Ricin: An Ancient Story for a Timeless Plant Toxin
Abstract
:1. Castor Bean in Traditional and Folk Medicine
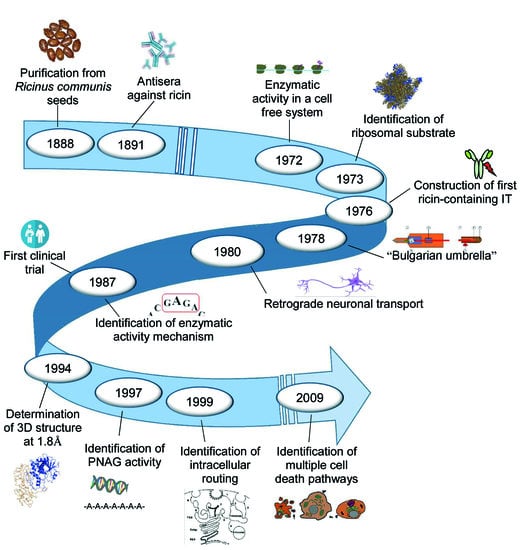
2. The Ricin Story
2.1. Ricin Structure
2.2. Ricin Enzymatic Activity
2.3. Ricin Cellular Uptake, Routing, and Toxicity
3. Ricin Toxicity in Humans and Animals
3.1. Oral Toxicity
3.2. Inhalation Toxicity
3.3. Parenteral Toxicity
4. Bioterrorism and Environmental Toxicity
5. Ricin-Containing Immunotoxins
6. Conclusions
Funding
Conflicts of Interest
References
- D’Errico, F.; Backwell, L.; Villa, P.; Degano, I.; Lucejko, J.J.; Bamford, M.K.; Higham, T.F.; Colombini, M.P.; Beaumont, P.B. Early evidence of San material culture represented by organic artifacts from Border Cave, South Africa. Proc. Natl. Acad. Sci. USA 2012, 109, 13214–13219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebers, G. Papyros Ebers: Das Hermetische Buch über die Arzneimittel der Alten Äegypter; Hinrichs, J.C., Ed.; Wilhelm Engelmann: Leipzig, Germany, 1875. [Google Scholar]
- Leake, C.D. The Old Egyptian Medical Papyri; University of Kansas Press: Lawrence, KS, USA, 1952. [Google Scholar]
- Carpenter, S.; Rigaud, M.; Barile, M.; Priest, T.J.; Perez, L.; Ferguson, J.B. An Interlinear Transliteration and English Translation of Portions of the Ebers Papyrus Possibly Having to do with Diabetes Mellitus; Bard College: Annandale-on-Hudson, NY, USA, 1998. [Google Scholar]
- Totelin, L.M.V. Hippocratic Recipes: Oral and Written Transmission of Pharmacological Knowledge in Fifth- and Fourth-Century Greece; Brill: Leiden, The Netherlands; Boston, MA, USA, 2009. [Google Scholar]
- Gunther, R.T. The Greek Herbal of Dioscorides/Illustrated by a Byzantine, A.D. 512; Englished by John Goodyer, A.D. 1655; Edited and First Printed, A.D. 1933; Gunther, R.W.T., Ed.; Oxford University Press: New York, NY, USA, 1934. [Google Scholar]
- Bostock, J.; Riley, H.T. The Natural History of Pliny, 1st ed.; Taylor and Francis: London, UK, 1855. [Google Scholar]
- Scarpa, A.; Guerci, A. Various uses of the castor oil plant (Ricinus communis L.). A review. J. Ethnopharmacol. 1982, 5, 117–137. [Google Scholar] [CrossRef]
- Ladda, P.L.; Kamthane, R.B. Ricinus communis (castor): An overview. Int. J. Res. Pharmacol. Pharmacother. 2014, 3, 136–144. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; Routledge: Abingdon-on-Thames, UK, 2012. [Google Scholar]
- Polito, L.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Bolognesi, A. Plants producing Ribosome-Inactivating Proteins in traditional medicine. Molecules 2016, 21, 1560. [Google Scholar] [CrossRef] [PubMed]
- Tunaru, S.; Althoff, T.F.; Nüsing, R.M.; Diener, M.; Offermanns, S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 9179–9184. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, O.S.; Omale, J.; Omeje, S.C.; Edino, V.O. Antidiarrheal activity of hexane extract of Citrus limon peel in an experimental animal model. J. Integr. Med. 2017, 15, 158–164. [Google Scholar] [CrossRef]
- Battelli, M.G. Cytotoxicity and toxicity to animals and humans of ribosome-inactivating proteins. Mini Rev. Med. Chem. 2004, 4, 513–521. [Google Scholar] [CrossRef]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1993, 1154, 237–282. [Google Scholar] [CrossRef]
- Dixson, T. Ricinus communis. Aust. Med. Gazz. 1887, 6, 137–138. [Google Scholar]
- Hartmut, F. The ricin story. Adv. Lectin Res. 1988, 1, 10–25. [Google Scholar]
