Relationships to production and clinical episodes of mastitis. Can. J. Comp. Med. 1984, 48, 130–135. [Google Scholar] [PubMed]
- Sharma, N.; Rho, G.J.; Hong, Y.H.; Kang, T.Y.; Lee, H.K.; Hur, T.-Y.; Jeong, D.K. Bovine mastitis: An Asian perspective. Asian J. Anim. Vet. Adv. 2012, 7, 454–476. [Google Scholar] [CrossRef]
- Schepers, A.J.; Lam, T.J.; Schukken, Y.H.; Wilmink, J.B. Estimation of variance components for somatic cell counts to determine thresholds for uninfected quarters. J. Dairy Sci. 1997, 80, 1833–1840. [Google Scholar] [CrossRef]
- Chaffer, M.; Friedman, S.; Saran, A.; Younis, A. An outbreak of Streptococcus canis mastitis in a dairy herd in Izrael. N. Z. Vet. J. 2005, 53, 261–264. [Google Scholar] [CrossRef]
- Hande, G.; Arzu, F.; Nilgün, G.; Serhat, A.S.; Alper, Ç.; Ece, K.; Murat, F. Investigation on the etiology of subclinical mastitis in jersey and hybrid jersey cows. Acta Vet. Beogr. 2015, 65, 358–370. [Google Scholar] [CrossRef]
- Plozza, K.; Lievaart, J.J.; Potts, G.; Barkema, H.W. Subclinical mastitis and associated risk factors on dairy farms in New South Wales. Aust. Vet. J. 2011, 89, 41–46. [Google Scholar] [CrossRef]
- Bergonier, D.; De Cremoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef]
- Rupp, R.; Biochard, D. Genetics of resistance to mastitis in dairy cattle. Vet. Res. 2003, 34, 671–688. [Google Scholar] [CrossRef]
- Juozaitene, V.; Juozaitis, A.; Mikicikeviciene, R. Relationship between somatic cell count and milk production or morphological traits of udder in black-and-white cows. Turk. J. Vet. Anim. Sci. 2006, 30, 47–51. [Google Scholar]
- Porcionato, M.A.D.F.; Soares, W.V.B.; Reis, C.B.M.D.; Cortinhas, C.S.; Mestieri, L.; Santos, M.V.D. Milk flow, teat morphology and subclinical mastitis prevalence in Gir cows. Pesq. Agropec. Bras. 2010, 45, 1507–1512. [Google Scholar] [CrossRef]
- Balaji, S.; Saravanan, R. Prevalence of subclinical mastitis in dairy cows of Salem district in Tamil Nadu. Int. J. Sci. Environ. Technol. 2017, 6, 1772–1776. [Google Scholar]
- Olde Riekerink, R.G.M.; Barkema, H.W.; Stryhn, H. The effect of season on somatic cell count and the incidence of clinical mastitis. J. Dairy Sci. 2007, 90, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Schukken, Y.H.; Weersink, A.; Leslie, K.E.; Martin, S.W. Dynamics and regulation of bulk milk somatic cell counts. Can. J. Vet. Res. 1993, 57, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Blount, W.P. Turkey “X” disease. J. Br. Turk. Fed. 1961, 9, 52–61. [Google Scholar]
- Forgacs, J. Mycotoxicoses—the neglected diseases. Feedstuffs 1962, 34, 124–134. [Google Scholar]
- Cole, R.J.; Cox, R.H. Handbook of Toxic Fungal Metabolites; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Council for Agricultural Science and Technology (CAST). Mycotoxins: Risks in Plant Animal, and Human Systems; CAST: Ames, IA, USA, 2003. [Google Scholar]
- Whitlow, L.W.; Hagler, W.M.J.; Diaz, D.E. Mycotoxins in feeds. Foodst. Mag. 2010, 74, 74–84. [Google Scholar]
- Nichea, M.; Palacios, S.; Chiacchiera, S.; Sulyok, M.; Krska, R.; Chulze, S.; Ramirez, M. Presence of multiple mycotoxins and other fungal metabolites in native grasses from a Wetland ecosystem in Argentina intended for grazing cattle. Toxins 2015, 7, 3309–3329. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Smedsgaard, J. Fungal metabolite screening: Database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography–UV–mass spectrometry methodology. J. Chromatogr. A 2003, 1002, 111–136. [Google Scholar] [CrossRef]
- Kononenko, G.P.; Burkin, A.A.; Gavrilova, O.P.; Gagkaeva, T.Y. Fungal species and multiple mycotoxin contamination of cultivated grasses and legumes crops. Agric. Biol. 2015, 24, 323–330. [Google Scholar] [CrossRef]
