Evaluation of the Spider (Phlogiellus genus) Phlotoxin 1 and Synthetic Variants as Antinociceptive Drug Candidates
Abstract
:1. Introduction
2. Results
2.1. Chemical Synthesis and In Vitro Folding of PhlTx1
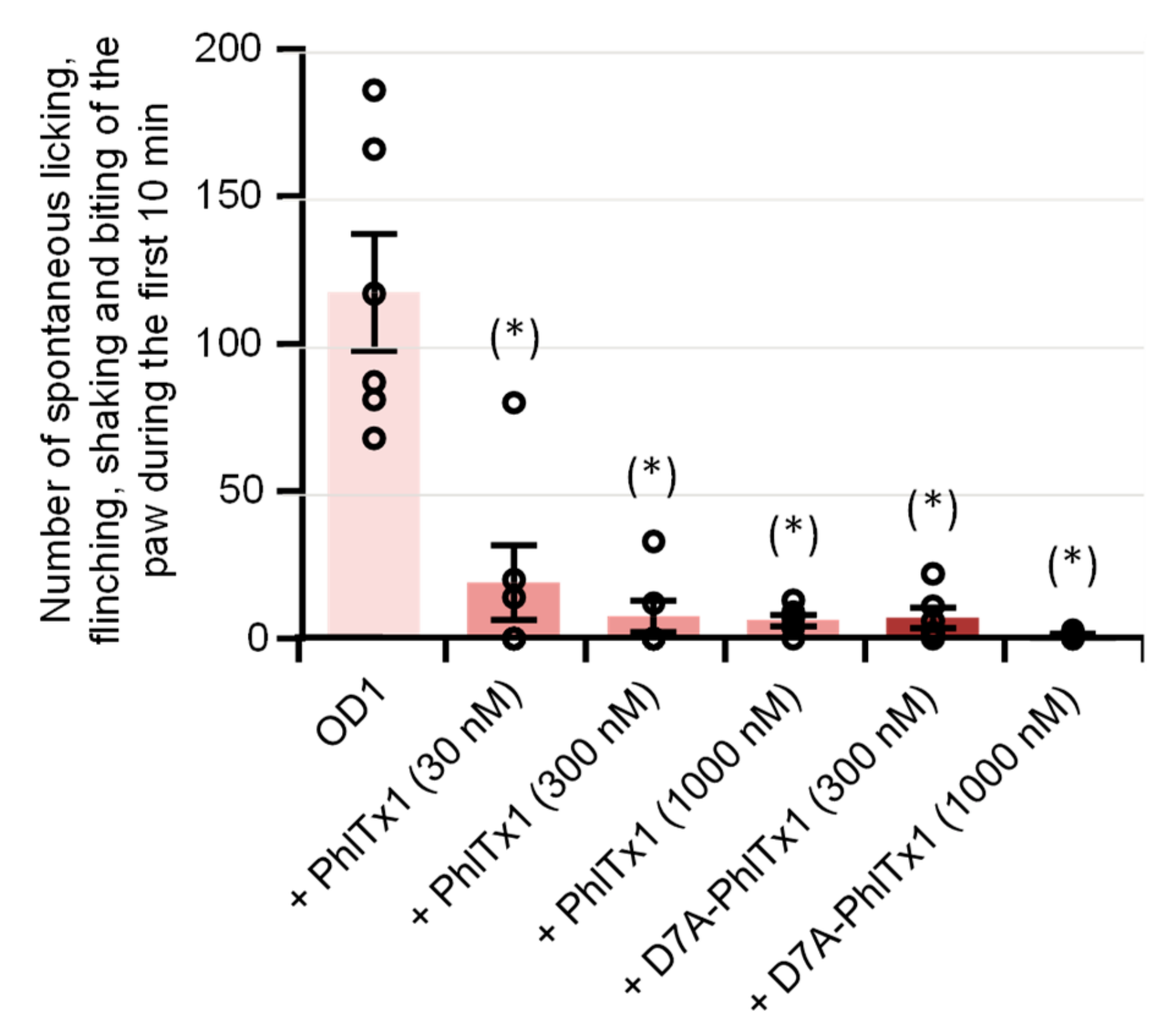
2.2. Effects of PhlTx1 on a Mouse Model of NaV1.7-Mediated Pain
2.3. Effects of PhlTx1 on hNaV1.1-1.8, hCaV1.2, and KV11.1 Channel Subtypes
2.4. Structure–Activity Relationships of PhlTx1 Using an Alanine-Substituted Approach
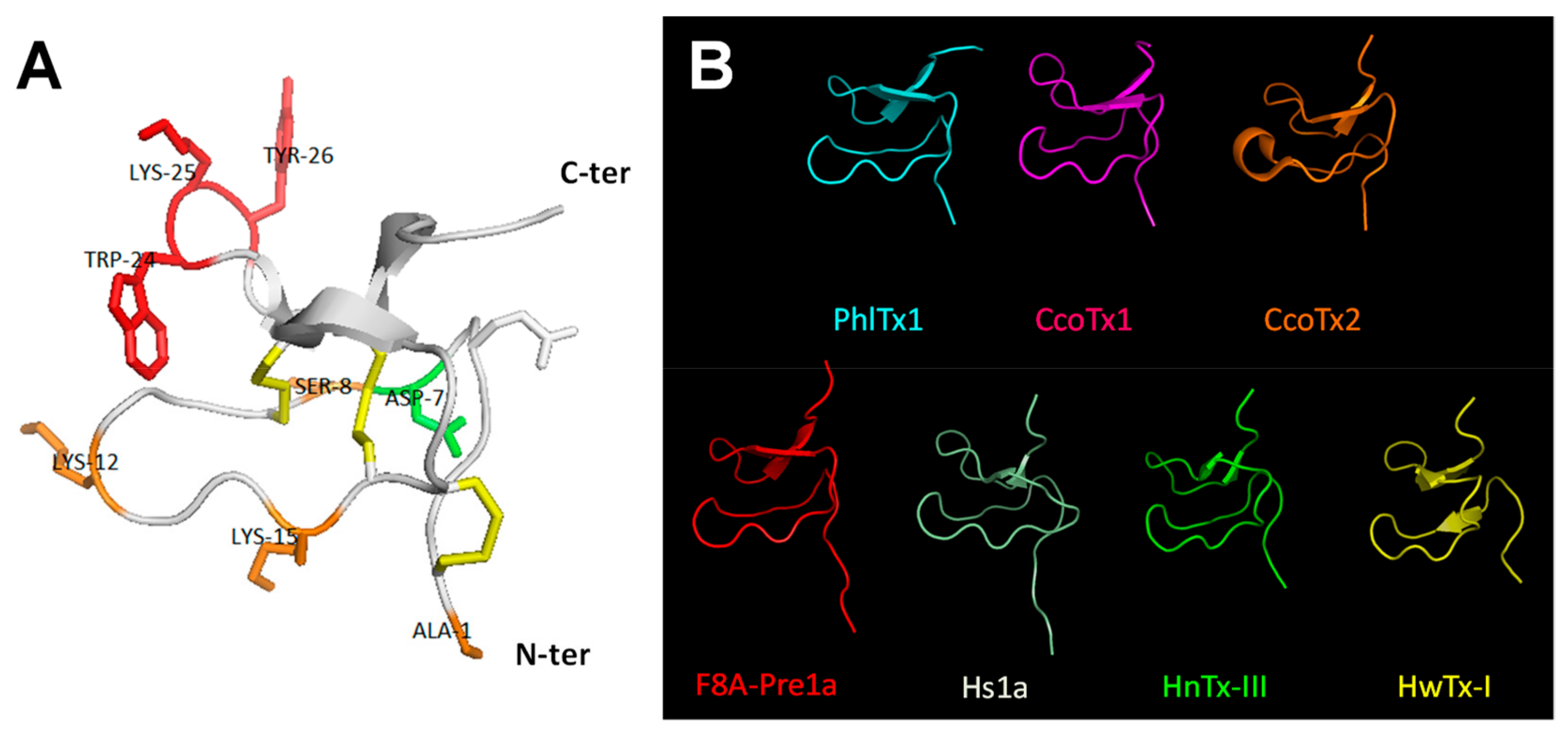
2.5. Comparison of Amino Acid Sequences and 3D-Structures of PhlTx1 with Closely Related Toxins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemical Synthesis and In Vitro Folding of PhlTx1 and Its Variants
5.2. Predicted 3D-Structure of PhlTx1
5.3. Toxins and Chemicals
5.4. Cell Lines Used for Functional Assays
5.5. Automated Whole-Cell Patch-Clamp Electrophysiology
5.6. Mouse Model of NaV1.7-Mediated Pain
5.7. Statistical Analyses
Author Contributions
Funding
Acknowledgments
References
- Goldberg, D.S.; Mcgee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.L.; Wu, Z.Z.; Zhou, H.Y.; Chen, S.R.; Zhang, H.M.; Li, D.P. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, D.L.; Woods, C.G. Painful and painless channelopathies. Lancet Neurol. 2014, 13, 587–599. [Google Scholar] [CrossRef]
- Waxman, S.G.; Zamponi, G.W. Regulating excitability of peripheral afferents: Emerging ion channel targets. Nat. Neurosci. 2014, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Spray, D.C.; Hanani, M. Gap junctions, pannexins and pain. Neurosci. Lett. 2017, 695, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, S.R.; Kirsh, K.L.; Passik, S.D. Challenges in using opioids to treat pain in persons with substance use disorders. Addict. Sci. Clin. Pract. 2008, 4, 4–25. [Google Scholar] [CrossRef]
- Negus, S.S. Addressing the opioid crisis: The importance of choosing translational endpoints in analgesic drug discovery. Trends Pharmacol. Sci. 2018, 39, 327–330. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- De Lera Ruiz, M.; Kraus, R.L. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Vetter, I.; Deuis, J.R.; Mueller, A.; Israel, M.R.; Starobova, H.; Zhang, A.; Rash, L.D.; Mobli, M. NaV1.7 as a pain target - From gene to pharmacology. Pharmacol. Ther. 2017, 172, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.C.; Benoit, E.; Partiseti, M.; Servent, D. The NaV1.7 channel subtype as an antinociceptive target for spider toxins in adult dorsal root ganglia neurons. Front. Pharmacol. 