- Stillmark, H. Über Ricin, ein Giftiges Ferment aus den Samen von Ricinus communis L. und Einigen Anderen Euphorbiaceen. Ph.D. Thesis, University of Dorpat, Dorpat, Estonia, 1888. [Google Scholar]
- Takahashi, T.; Funatsu, G.; Funatsu, M. Biochemical studies on castor bean hemagglutinin. I. Separation and purification. J. Biochem. 1962, 51, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P. Experimentelle Untersuchungen über Immunität I. Über Ricin. DMW-Deutsche Med. Wochenschr. 1891, 17, 976–979. [Google Scholar] [CrossRef]
- Ehrlich, P. Experimentelle Untersuchungen über Immunität II. Über Abrin. DMW-Deutsche Med. Wochenschr. 1891, 17, 1218–1219. [Google Scholar] [CrossRef]
- Silverstein, A.M. Paul Ehrlich: The founding of pediatric immunology. Cell Immunol. 1996, 174, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P. Partial Cell Functions. In Nobel Lectures, Physiology or Medicine 1901–1921; Elsevier Publishing Company: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Lin, J.Y.; Tserng, K.Y.; Chen, C.C.; Lin, L.T.; Tung, T.C. Abrin & Ricin: New anti-tumour substances. Nature 1970, 227, 292–293. [Google Scholar] [PubMed]
- Lin, J.Y.; Liu, K.; Chen, C.C.; Tung, T.C. Effect of crystalline ricin on the biosynthesis of protein, RNA, and DNA in experimental tumor cells. Cancer Res. 1971, 31, 921–924. [Google Scholar] [PubMed]
- Olsnes, S.; Pihl, A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry 1973, 12, 3121–3126. [Google Scholar] [CrossRef]
- Olsnes, S.; Pihl, A. Inhibition of peptide chain elongation. Nature 1972, 238, 459–461. [Google Scholar] [CrossRef]
- Funatsu, G.; Yoshitake, S.; Funatsu, M. Primary Structure of Ile Chain of Ricin D. Agric. Biol. Chem. 1978, 42, 501–503. [Google Scholar] [CrossRef]
- Funatsu, G.; Kimura, M.; Funatsu, M. Primary Structure of Ala Chain of Ricin D. Agric. Biol. Chem. 1979, 43, 2221–2224. [Google Scholar] [CrossRef]
- Montfort, W.; Villafranca, J.E.; Monzingo, A.F.; Ernst, S.R.; Katzin, B.; Rutenber, E.; Xuong, N.H.; Hamlin, R.; Robertus, J.D. The three-dimensional structure of ricin at 2.8 A. J. Biol. Chem. 1987, 262, 5398–5403. [Google Scholar] [PubMed]
- Rutenber, E.; Katzin, B.J.; Ernst, S.; Collins, E.J.; Mlsna, D.; Ready, M.P.; Robertus, J.D. Crystallographic refinement of ricin to 2.5 A. Proteins 1991, 10, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Katzin, B.J.; Collins, E.J.; Robertus, J.D. Structure of ricin A-chain at 2.5 A. Proteins 1991, 10, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Rutenber, E.; Robertus, J.D. Structure of ricin B-chain at 2.5 A resolution. Proteins 1991, 10, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Weston, S.A.; Tucker, A.D.; Thatcher, D.R.; Derbyshire, D.J.; Pauptit, R.A. X-ray structure of recombinant ricin A-chain at 1.8 A resolution. J. Mol. Biol. 1994, 244, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.A.; Bartle, L.M.; Leszyk, J.D.; Lambert, J.M.; Goldmacher, V.S. Ricin A chain can be chemically cross-linked to the mammalian ribosomal proteins L9 and L10e. J. Biol. Chem. 1995, 270, 12933–12940. [Google Scholar] [CrossRef] [PubMed]
- Monzingo, A.F.; Robertus, J.D. X-ray analysis of substrate analogs in the ricin A-chain active site. J. Mol. Biol. 1992, 227, 1136–1145. [Google Scholar] [CrossRef]
- Ready, M.P.; Kim, Y.; Robertus, J.D. Site-directed mutagenesis of ricin A-chain and implications for the mechanism of action. Proteins 1991, 10, 270–278. [Google Scholar] [CrossRef]
- Olsnes, S.; Pihl, A. Ricin-a potent inhibitor of protein synthesis. FEBS Lett. 1972, 20, 327–329. [Google Scholar] [CrossRef]
