The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial
Abstract
:1. Introduction
2. Results
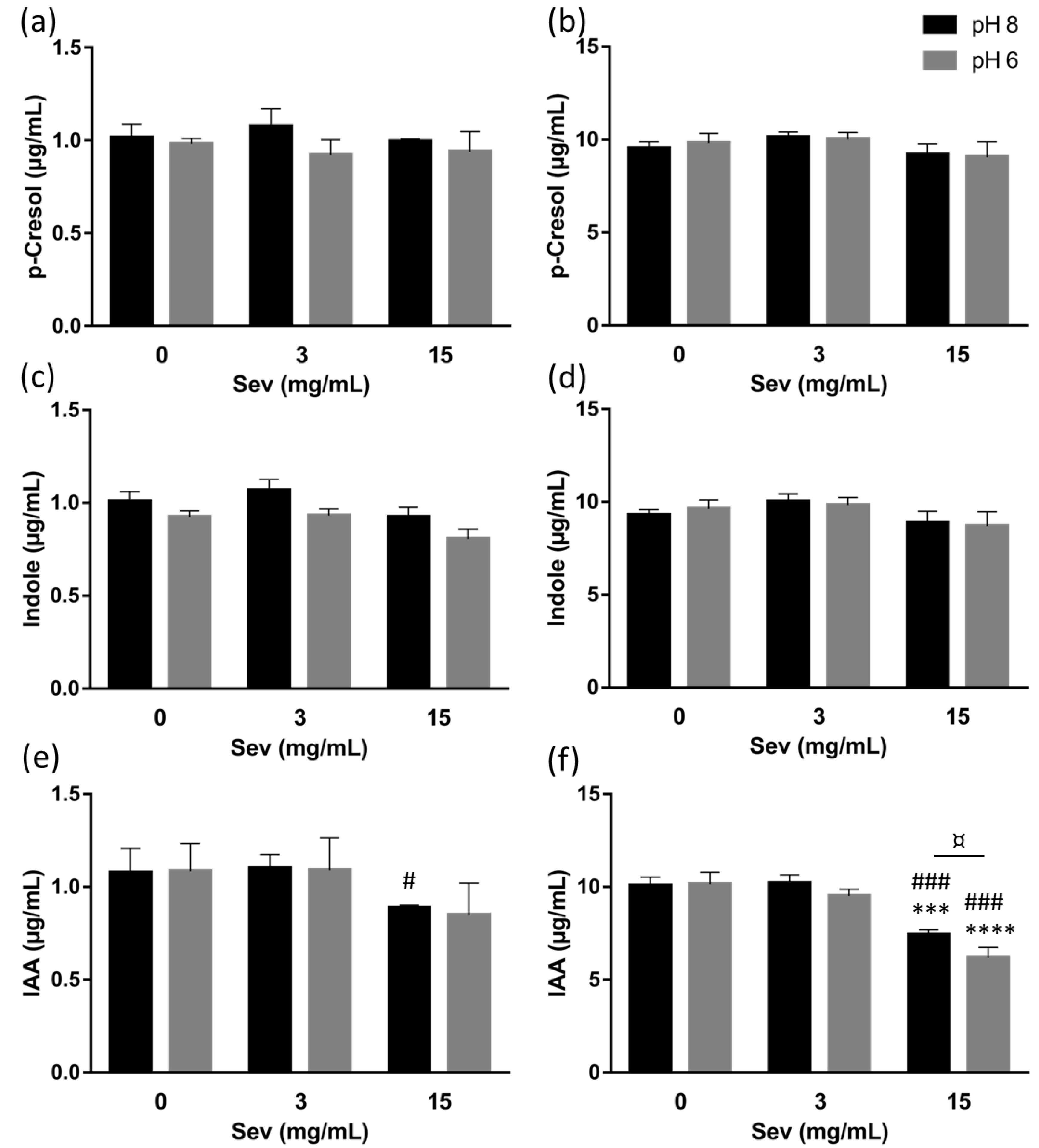
2.1. The Impact of Sevelamer In Vitro
2.2. Impact of Sevelamer in Chronic Kidney Disease Patients
3. Discussion
4. Methods
4.1. The In Vitro Study
4.1.1. Chemicals
4.1.2. Adsorption Experiments
4.1.3. Analysis of Uremic Toxin Concentrations in Filtrates
4.1.4. Analysis of The Inorganic Phosphorus Concentration in Filtrates
4.2. Clinical Study
4.2.1. Study Design
4.2.2. Sample Size Determination
4.2.3. Study Procedures
4.2.4. Clinical and Laboratory Evaluations
4.2.5. Analysis of Uremic Toxins in Serum Samples
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argiles, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Briskey, D.; Tucker, P.; Johnson, D.W.; Coombes, J.S. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin. Exp. Nephrol. 2017, 21, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Koga, J.; Syono, K.; Ichikawa, T.; Adachi, T. Involvement of L-tryptophan aminotransferase in indole-3-acetic acid biosynthesis in Enterobacter cloacae. Biochim. Biophys. Acta 1994, 1209, 241–247. [Google Scholar] [CrossRef]
- Williams, R.T.; Millburn, P.; Smith, R.L. The influence of enterohepatic circulation on toxicity of drugs. Ann. N. Y. Acad. Sci. 1965, 123, 110–124. [Google Scholar] [CrossRef]
- Morimoto, K.; Tominaga, Y.; Agatsuma, Y.; Miyamoto, M.; Kashiwagura, S.; Takahashi, A.; Sano, Y.; Yano, K.; Kakinuma, C.; Ogihara, T.; et al. Intestinal secretion of indoxyl sulfate as a possible compensatory excretion pathway in chronic kidney disease. Biopharm. Drug Dispos. 2018, 39, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Yamamoto, T.; Matsuo, H.; Tan, J.K.; Ooyama, K.; Sakiyama, M.; Miyata, H.; Yamanashi, Y.; Toyoda, Y.; Higashino, T.; et al. Identification of ABCG2 as an exporter of uremic toxin indoxyl sulfate in mice and as a crucial factor influencing CKD progression. Sci. Rep. 2018, 8, 11147. [Google Scholar] [CrossRef] [PubMed]
- Deichmann, W.; Keplinger, M. Phenols and phenolic compounds. In Patty’s Industrial Hygiene and Toxicology, 3rd ed.; Clayton, G.D., Clayton, F.E., Eds.; Wiley and Sons: Hoboken, NJ, USA, 1981; Volume 2A, pp. 2597–2601. ISBN 0-471-16042-3. [Google Scholar]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. JASN 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic Kidney disease patients. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Liabeuf, S.; Glorieux, G.; Lenglet, A.; Diouf, M.; Schepers, E.; Desjardins, L.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin (EUTox) Work Group. Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS ONE 2013, 8, e67168. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.C.; Eloot, S.; Glorieux, G.L.R.L. Future avenues to decrease uremic toxin concentration. Am. J. Kidney Dis. 2016, 67, 664–676. [Google Scholar] [CrossRef]