2018, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Klint, J.K.; Smith, J.J.; Vetter, I.; Rupasinghe, D.B.; Er, S.Y.; Senff, S.; Herzig, V.; Mobli, M.; Lewis, R.J.; Bosmans, F.; et al. Seven novel modulators of the analgesic target NaV 1.7 uncovered using a high-throughput venom-based discovery approach. Br. J. Pharmacol. 2015, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Dekan, Z.; Rosengren, K.J.; Erickson, A.; Vetter, I.; Deuis, J.R.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Identification and characterization of ProTx-III [mu-TRTX-Tp1a], a new voltage-gated sodium channel inhibitor from venom of the tarantula Thrixopelma pruriens. Mol. Pharmacol. 2015, 88, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.C.; Benoit, E.; Kurz, M.; Lucarain, L.; Fouconnier, S.; Combemale, S.; Jaquillard, L.; Schombert, B.; Chambard, J.M.; Boukaiba, R.; et al. From identification to functional characterization of cyriotoxin-1a, an antinociceptive toxin from Cyriopagopus schioedtei spider. Br. J. Pharmacol. 2019, 176, 1298–1314. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Lewis, R.J. Structure-function and therapeutic potential of spider venom-derived cysteine knot peptides targeting sodium channels. Front. Pharmacol. 2019, 10, 366. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Zhang, Y.; Xun, X.; Tang, D.; Peng, D.; Yi, J.; Liu, Z.; Shi, X. Synthesis and analgesic effects of mu-TRTX-Hhn1b on models of inflammatory and neuropathic pain. Toxins 2014, 6, 2363–2378. [Google Scholar] [CrossRef]
- Deuis, J.R.; Wingerd, J.S.; Winter, Z.; Durek, T.; Dekan, Z.; Sousa, S.R.; Zimmermann, K.; Hoffmann, T.; Weidner, C.; Nassar, M.A.; et al. Analgesic effects of GpTx-1, PF-04856264 and CNV1014802 in a mouse model of NaV1.7-mediated pain. Toxins 2016, 8, 78. [Google Scholar] [CrossRef]
- Flinspach, M.; Xu, Q.; Piekarz, A.D.; Fellows, R.; Hagan, R.; Gibbs, A.; Liu, Y.; Neff, R.A.; Freedman, J.; Eckert, W.A.; et al. Insensitivity to pain induced by a potent selective closed-state NaV1.7 inhibitor. Sci. Rep. 2017, 7, 39662. [Google Scholar]
- Rahnama, S.; Deuis, J.R.; Cardoso, F.C.; Ramanujam, V.; Lewis, R.J.; Rash, L.D.; King, G.F.; Vetter, I.; Mobli, M. The structure, dynamics and selectivity profile of a NaV1.7 potency-optimised huwentoxin-IV variant. PLoS ONE 2017, 12, e0173551. [Google Scholar] [CrossRef]