- Sperti, S.; Montanaro, L.; Mattioli, A.; Stirpe, F. Inhibition by ricin of protein synthesis in vitro: 60 S ribosomal subunit as the target of the toxin. Biochem. J. 1973, 136, 813–815. [Google Scholar] [CrossRef]
- Montanaro, L.; Sperti, S.; Stirpe, F. Inhibition by ricin of protein synthesis in vitro. Ribosomes as the target of the toxin. Biochem. J. 1973, 136, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperti, S.; Montanaro, L.; Mattioli, A.; Testoni, G. Relationship between elongation factor I- and elongation factor II—Dependent guanosine triphosphatase activities of ribosomes. Inhibition of both activities by ricin. Biochem. J. 1975, 148, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; Olsnes, S.; Pihl, A.; Skorve, J.; Abraham, A.K. On the mechanism of protein-synthesis inhibition by abrin and ricin. Inhibition of the GTP-hydrolysis site on the 60-S ribosomal subunit. Eur. J. Biochem. 1975, 59, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S.; Fernandez-Puentes, C.; Carrasco, L.; Vazquez, D. Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur. J. Biochem. 1975, 60, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Stirpe, F. Ribosome-inactivating proteins from plants: Properties and possible uses. Cancer Surv. 1982, 1, 489–520. [Google Scholar]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef]
- Endo, Y.; Tsurugi, K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987, 262, 8128–8130. [Google Scholar]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar]
- Barbieri, L.; Valbonesi, P.; Bonora, E.; Gorini, P.; Bolognesi, A.; Stirpe, F. Polynucleotide:adenosine glycosidase activity of ribosome-inactivating proteins: Effect on DNA, RNA and poly(A). Nucleic Acids Res. 1997, 25, 518–522. [Google Scholar] [CrossRef]
- Barbieri, L.; Bolognesi, A.; Valbonesi, P.; Polito, L.; Olivieri, F.; Stirpe, F. Polynucleotide: Adenosine glycosidase activity of immunotoxins containing ribosome-inactivating proteins. J. Drug Target. 2000, 8, 281–288. [Google Scholar] [CrossRef]
- Barbieri, L.; Brigotti, M.; Perocco, P.; Carnicelli, D.; Ciani, M.; Mercatali, L.; Stirpe, F. Ribosome-inactivating proteins depurinate poly(ADP-ribosyl)ated poly(ADP-ribose) polymerase and have transforming activity for 3T3 fibroblasts. FEBS Lett. 2003, 538, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Brigotti, M.; Alfieri, R.; Sestili, P.; Bonelli, M.; Petronini, P.G.; Guidarelli, A.; Barbieri, L.; Stirpe, F.; Sperti, S. Damage to nuclear DNA induced by Shiga toxin 1 and ricin in human endothelial cells. FASEB J. 2002, 16, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Olsnes, S.; Pihl, A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J. Biol. Chem. 1976, 251, 3977–3984. [Google Scholar] [PubMed]
- van Deurs, B.; Petersen, O.W.; Sundan, A.; Olsnes, S.; Sandvig, K. Receptor-mediated endocytosis of a ricin-colloidal gold conjugate in Vero cells: Intracellular routing to vacuolar and tubulo-vesicular portions of the endosomal system. Exp. Cell Res. 1985, 159, 287–304. [Google Scholar] [CrossRef]
- Moya, M.; Dautry-Varsat, A.; Goud, B.; Louvard, D.; Boquet, P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin. J. Cell Biol. 1985, 101, 548–559. [Google Scholar] [CrossRef] [PubMed]
- van Deurs, B.; Sandvig, K.; Petersen, O.W.; Olsnes, S.; Simons, K.; Griffiths, G. Routing of internalised ricin and ricin conjugates to the Golgi complex. J. Cell Biol. 1986, 102, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; van Deurs, B. Endocytosis, intracellular transport, and cytotoxic action of Shiga toxin and ricin. Physiol. Rev. 1996, 76, 949–966. [Google Scholar] [CrossRef]