- Sotnichenko, A.I.; Okhanov, V.V. Non-polar toxins in feeds. Strategy of struggle. Kombikorma 2016, 1, 106–109. [Google Scholar]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Oswald, I.P. Current situation of mycotoxin contamination and Co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Addit. Contam. Part B 2011, 4, 168–179. [Google Scholar] [CrossRef]
- Alonso, V.A.; Pereyra, C.M.; Keller, L.A.M. Fungi and mycotoxins in silage: An overview. J. Appl. Microbiol. 2013, 115, 637–643. [Google Scholar] [CrossRef]
- Gill, R.; Howard, W.H.; Leslie, K.E.; Lissemore, K. Economics of mastitis control. J. Dairy Sci. 1990, 73, 3340–3348. [Google Scholar] [CrossRef]
- Obremski, K.; Zielonka, L.; Gajecka, M.; Jakimiuk, E.; Gajecki, M. Mycotoxins—Dairy cattle breeding problem. Bull. Vet. Inst. Pulawy 2009, 53, 221–224. [Google Scholar]
- Sumantri, I.; Murti, T.W.; Van der Poel, A.F.B.; Boehm, J.; Agus, A. Carry-over of aflatoxin B1-feed into aflatoxin M1-milk in dairy cows treated with natural sources of aflatoxin and bentonite. J. Indones. Trop. Anim. Agric. 2012, 37, 271–277. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; Lizarraga, E.; de Cerain, A.L.; Gonzales-Penas, E. Presence of mycotoxins in animal milk: A review. Food Control 2015, 53, 163–176. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Vulić, A.; Zadravec, M.; Lešić, T.; Benić, M.; Jaki Tkalec, V.; Vahčić, N. Presence of Fusarium mycotoxins in feedstuffs and cow milk sampled from Croatian farms during 2015. Mljekarstvo 2017, 67, 102–111. [Google Scholar] [CrossRef]
- Kan, C.A. Factors affecting absorption of harmful substances from the digestive tract of poultry and their level in poultry products. World’s Poult. Sci. J. 1994, 50, 39–53. [Google Scholar] [CrossRef]
- Kan, C.A.; Meijer, G.A.L. The risk of contamination of food with toxic substances present in animal feed. Anim. Feed Sci. Technol. 2007, 133, 84–108. [Google Scholar] [CrossRef]
- Völkel, I.; Schröer-Merker, E.; Czerny, C.-P. The carry-over of mycotoxins in products of animal origin with special regard to its implications for the European food safety legislation. Food Nutr. Sci. 2011, 2, 852–867. [Google Scholar] [CrossRef]
- Dänicke, S. Prevention and control of mycotoxins in the poultry production chain: An European view. World’s Poult. Sci. Assoc. J. 2002, 58, 451–474. [Google Scholar] [CrossRef]
- Schatzmayr, G.; Streit, E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Galvano, F.; Piva, A.; Ritieni, A. Dietary strategies to counteract the effects of mycotoxins: A review. J. Food Protect. 2001, 64, 120–131. [Google Scholar] [CrossRef]
- Galvano, F.; Ritieni, A.; Piva, G.; Pietri, A. Mycotoxins in the human food chain. In The Mycotoxin Blue Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; Volume 1, pp. 187–224. ISBN 1-904761-19-4. [Google Scholar]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the presence of mycotoxins in biological samples: An overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef]
- Jones, K.C.; de Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Rychen, G.; Jurjanz, S.; Fournier, A. Exposure of ruminants to persistent organic pollutants and potential of decontamination. Environ. Sci. Pollut. Res. 2014, 21, 6440–6447. [Google Scholar] [CrossRef] [PubMed]
- Beek, B. Bioaccumulation: New aspects and developments. In Handbook of Environmental Chemistry; Hutzinger, O., Ed.; Springer: New York, NY, USA, 1986; Volume 2, p. 298. ISBN 3540625755. [Google Scholar]
- Rychen, G.; Jurjanz, S.; Toussaint, H.; Feidt, C. Dairy ruminant exposure to persistent organic pollutants and excretion to milk. Animal 2008, 2, 312–323. [Google Scholar] [CrossRef]