- Gonçalves, T.C.; Boukaiba, R.; Molgὀ, J.; Amar, M.; Partiseti, M.; Servent, D.; Benoit, E. Direct evidence for high affinity blockade of NaV1.6 channel subtype by huwentoxin-IV spider peptide, using multiscale functional approaches. Neuropharmacology 2018, 133, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Escoubas, P.; Diochot, S.; Mebs, D.; Craik, D.; Hill, J.; Nakajima, T.; Lazdunski, M.; Tytgat, J. Isolation and characterization of Phlotoxin 1 (PhlTx1), a novel peptide active on voltage-gated sodium channels. In Proceedings of the 13èmes Rencontres en Toxinologie “Toxines et douleur”, Paris, France, 1–2 December 2005. [Google Scholar]
- Escoubas, P.; Bosmans, F.; Cuypers, E.; Diochot, S.; Mebs, D.; Craik, D.; Hill, J.; Nakajima, T.; Lazdunski, M.; Tytgat, J. Phlotoxin 1, a toxin from tarantula venom, is a potent modulator of NaV1.7 sodium channels and a potential analgesic. In Proceedings of the 15th World congress on Animal, Plant & Microbial Toxins, Glasgow, UK, 23–28 July 2006. [Google Scholar]
- Nicolas, S.; Zoukimian, C.; Bosmans, F.; Montnach, J.; Diochot, S.; Cuypers, E.; De Waard, S.; Béroud, R.; Mebs, D.; Craik, D.; et al. Chemical synthesis, proper folding, NaV channel selectivity profile and analgesic properties of the spider peptide Phlotoxin 1. Toxins 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Luiz, A.P.; Wood, J.N. NaV1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin. Ther. Targets 2016, 20, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Dekan, Z.; Wingerd, J.S.; Smith, J.J.; Munasinghe, N.R.; Bhola, R.F.; Imlach, W.L.; Herzig, V.; Armstrong, D.A.; Rosengren, K.J.; et al. Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a. Sci. Rep. 2017, 7, 40883. [Google Scholar] [CrossRef] [PubMed]
- Minett, M.S.; Pereira, V.; Sikandar, S.; Matsuyama, A.; Lolignier, S.; Kanellopoulos, A.H.; Mancini, F.; Iannetti, G.D.; Bogdanov, Y.D.; Santana-Varela, S.; et al. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nat. Commun. 2015, 6, 8967. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Cai, T.; Zhu, Q.; Deng, M.; Li, J.; Zhou, X.; Zhang, F.; Li, D.; Li, J.; Liu, Y.; et al. Structure and function of hainantoxin-III, a selective antagonist of neuronal tetrodotoxin-sensitive voltage-gated sodium channels isolated from the Chinese bird spider Ornithoctonus hainana. J. Biol. Chem. 2013, 288, 20392–20403. [Google Scholar] [CrossRef] [PubMed]