- Cosson, P.; Letourneur, F. Coatamer interaction with di-lysine endoplasmic reticulum retention motifs. Science 1994, 263, 1629–1631. [Google Scholar] [CrossRef]
- Chen, A.; Hu, T.; Mikoryak, C.; Draper, R.K. Retrograde transport of protein toxins under conditions of COPI dysfunction. Biochim. Biophys. Acta 2002, 1589, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Wesche, J.; Rapak, A.; Olsnes, S. Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 1999, 274, 34443–34449. [Google Scholar] [CrossRef]
- Sandvig, K.; Bergan, J.; Kavaliauskiene, S.; Skotland, T. Lipid requirements for entry of protein toxins into cells. Prog. Lipid Res. 2014, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.M.; Smith, D.C. Ricin: The endoplasmic reticulum connection. Toxicon 2004, 44, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Eiklid, K.; Olsnes, S.; Pihl, A. Entry of lethal doses of abrin, ricin and modeccin into the cytosol of HeLa cells. Exp. Cell Res. 1980, 126, 321–326. [Google Scholar] [CrossRef]
- Harper, C.G.; Gonatas, J.O.; Mizutani, T.; Gonatas, N.K. Retrograde transport and effects of toxic ricin in the autonomic nervous system. Lab. Investig. 1980, 42, 396–404. [Google Scholar] [PubMed]
- Polito, L.; Bortolotti, M.; Farini, V.; Battelli, M.G.; Barbieri, L.; Bolognesi, A. Saporin induces multiple death pathways in lymphoma cells with different intensity and timing as compared to ricin. Int. J. Biochem. Cell Biol. 2009, 41, 1055–1061. [Google Scholar] [CrossRef]
- Tazzari, P.L.; Bolognesi, A.; de Totero, D.; Falini, B.; Lemoli, R.M.; Soria, M.R.; Pileri, S.; Gobbi, M.; Stein, H.; Flenghi, L.; et al. Ber-H2 (anti-CD30)-saporin immunotoxin: A new tool for the treatment of Hodgkin’s disease and CD30+ lymphoma: In vitro evaluation. Br. J. Haematol. 1992, 81, 203–211. [Google Scholar] [CrossRef]
- Ngo, T.N.; Nguyen, T.T.; Bui, D.T.-T.; Hoang, N.T.-M.; Nguyen, T.D. Effects of ricin extracted from seeds of the castor bean (Ricinus communis) on cytotoxicity and tumorigenesis of melanoma cells. Biomed. Res. Ther. 2016, 3, 633–644. [Google Scholar]
- Polito, L.; Bortolotti, M.; Pedrazzi, M.; Mercatelli, D.; Battelli, M.G.; Bolognesi, A. Apoptosis and necroptosis induced by stenodactylin in neuroblastoma cells can be completely prevented through caspase inhibition plus catalase or necrostatin-1. Phytomedicine 2016, 23, 32–41. [Google Scholar] [CrossRef]
- Roxas-Duncan, V.I.; Smith, L.A. Of Beans and Beads: Ricin and abrin in bioterrorism and biocrime. J. Bioterr. Biodef. 2012, S2, 002. [Google Scholar] [CrossRef]
- Whitfield, S.J.C.; Griffiths, G.D.; Jenner, D.C.; Gwyther, R.J.; Stahl, F.M.; Cork, L.J.; Holley, J.L.; Green, A.C.; Clark, G.C. Production, characterisation and testing of an ovine antitoxin against ricin; efficacy, potency and mechanisms of action. Toxins 2017, 9, 329. [Google Scholar] [CrossRef]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning-a comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.L.; David, J.; Griffiths, G.D. Retrospective identification of ricin in animal tissues following administration by pulmonary and oral routes. Toxicology 2006, 223, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.J.; Song, K.; Sivasubramani, S.K.; Gardner, D.J.; Pincus, S.H. Animal models of ricin toxicosis. Curr. Top. Microbiol. Immunol. 2012, 357, 243–257. [Google Scholar] [PubMed]