- Thomas, G.O.; Sweetman, A.J.; Jones, K.C. Input–output balance of polychlorinated biphenyls in a long-term study of lactating daily cows. Environ. Sci. Technol. 1999, 33, 104–112. [Google Scholar] [CrossRef]
- Desiato, R.; Bertolini, S.; Baioni, E.; Crescio, M.I.; Scortichini, G.; Ubaldi, A.; Ru, G. Data on milk dioxin contamination linked with the location of fodder croplands allow to hypothesize the origin of the pollution source in an Italian valley. Sci. Total Environ. 2014, 499, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ahmadkhaniha, R.; Nodehi, R.N.; Rastkari, N.; Aghamirloo, H.M. Polychlorinated biphenyls (PCBs) residues in commercial pasteurized cows’ milk in Tehran, Iran. J. Environ. Health Sci. Eng. 2017, 15, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Klarić, M.S.; Rašić, D.; Peraica, M. Deleterious effects of mycotoxin combinations involving ochratoxin, A. Toxins 2013, 5, 1965–1987. [Google Scholar] [CrossRef]
- Pedrosa, K.; Borutova, R. Synergistic effects of mycotoxins discussed. Feedstuffs 2011, 83, 1–3. [Google Scholar]
- Kadikov, I.R.; Novikov, V.A.; Tremasov, M.Y.; Papunidi, K.K. The combined effect of T-2 toxin and dioxin on the rabbits. Immunopathol. Allergol. Infectol. 2010, 1, 193–194. [Google Scholar]
- Gallo, A.; Giuberti, G.; Frisvad, J.; Bertuzzi, T.; Nielsen, K. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Solfrizzo, M.; Visconti, A. Recent advances on the use of adsorbent materials for detoxification of Fusarium mycotoxins. Food Addit. Contam. 2005, 22, 379–388. [Google Scholar] [CrossRef]
- De Mil, T.; Devreese, M.; De Baere, S.; Van Ranst, E.; Eeckhout, M.; De Backer, P.; Croubels, S. Characterization of 27 mycotoxin binders and the relation with in vitro zearalenone adsorption at a single concentration. Toxins 2015, 7, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Huwig, A.; Freimund, S.; Kappeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, A.; Stroka, J. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin J. 2011, 4, 225–256. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other adsorbent materials. Food Chem. Toxicol. 2004, 42, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Assessment of the multi-mycotoxin-binding efficacy of a carbon/aluminosilicate-based product in an in vitro gastrointestinal model. J. Agric. Food Chem. 2007, 55, 4810–4819. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Lai, L. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- PubChem Data Base. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 1 October 2015).
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Jovaisiene, J.; Bakutis, B.; Baliukoniene, V.; Gerulis, G. Fusarium and Aspergillus mycotoxins effects on dairy cow health, performance and the efficacy of anti-mycotoxin additive. Polish J. Vet. Sci. 2016, 19, 79–87. [Google Scholar] [CrossRef]
- Korosteleva, S.N.; Smith, T.K.; Boermans, H.J. Effects of feed naturally contaminated with Fusarium mycotoxins on metabolism and immunity of dairy cows. J. Dairy Sci. 2009, 92, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Storm, I.M.L.D.; Rasmussen, R.R.; Rasmussen, P.H. Occurrence of pre- and post-harvest mycotoxins and other secondary metabolites in danish maize silage. Toxins 2014, 6, 2256–2269. [Google Scholar] [CrossRef] [PubMed]
- Bush, L.P.; Wilkinson, H.; Schardl, C.L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guerre, P. Ergot alkaloids produced by endophytic fungi of the genus epichloë. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Guerre, P.; Lolitrem, B. Indole diterpene alkaloids produced by endophytic fungi of the genus epichloë and their toxic effects in livestock. Toxins 2016, 8, 47. [Google Scholar] [CrossRef]