- Fujii, H.; Nishijima, F.; Goto, S.; Sugano, M.; Yamato, H.; Kitazawa, R.; Kitazawa, S.; Fukagawa, M. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol. Dial. Transplant. 2009, 24, 2089–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, I.; Yamaguchi, Y.; Saikawa, H.; Uetake, D.; Hikita, M.; Okabe, H.; Ichida, K.; Hosoya, T. Sevelamer decreases serum uric acid concentration through adsorption of uric acid in maintenance hemodialysis patients. Intern. Med. 2009, 48, 415–420. [Google Scholar] [CrossRef]
- Braunlin, W.; Zhorov, E.; Guo, A.; Apruzzese, W.; Xu, Q.; Hook, P.; Smisek, D.L.; Mandeville, W.H.; Holmes-Farley, S.R. Bile acid binding to sevelamer HCl. Kidney Int. 2002, 62, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Takagi, K.; Masuda, K.; Yamazaki, M.; Kiyohara, C.; Itoh, S.; Wasaki, M.; Inoue, H. Metal ion and vitamin adsorption profiles of phosphate binder ion-exchange resins. Clin. Nephrol. 2010, 73, 30–35. [Google Scholar] [CrossRef]
- Kays, M.B.; Overholser, B.R.; Mueller, B.A.; Moe, S.M.; Sowinski, K.M. Effects of sevelamer hydrochloride and calcium acetate on the oral bioavailability of ciprofloxacin. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 42, 1253–1259. [Google Scholar] [CrossRef]
- Arnadottir, M.; Johannesson, A.J. Phosphate binders and timing of levothyroxine administration. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2008, 23, 420. [Google Scholar] [CrossRef]
- De Smet, R.; Thermote, F.; Lameyre, N.; Vanholder, R. Sevelamer hydrochloride (Renagel) adsorbs the uremic compounds indoxyl sulfate, indeole and p-cresol. J. Am. Soc. Nephrol. 2004, 15, 505. [Google Scholar]
- Brandenburg, V.M.; Schlieper, G.; Heussen, N.; Holzmann, S.; Busch, B.; Evenepoel, P.; Vanholder, R.; Meijers, B.; Meert, N.; Fassbender, W.J.; et al. Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: A prospective study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2010, 25, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; Cataldi, M.; Riccio, E.; Grumetto, L.; Pota, A.; Borrelli, S.; Memoli, A.; Barbato, F.; Argentino, G.; Salerno, G.; et al. Plasma p-cresol lowering effect of sevelamer in peritoneal dialysis patients: Evidence from a cross-sectional observational study. PLoS ONE 2013, 8, e73558. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. JASN 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Liabeuf, S.; Ryckelynck, J.-P.; El Esper, N.; Ureña, P.; Combe, C.; Dussol, B.; Fouque, D.; Vanhille, P.; Frimat, L.; Thervet, E.; et al. Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 1930–1940. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef]