- He, X.; Carter, J.M.; Brandon, D.L.; Cheng, L.W.; McKeon, T.A. Application of a real time polymerase chain reaction method to detect castor toxin contamination in fluid milk and eggs. J. Agric. Food Chem. 2007, 55, 6897–6902. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S. The history of ricin, abrin and related toxins. Toxicon 2004, 44, 361–370. [Google Scholar] [CrossRef]
- Wedin, G.P.; Neal, J.S.; Everson, G.W.; Krenzelok, E.P. Castor bean poisoning. Am. J. Emerg. Med. 1986, 4, 259–261. [Google Scholar] [CrossRef]
- Worbs, S.; Köhler, K.; Pauly, D.; Avondet, M.A.; Schaer, M.; Dorner, M.B.; Dorner, B.G. Ricinus communis intoxications in human and veterinary medicine-a summary of real cases. Toxins 2011, 3, 1332–1372. [Google Scholar] [CrossRef]
- Grimshaw, B.; Wennike, N.; Dayer, M. Ricin poisoning: A case of internet-assisted parasuicide. Br. J. Hosp. Med. 2013, 74, 532–533. [Google Scholar] [CrossRef]
- Bradberry, S. Ricin and abrin. Medicine 2016, 44, 109–110. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Dickers, K.J.; Rice, P.; Griffiths, G.D.; Vale, J.A. Ricin poisoning. Toxicol. Rev. 2003, 22, 65–70. [Google Scholar] [CrossRef]
- OPCW-Organisation for the Prohibition of Chemical Weapons. Ricin Fact Sheet 2014. Available online: https://www.opcw.org/sites/default/files/documents/SAB/en/sab-21-wp05_e_.pdf (accessed on 15 April 2019).
- Pincus, S.H.; Bhaskaran, M.; Brey, R.N., 3rd; Didier, P.J.; Doyle-Meyers, L.A.; Roy, C.J. Clinical and Pathological Findings Associated with Aerosol Exposure of Macaques to Ricin Toxin. Toxins 2015, 7, 2121–2133. [Google Scholar] [CrossRef]
- Franz, D.R.; Jaax, N.K. Ricin Toxin. In Medical Aspects of Chemical and Biological Warfare; Sidell, F.R., Takafuji, E.T., Franz, D.R., Eds.; Walter Reed Army Medical Center, Borden Institute: Washington, DC, USA, 1997; Volume 3, pp. 631–642. [Google Scholar]
- Millard, C.; LeClaire, R. Ricin and related toxins: Review and perspective. In Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics, 2nd ed.; Brian, J., Lukey, J.A., Romano, J.A., Jr., Harry Salem, R., Lukey, B.J., Salem, H., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 424–452. [Google Scholar]
- Crompton, R.; Gall, D. Georgi Markov-death in a pellet. Med. Leg. J. 1980, 48, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Kortepeter, M.G.; Parker, G.W. Potential biological weapons threats. Emerg. Infect. Dis. 1999, 5, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Ordman, D. An outbreak of bronchial asthma in South Africa, affecting more than 200 persons, caused by castor bean dust from an oil processing factory. Int. Arch. Allergy Appl. Immunol. 1955, 7, 10–24. [Google Scholar] [CrossRef]
- Panzani, R.C. Respiratory castor bean dust allergy in the south of France with special reference to Marseilles. Int. Arch. Allergy Appl. Immunol. 1957, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.A. Castor. In Oilseed Crops, 2nd ed.; Blackwell Science Ltd.: Oxford, UK, 2000; pp. 31–36. [Google Scholar]
- Vashst, D.; Amhad, M. Statistical analysis of diesel engine performance for castor and jatropha biodiesel-blended fuel. IJAME 2014, 10, 2155–2169. [Google Scholar] [CrossRef]
- Falk, S. World Outlook for Castor Oil 2018. In Proceedings of the Global Castor Conference 2018, Ahmedabad, India, 23–24 February 2018. [Google Scholar]
- Food and Agriculture Organization of United States (FAO). 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 April 2019).