- Klotz, J.L. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins 2015, 7, 2801–2821. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.G.; Petroski, R.J. Alkaloid toxins in endophyte-infected grasses. Nat. Toxins 1992, 1, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Tangni, E.K.; Pussemier, L.; Bastiaanse, H.; Haesaert, G.; Foucart, G.; Van Hove, F. Presence of mycophenolic acid, roquefortine C, citrinin and ochratoxin A in maize and grass silages supplied to dairy cattle in belgium. J. Anim. Sci. Adv. 2013, 3, 598–612. [Google Scholar]
- Collander, R. The partition of organic compounds between higher alcohols and water. Acta Chem. Scand. 1951, 5, 774–780. [Google Scholar] [CrossRef]
- Fujita, T.; Iwasa, J.; Hansch, C. A new substituent constant, π, derived from partition coefficients. J. Am. Chem. Soc. 1964, 86, 5175–5180. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Sutherland, J.B.; Tanner, L.A.; Henderson, A.L.; Cerniglia, C.E. Use of a bioassay to evaluate the toxicity of beauvericin to bacteria. World J. Microb. Biot. 1999, 15, 119–121. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Zhao, J.; Li, P.; Shan, T.; Wang, J.; Zhou, L. Beauvericin from the endophytic fungus, Fusarium redolens, isolated from Dioscorea zingiberensis and its antibacterial activity. Nat. Prod. Commun. 2010, 5, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Meca, G.; Sospedra, I.; Soriano, J.M.; Ritieni, A.; Moretti, A.; Manes, J. Antibacterial effect of the bioactive compound beauvericin produced by Fusarium proliferatum on solid medium of wheat. Toxicon 2010, 56, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Meca, G.; Sospedra, I.; Valero, M.A.; Mañes, J.; Font, G.; Ruiz, M.J. Antibacterial activity of the enniatin B, produced by Fusarium tricinctum in liquid culture, and cytotoxic effects on Caco-2 cells. Toxicol. Mech. Methods 2011, 21, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.; Jestoi, M.; Anthoni, M. Fusarium mycotoxin enniatin B: Cytotoxic effects and changes in gene expression profile. Toxicol. In Vitro 2016, 34, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Heilos, D.; Rodríguez-Carrasco, Y.; Englinger, B. The natural fungal metabolite beauvericin exerts anticancer activity in vivo: A pre-clinical pilot study. Toxins 2017, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Manyes, L.; Font, G.; Juan-García, A. Evaluation of immunologic effect of enniatin A and quantitative determination in feces, urine and serum on treated Wistar rats. Toxicon 2014, 87, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Juan-García, A.; Font, G.; Ruiz, M.J. Beauvericin-induced cytotoxicity via ROS production and mitochondrial damage in Caco-2 cells. Toxicol. Lett. 2013, 222, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ficheux, A.S.; Sibiril, Y.; Parent-Massin, D. Effects of beauvericin, enniatin b and moniliformin on human dendritic cells and macrophages: An in vitro study. Toxicon 2013, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.T.; Roth, S.; Deyerup, S.T.; Gloer, J.B. A protective endophyte of maize: Acremonium zeae antibiotics inhibitory to Aspergillus flavus and Fusarium verticillioides. Mycol. Res. 2005, 109, 610–618. [Google Scholar] [CrossRef]
- Grimm, F.A.; Hu, D.; Kania-Korwel, I.; Lehmler, H.J.; Ludewig, G.; Hornbuckle, K.C.; Robertson, L.W. Metabolism and metabolites of polychlorinated biphenyls (PCBs). Crit. Rev. Toxicol. 2015, 45, 245–272. [Google Scholar] [CrossRef]
- Hu, K.; Bunce, N.J. Metabolism of polychlorinated dibenzo-p-dioxins and related dioxin-like compounds. J. Toxicol. Environ. Health Part B Crit. Rev. 1999, 2, 183–210. [Google Scholar] [CrossRef]