- Markopoulos, C.; Andreas, C.J.; Vertzoni, M.; Dressman, J.; Reppas, C. In-vitro simulation of luminal conditions for evaluation of performance of oral drug products: Choosing the appropriate test media. Eur. J. Pharm. Biopharm. 2015, 93, 173–182. [Google Scholar] [CrossRef]
- Yang, Y.; Mohammad, A.; Berendt, R.T.; Carlin, A.; Khan, M.A.; Faustino, P.J. Evaluation of the in vitro efficacy of sevelamer hydrochloride and sevelamer carbonate. J. Pharm. Sci. 2016, 105, 864–875. [Google Scholar] [CrossRef]
- Chinnappa, S.; Tu, Y.-K.; Yeh, Y.C.; Glorieux, G.; Vanholder, R.; Mooney, A. Association between protein-bound uremic toxins and asymptomatic cardiac dysfunction in patients with chronic kidney disease. Toxins 2018, 10, 520. [Google Scholar] [CrossRef]
- Phan, O.; Ivanovski, O.; Nguyen-Khoa, T.; Mothu, N.; Angulo, J.; Westenfeld, R.; Ketteler, M.; Meert, N.; Maizel, J.; Nikolov, I.G.; et al. Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E–deficient mice. Circulation 2005, 112, 2875–2882. [Google Scholar] [CrossRef]
- Lin, C.-J.; Pan, C.-F.; Chuang, C.-K.; Liu, H.-L.; Huang, S.-F.; Chen, H.-H.; Wu, C.-J. Effects of sevelamer hydrochloride on uremic toxins serum indoxyl sulfate and P-cresyl sulfate in hemodialysis patients. J. Clin. Med. Res. 2017, 9, 765–770. [Google Scholar] [CrossRef]
- Riccio, E.; Sabbatini, M.; Bruzzese, D.; Grumetto, L.; Marchetiello, C.; Amicone, M.; Andreucci, M.; Guida, B.; Passaretti, D.; Russo, G.; et al. Plasma p-cresol lowering effect of sevelamer in non-dialysis CKD patients: Evidence from a randomized controlled trial. Clin. Exp. Nephrol. 2018, 22, 529–538. [Google Scholar] [CrossRef]
- Vanholder, R.; Bammens, B.; de Loor, H.; Glorieux, G.; Meijers, B.; Schepers, E.; Massy, Z.; Evenepoel, P. Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 1464–1467. [Google Scholar] [CrossRef]

| Variables | All n = 78 | Placebo n = 39 | Sevelamer n = 39 | p |
|---|---|---|---|---|
| Age (years) | 63 ± 13 | 63 ± 14 | 63 ± 13 | 0.918 |
| Male gender, n (%) | 55 (70.5%) | 28 (71.8%) | 27 (69.2%) | 0.804 |
| GFR MDRD (mL/min per 1.73 m2) | 27.0 ± 9.1 | 28.1 ± 9.9 | 25.4 ± 8.1 | 0.193 |
| Serum phosphorus (mmol/L) | 1.24 ± 0.17 | 1.25 ± 0.16 | 1.23 ± 0.18 | 0.705 |
| Serum calcium (mmol/L) | 2.34 ± 0.11 | 2.33 ± 0.11 | 2.35 ± 0.11 | 0.486 |
| Serum creatinine (µmol/L) | 234 ± 76 | 227 ± 75 | 241 ± 79 | 0.379 |