- Patel, V.R.; Dumancas, G.G.; Kasi Viswanath, L.C.; Maples, R.; Subong, B.J. Castor oil: Properties, uses, and optimization of processing parameters in commercial production. Lipid Insights 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Férnandez-Cruz, M.L.; Fürst, P.; Fink-Gremmels, J.; Galli, C.L.; et al. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on ricin (from Ricinus communis) as undesirable substances in animal feed. EFSA J. 2008, 726, 9–38. [Google Scholar] [CrossRef]
- Anandan, S.; Kumar, G.; Ghosh, J.; Ramachandra, K. Effect of different physical and chemical treatments on detoxification of ricin in castor cake. Anim. Feed Sci. Tech. 2004, 120, 159–168. [Google Scholar] [CrossRef]
- Smallshaw, J.E.; Vitetta, E.S. Ricin vaccine development. Curr. Top. Microbiol. Immunol. 2012, 357, 259–272. [Google Scholar] [PubMed]
- Rainey, G.J.; Young, J.A. Antitoxins: Novel strategies to target agents of bioterrorism. Nat. Rev. Microbiol. 2004, 2, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Stechmann, B.; Bai, S.K.; Gobbo, E.; Lopez, R.; Merer, G.; Pinchard, S.; Panigai, L.; Tenza, D.; Raposo, G.; Beaumelle, B.; et al. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell 2010, 141, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Barbier, J.; Bouclier, C.; Johannes, L.; Gillet, D. Inhibitors of the cellular trafficking of ricin. Toxins 2012, 4, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Gal, Y.; Mazor, O.; Alcalay, R.; Seliger, N.; Aftalion, M.; Sapoznikov, A.; Falach, R.; Kronman, C.; Sabo, T. Antibody/doxycycline combined therapy for pulmonary ricinosis: Attenuation of inflammation improves survival of ricin-intoxicated mice. Toxicol. Rep. 2014, 1, 496–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, J.M.; Whaley, K.; Pauly, M.; Zeitlin, L.C.; Mantis, N. Plant-based expression of a partially humanized neutralizing monoclonal IgG directed against an immunodominant epitope on the ricin toxin A subunit. Vaccine 2012, 30, 1239–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sully, E.K.; Whaley, K.J.; Bohorova, N.; Goodman, C.; Kim, D.H.; Pauly, M.H.; Velasco, J.; Hiatt, E.; Morton, J.; Swope, K.; et al. Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin. Vaccine Immunol. 2014, 21, 777–782. [Google Scholar] [CrossRef]
- Van Slyke, G.; Sully, E.K.; Bohorova, N.; Bohorov, O.; Kim, D.; Pauly, M.; Whaley, K.; Zeitlin, L.; Mantis, N.J. Humanized monoclonal antibody that passively protects mice 3 against systemic and intranasal ricin toxin challenge. Clin. Vaccine Immunol. 2016, 23, 795–799. [Google Scholar] [CrossRef]
- Pratt, T.S.; Pincus, S.H.; Hale, M.L.; Moreira, A.L.; Roy, C.J.; Tchou-Wong, K.-M. Oropharyngeal aspiration of ricin as a lung challenge model for evaluation of the therapeutic index of antibodies against ricin A chain for post-exposure treatment. Exp. Lung Res. 2007, 33, 459–481. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Alcalay, R.; Epstein, E.; Sabo, T.; Kronman, C. Extended therapeutic window for post exposure treatment of ricin intoxication conferred by the use of high-affinity antibodies. Toxicon 2017, 127, 100–105. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Rosenfeld, R.; Ariel, N.; Epstein, E.; Alcalay, R.; Zvi, A.; Kronman, C.; Ordentlich, A.; Mazor, O. Isolation of anti-ricin protective antibodies exhibiting high affinity from immunized non-human primates. Toxins 2016, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Moolten, F.; Zajdel, S.; Cooperband, S. Immunotherapy of experimental animal tumors with antitumor antibodies conjugated to diphtheria toxin or ricin. Ann. N. Y. Acad. Sci. 1976, 277, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Weng, A.; Mallinckrodtm, B.; Melzig, M.F.; Fuchs, H.; Thakur, M. Immunotoxins constructed with ribosome-inactivating proteins and their enhancers: A lethal cocktail with tumor specific efficacy. Curr. Pharm. Des. 2014, 20, 6584–6643. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Tyagi, M.; Pachauri, M.; Ghosh, P.C. Potential therapeutic applications of plant toxin-ricin in cancer: Challenges and advances. Tumour Biol. 2015, 36, 8239–8246. [Google Scholar] [CrossRef] [PubMed]
- Blythman, H.E.; Casellas, P.; Gros, O.; Gros, P.; Jansen, F.K.; Paolucci, F.; Pau, B.; Vidal, H. Immunotoxins: Hybrid molecules of monoclonal antibodies and a toxin subunit specifically kill tumour cells. Nature 1981, 290, 145–146. [Google Scholar] [CrossRef]
- Weil-Hillman, G.; Runge, W.; Jansen, F.K.; Vallera, D.A. Cytotoxic effect of anti-Mr 67,000 protein immunotoxins on human tumors in a nude mouse model. Cancer Res. 1985, 45, 1328–1336. [Google Scholar]
- Ghetie, V.; Ghetie, M.A.; Uhr, J.W.; Vitetta, E.S. Large scale preparation of immunotoxins constructed with the Fab’ fragment of IgG1 murine monoclonal antibodies and chemically deglycosylated ricin A chain. J. Immunol. Methods 1988, 112, 267–277. [Google Scholar] [CrossRef]
- Ghetie, M.A.; Richardson, J.; Tucker, T.; Jones, D.; Uhr, J.W.; Vitetta, E.S. Antitumor activity of Fab’ and IgG-anti-CD22 immunotoxins in disseminated human B lymphoma grown in mice with severe combined immunodeficiency disease: Effect on tumor cells in extranodal sites. Cancer Res. 1991, 51, 5876–5880. [Google Scholar]
- FitzGerald, D.J.; Bjorn, M.J.; Ferris, R.J.; Winkelhake, J.L.; Frankel, A.E.; Hamilton, T.C.; Ozols, R.F.; Willingham, M.C.; Pastan, I. Antitumor activity of an immunotoxin in a nude mouse model of human ovarian cancer. Cancer Res. 1987, 47, 1407–1410. [Google Scholar]
- Wang, D.; Li, Q.; Hudson, W.; Berven, E.; Uckun, F.; Kersey, J.H. Generation and characterization of an anti-CD19 single-chain Fv immunotoxin composed of C-terminal disulfide-linked dgRTA. Bioconj. Chem. 1997, 8, 878–884. [Google Scholar] [CrossRef]
- Hertler, A.A.; Splitler, L.; Frankel, A. Humoral immune response to a ricin A chain immunotoxin in patients with metastatic melanoma. Cancer Drug Deliv. 1987, 4, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Spitler, L.E.; del Rio, M.; Khentigan, A.; Wedel, N.I.; Brophy, N.A.; Miller, L.L.; Harkonen, W.S.; Rosendorf, L.L.; Lee, H.M.; Mischak, R.P.; et al. Therapy of patients with malignant melanoma using a monoclonal antimelanoma antibody-ricin A chain immunotoxin. Cancer Res. 1987, 47, 1717–1723. [Google Scholar] [PubMed]