- Shcherbatko, A.; Rossi, A.; Foletti, D.; Zhu, G.; Bogin, O.; Galindo Casas, M.; Rickert, M.; Hasa-Moreno, A.; Bartsevich, V.; Crameri, A.; et al. Engineering highly potent and selective microproteins against NaV1.7 sodium channel for treatment of pain. J. Biol. Chem. 2016, 291, 13974–13986. [Google Scholar] [CrossRef]
- Sousa, S.R.; Wingerd, J.S.; Brust, A.; Bladen, C.; Ragnarsson, L.; Herzig, V.; Deuis, J.R.; Dutertre, S.; Vetter, I.; Zamponi, G.W.; et al. Discovery and mode of action of a novel analgesic beta-toxin from the African spider Ceratogyrus darlingi. PLoS ONE 2017, 12, e0182848. [Google Scholar] [CrossRef]
- Herzig, V.; Wood, D.L.; Newell, F.; Chaumeil, P.A.; Kaas, Q.; Binford, G.J.; Nicholson, G.M.; Gorse, D.; King, G.F. ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 2011, 39, D653–D657. [Google Scholar] [CrossRef]
- Agwa, A.J.; Peigneur, S.; Chow, C.Y.; Lawrence, N.; Craik, D.J.; Tytgat, J.; King, G.F.; Henriques, S.T.; Schroeder, C.I. Gating modifier toxins isolated from spider venom: Modulation of voltage-gated sodium channels and the role of lipid membranes. J. Biol. Chem. 2018, 293, 9041–9052. [Google Scholar] [CrossRef] [Green Version]
- Wingerd, J.S.; Mozar, C.A.; Ussing, C.A.; Murali, S.S.; Chin, Y.K.; Cristofori-Armstrong, B.; Durek, T.; Gilchrist, J.; Vaughan, C.W.; Bosmans, F.; et al. The tarantula toxin beta/delta-TRTX-Pre1a highlights the importance of the S1-S2 voltage-sensor region for sodium channel subtype selectivity. Sci. Rep. 2017, 7, 974. [Google Scholar] [CrossRef] [PubMed]
- Klint, J.K.; Castro, J.; Vetter, I.; Er, S.Y.; Cardoso, F.; Liu, Y.; Hagan, R.; Neff, R.; Minassian, N.; Huang, J.X.; et al. NaV1.7 inhibitors normalise mechanical responses in chronic visceral hypersensitivity. Protein Data Bank 2015. submitted. [Google Scholar]
- Meir, A.; Cherki, R.S.; Kolb, E.; Langut, Y.; Bajayo, N. Novel peptides isolated from spider venom, and uses thereof. UniProtKB 2012. submitted. [Google Scholar]