- Inouye, K.; Shinkyo, R.; Takita, T.; Ohta, M.; Sakaki, T. Metabolism of polychlorinated dibenzo-p-dioxins (PCDDs) by human cytochrome P450-dependent monooxygenase systems. J. Agric. Food Chem. 2002, 50, 5496–5502. [Google Scholar] [CrossRef] [PubMed]
- Sotnichenko, A.I.; Saprin, A.N. Microsomal metabolism of 3,4-benzpyrene. III. The limited nature of the substrate-positional specificity of arene epoxidase and its dependence on the properties of the oxidizable bond. Chem. Pharm. J. 1986, 12, 1429–1437. [Google Scholar]
- Ullah, H.A.; Durrani, A.Z.; Ijaz, M.; Javeed, A.; Sadique, U.; Hassan, Z.U.; Khattak, I. Dietary mycotoxins binders: A strategy to reduce aflatoxin M1 residues and improve milk quality of lactating Beetal goats. J. Verbr. Lebensm. 2016, 11, 305–309. [Google Scholar] [CrossRef]
- Kutz, R.E.; Sampson, J.D.; Pompeu, L.B.; Ledoux, D.R.; Spain, J.N.; Vazquez-Anon, M.; Rottinghaus, G.E. Efficacy of solis, Novasil PLUS, and MTB-100 to reduce aflatoxin M1 levels in milk of early to mid lactation dairy cows fed aflatoxin B1. J. Dairy Sci. 2009, 92, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Yamagami, C.; Kawase, K.; Iwaki, K. Hydrophobicity parameters determined by reversed-phase liquid chromatography. XV: Optimal conditions for prediction of log poct by using RP-HPLC procedures. Chem. Pharm. Bull. 2002, 50, 1578–1583. [Google Scholar] [CrossRef]
- Sotnichenko, A.I. A Method for Obtaining of Reversed-Phase Hydrophobized Polysilicate Adsorbents and Adsorbents Obtained by This Method. The Patent of the Russian Federation № 2538897, 24 October 2012. [Google Scholar]
- Morozenko, A.A.; Tyulkov, A.V.; Yukanova, T.I.; Okhanov, V.V.; Sotnichenko, A.I. Application of a feed additive Alvisorb® in a herd of lactating cows. Veterinary 2018, 2, 25–32. [Google Scholar]
- Buchta, S. The Influence of Fix-A-Tox on SCC in Cow’s Milk. Available online: http://www.auw-nutrition.at/fileadmin/alvetra_und_werfft/Benutzerdaten/PDFs/Produktdatenblaetter/Fix-A-Tox_the_Influence.pdf (accessed on 5 May 2019).
- Andrieu, S.; Agovino, M. Effect of modified glucomannan fraction from yeast cell wall extract (Mycosorb) on milk production in dairy herds in south Italy. In Proceedings of the Abstract of 59th Annual Meeting of the European Association for Animal Production, Vilnius, Lithuania, 24–27 August 2008. [Google Scholar]
- Gerlach, H.; Gerlach, A.; Schrödl, W.; Haufe, S.; Schottdorf, B.; Shehata, A.A.; Krüger, M. Oral application of charcoal and humic acids influence selected gastrointestinal microbiota, enzymes, electrolytes, and substrates in the blood of dairy cows challenged with glyphosate in GMO feeds. J Environ Anal Toxicol. 2014, 5, 256–262. [Google Scholar] [CrossRef]
- Ferreira, F.C.; De Vries, A. Effects of season and herd milk volume on somatic cell counts of Florida dairy farms. J. Dairy Sci. 2015, 98, 4182–4197. [Google Scholar] [CrossRef]
- Corrier, D.E. Mycotoxicosis: Mechanisms of immunosuppression. Vet. Immunol. Immunopathol. 1991, 30, 73–87. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Croubels, S. The impact of fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health Part B 2000, 3, 109–143. [Google Scholar] [CrossRef]
- Silkworth, J.B.; Lipinskas, T.; Stoner, C.R. Immunosuppressive potential of several polycyclic aromatic hydrocarbons (PAHs) found at a Superfund site: New model used to evaluate additive interactions between benzo[a]pyrene and TCDD. Toxicology 1995, 105, 375–386. [Google Scholar] [CrossRef]
- Burchiel, S.; Li, Q.; Lauer, F.; Hudson, L.; Liu, K.J. Low dose synergistic immunosuppression of T-dependent antibody responses by polycyclic aromatic hydrocarbons and arsenic in C57BL/6J Mice Spleen. Cells Toxicol. Appl. Pharmacol. 2010, 245, 344–351. [Google Scholar] [CrossRef]