- Mischak, R.P.; Foxall, C.; Rosendorf, L.L.; Knebel, K.; Scannon, P.J.; Spitler, L.E. Human antibody responses to components of the monoclonal antimelanoma antibody ricin A chain immunotoxin Xoma-Zyme-MEL. Mol. Biother. 1990, 2, 104–109. [Google Scholar] [PubMed]
- Strand, V.; Lipsky, P.E.; Cannon, G.W.; Calabrese, L.H.; Wiesenhutter, C.; Cohen, S.B.; Olsen, N.J.; Lee, M.L.; Lorenz, T.J.; Nelson, B. Effects of administration of an anti-CD5 plus immunoconjugate in rheumatoid arthritis. Results of two phase II studies. The CD5 plus rheumatoid arthritis investigators group. Arthritis Rheum. 1993, 36, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Stafford, F.J.; Fleisher, T.A.; Lee, G.; Brown, M.; Strand, V.; Austin, H.A.; Balow, J.E.; Klippel, J.H. A pilot study of anti-CD5 ricin A chain immunoconjugate in systemic lupus erythematosus. J. Rheumatol. 1994, 21, 2068–2070. [Google Scholar] [PubMed]
- Polito, L.; Djemil, A.; Bortolotti, M. Plant Toxin-Based Immunotoxins for Cancer Therapy: A Short Overview. Biomedicines 2016, 4, E12. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Farajnia, S.; Ahdi Khosroshahi, S.; Safari, F.; Yousefi, M.; Dariushnejad, H.; Rahbarnia, L. Immunotoxins in cancer therapy: Review and update. Int. Rev. Immunol. 2017, 36, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L.; et al. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 2011, 5, 5729–5745. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.; Pallarès, V.; Cano-Garrido, O.; Serna, N.; Sánchez-García, L.; Falgàs, A.; Pesarrodona, M.; Unzueta, U.; Sánchez-Chardi, A.; Sánchez, J.M.; et al. Selective CXCR4(+) cancer cell targeting and potent antineoplastic effect by a nanostructured version of recombinant ricin. Small 2018, 14, 1800665. [Google Scholar] [CrossRef]
- Polito, L.; Mercatelli, D.; Bortolotti, M.; Maiello, S.; Djemil, A.; Battelli, M.G.; Bolognesi, A. Two Saporin-Containing Immunotoxins Specific for CD20 and CD22 Show Different Behavior in Killing Lymphoma Cells. Toxins 2017, 9, 182. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, Y.; Yusufali, A.H.; Ashby, F.; Zhang, D.; Yin, Z.F.; Aslanidi, G.V.; Srivastava, A.; Ling, C.Q.; Ling, C. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J. Integr. Med. 2014, 12, 483–494. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polito, L.; Bortolotti, M.; Battelli, M.G.; Calafato, G.; Bolognesi, A. Ricin: An Ancient Story for a Timeless Plant Toxin. Toxins 2019, 11, 324. https://doi.org/10.3390/toxins11060324
Polito L, Bortolotti M, Battelli MG, Calafato G, Bolognesi A. Ricin: An Ancient Story for a Timeless Plant Toxin. Toxins. 2019; 11(6):324. https://doi.org/10.3390/toxins11060324
Chicago/Turabian StylePolito, Letizia, Massimo Bortolotti, Maria Giulia Battelli, Giulia Calafato, and Andrea Bolognesi. 2019. "Ricin: An Ancient Story for a Timeless Plant Toxin" Toxins 11, no. 6: 324. https://doi.org/10.3390/toxins11060324
APA StylePolito, L., Bortolotti, M., Battelli, M. G., Calafato, G., & Bolognesi, A. (2019). Ricin: An Ancient Story for a Timeless Plant Toxin. Toxins, 11(6), 324. https://doi.org/10.3390/toxins11060324