| Serum albumin (g/L) | 41.7 ± 3.4 | 41.5 ± 3.7 | 41.9 ± 3.1 | 0.645 |
| Serum intact PTH (pg/mL) | 97 (61; 127) | 103 (71; 141) | 91 (58; 123) | 0.169 |
| Serum total cholesterol (mmol/L) | 4.7 ± 1.1 | 4.7 ± 1.0 | 4.7 ± 1.2 | 0.522 |
| Serum LDL cholesterol (mmol/L) | 2.5 ± 1.0 | 2.4 ± 1.0 | 2.6 ± 1.0 | 0.430 |
| Serum indoxyl sulfate (µg/mL) * 0.53 ± 0.29 µg/mL in healthy adults # [23] | 3.57 (1.32; 6.31) | 3.45 (1.07; 5.61) | 4.26 (1.77; 7.35) | 0.119 |
| Serum p-cresyl sulfate (µg/mL) * 1.9 ± 1.3 µg/mL in healthy adults # [23] | 10.65 (6.05; 17.69) | 10.34 (5.12; 21.00) | 11.34 (7.82; 16.44) | 0.681 |
| Serum indole acetic acid (µg/mL) * 0.5 ± 0.3 µg/mL in healthy adults # [23] | 1.02 (0.58; 1.44) | 0.96 (0.59; 1.47) | 1.08 (0.57; 1.44) | 0.812 |
| Toxin | Visit 1 (V1) | Visit 5 (V5) | V5-V1 |
|---|---|---|---|
| Phosphorus (mmol/L) | |||
| Placebo | 1.25 ± 0.16 | 1.26 ± 0.19 | 0.01 ± 0.16 |
| Sevelamer | 1.23 ± 0.18 | 1.20 ± 0.19 | −0.04 ± 0.20 |
| pCS (µg/mL) | |||
| Placebo | 10.34 (5.12; 21.00) | 11.69 (7.97; 25.37) | 1.97 (−1.33; 7.89) |
| Sevelamer | 11.34 (7.82; 16.44) | 11.17 (8.89; 16.13) | −0.12 (−2.70; 4.30) |
| IS (µg/mL) | |||
| Placebo | 3.45 (1.07; 5.61) | 3.46 (1.65; 9.99) | 0.38 (−0.36; 4.39) |
| Sevelamer | 4.26 (1.77; 7.35) | 3.84 (2.10; 7.49) | 0.26 (−0.85; 2.20) |
| IAA (µg/mL) | |||
| Placebo | 0.96 (0.59; 1.47) | 0.99 (0.58; 1.65) | 0.05 (−0.07; 0.16) |
| Sevelamer | 1.08 (0.57; 1.44) | 0.92 (0.57; 1.44) | −0.06 (−0.12; 0.05) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennis, Y.; Cluet, Y.; Titeca-Beauport, D.; El Esper, N.; Ureña, P.; Bodeau, S.; Combe, C.; Dussol, B.; Fouque, D.; Choukroun, G.; et al. The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Toxins 2019, 11, 279. https://doi.org/10.3390/toxins11050279
Bennis Y, Cluet Y, Titeca-Beauport D, El Esper N, Ureña P, Bodeau S, Combe C, Dussol B, Fouque D, Choukroun G, et al. The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Toxins. 2019; 11(5):279. https://doi.org/10.3390/toxins11050279
Chicago/Turabian StyleBennis, Youssef, Yan Cluet, Dimitri Titeca-Beauport, Najeh El Esper, Pablo Ureña, Sandra Bodeau, Christian Combe, Bertrand Dussol, Denis Fouque, Gabriel Choukroun, and et al. 2019. "The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial" Toxins 11, no. 5: 279. https://doi.org/10.3390/toxins11050279
APA StyleBennis, Y., Cluet, Y., Titeca-Beauport, D., El Esper, N., Ureña, P., Bodeau, S., Combe, C., Dussol, B., Fouque, D., Choukroun, G., & Liabeuf, S. (2019). The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Toxins, 11(5), 279. https://doi.org/10.3390/toxins11050279