- Ebtekar, M. Effects of persistent organic pollutants on the immune system: The case of dioxins. Iranian J. Environ. Health Sci. Eng. 2004, 1, 1–7. [Google Scholar]
- Serdar, B.; LeBlanc, W.G.; Norris, J.M.; Dickinson, L.M. Potential effects of polychlorinated biphenyls (PCBs) and selected organochlorine pesticides (OCPs) on immune cells and blood biochemistry measures: A cross-sectional assessment of the NHANES 2003-2004 data. Environ. Health 2014, 13, 114–126. [Google Scholar] [CrossRef]
- Bladt, T.; Frisvad, J.; Knudsen, P.; Larsen, T. Anticancer and antifungal compounds from aspergillus, penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.S.; Thampson, C.G.; Abramoritz, M. Artemia salina as a test organism for bioassay. Science 1956, 123, 464. [Google Scholar] [CrossRef]
- Sarah, Q.S.; Anny, F.C.; Misbahuddin, M. Brine shrimp lethality assay. Bangladesh J. Pharmacol. 2017, 12, 186–189. [Google Scholar] [CrossRef]
- Feeds, compound feeds, materials for compound feeds. Methods for the determination of total toxicity. GOST 31674-2012. 2014. (rus). Available online: https://files.stroyinf.ru/Data2/1/4293787/4293787722.pdf (accessed on 6 May 2019).
- Phenomenex (TN 20027). Multi-Class Screening of 243 Mycotoxins by LC/MS/MS. Available online: http://www.phenomenex.com/Application/Detail/20027 (accessed on 21 January 2016).
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol. 2004, 49, 201–241. [Google Scholar]
- Varga, E.; Glauner, T.; Berthiller, F.; Krska, R.; Schuhmacher, R.; Sulyok, M. Development and validation of a (semi-)quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013, 405, 5087–5104. [Google Scholar] [CrossRef]
- Tian, W.; Deng, Z.; Hong, K. The biological activities of sesterterpenoid-type ophiobolins. Mar. Drugs 2017, 15, 229. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium Mycotoxins. Chemistry, Genetics, and Biology; American Phytopathological Society Press: St. Paul, MN, USA, 2006; p. 268. ISBN 0-89-54-335-6. [Google Scholar]
- Houbraken, J.; Wang, L.; Lee, H.B.; Frisvad, J.C. New sections in Penicillium containing novel species producing patulin, pyripyropens or other bioactive compounds. Persoonia 2016, 36, 299–314. [Google Scholar] [CrossRef] [PubMed]


| Substance | Pesticide Concentration (µg/kg) | |
|---|---|---|
| Control group | Experimental group | |
| Aldrin | 10.6 ± 0.35 | n.d.* |
| Dieldrin | 5.70 ± 0.21 | n.d. |
| Heptachlor | 5.85 ± 0.24 | n.d. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotnichenko, A.; Pantsov, E.; Shinkarev, D.; Okhanov, V. Hydrophobized Reversed-Phase Adsorbent for Protection of Dairy Cattle against Lipophilic Toxins from Diet. Efficiensy In Vitro and In Vivo. Toxins 2019, 11, 256. https://doi.org/10.3390/toxins11050256
Sotnichenko A, Pantsov E, Shinkarev D, Okhanov V. Hydrophobized Reversed-Phase Adsorbent for Protection of Dairy Cattle against Lipophilic Toxins from Diet. Efficiensy In Vitro and In Vivo. Toxins. 2019; 11(5):256. https://doi.org/10.3390/toxins11050256
Chicago/Turabian StyleSotnichenko, Alexander, Evgeny Pantsov, Dmitry Shinkarev, and Victor Okhanov. 2019. "Hydrophobized Reversed-Phase Adsorbent for Protection of Dairy Cattle against Lipophilic Toxins from Diet. Efficiensy In Vitro and In Vivo" Toxins 11, no. 5: 256. https://doi.org/10.3390/toxins11050256
APA StyleSotnichenko, A., Pantsov, E., Shinkarev, D., & Okhanov, V. (2019). Hydrophobized Reversed-Phase Adsorbent for Protection of Dairy Cattle against Lipophilic Toxins from Diet. Efficiensy In Vitro and In Vivo. Toxins, 11(5), 256. https://doi.org/10.3390/toxins11050256