- Wang, M.; Rong, M.; Xiao, Y.; Liang, S. The effects of huwentoxin-I on the voltage-gated sodium channels of rat hippocampal and cockroach dorsal unpaired median neurons. Peptides 2012, 34, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Bingham, J.P.; Zhu, W.; Moczydlowski, E.; Liang, S.; Cummins, T.R. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain II voltage sensor in the closed configuration. J. Biol. Chem. 2008, 283, 27300–27313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Wu, Z.; Li, J.; Nie, D.; Xiang, Y.; Liu, Z. A positively charged surface patch is important for hainantoxin-IV binding to voltage-gated sodium channels. J. Pept. Sci. 2012, 18, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.K.; Ligutti, J.; Liu, D.; Zou, A.; Poppe, L.; Li, H.; Andrews, K.L.; Moyer, B.D.; Mcdonough, S.I.; Favreau, P.; et al. Engineering potent and selective analogues of GpTx-1, a tarantula venom peptide antagonist of the NaV1.7 sodium channel. J. Med. Chem. 2015, 58, 2299–2314. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.K.; Long, J.; Zou, A.; Ligutti, J.; Andrews, K.L.; Poppe, L.; Biswas, K.; Moyer, B.D.; Mcdonough, S.I.; Miranda, L.P. Single residue substitutions that confer voltage-gated sodium ion channel subtype selectivity in the NaV1.7 inhibitory peptide GpTx-1. J. Med. Chem. 2016, 59, 2704–2717. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Dekan, Z.; Smith, J.J.; Deuis, J.R.; Vetter, I.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Modulatory features of the novel spider toxin mu-TRTX-Df1a isolated from the venom of the spider Davus fasciatus. Br. J. Pharmacol. 2017, 174, 2528–2544. [Google Scholar] [CrossRef]
- Minassian, N.A.; Gibbs, A.; Shih, A.Y.; Liu, Y.; Neff, R.A.; Sutton, S.W.; Mirzadegan, T.; Connor, J.; Fellows, R.; Husovsky, M.; et al. Analysis of the structural and molecular basis of voltage-sensitive sodium channel inhibition by the spider toxin huwentoxin-IV (mu-TRTX-Hh2a). J. Biol. Chem. 2013, 288, 22707–22720. [Google Scholar] [CrossRef]
- Agwa, A.J.; Lawrence, N.; Deplazes, E.; Cheneval, O.; Chen, R.M.; Craik, D.J.; Schroeder, C.I.; Henriques, S.T. Spider peptide toxin HwTx-IV engineered to bind to lipid membranes has an increased inhibitory potency at human voltage-gated sodium channel hNaV1.7. Biochim. Biophys. Acta 2017, 1859, 835–844. [Google Scholar] [CrossRef] [PubMed]



| PhlTx1 | Production | Biochemical Characterization | Pharmacological Characterization 1 | |||
|---|---|---|---|---|---|---|
| Linear (mg) | Oxidized (mg) | Folding yield (%) | Theo. Mass | Exp. Mass | hNaV1.7 IC50 (nM) | |
| WT | 150 | 17.3 | 12 | 4055.72 | 4055.73 | 254.3 ± 147.6 (5) |
| A1Z * | 9.8 | 1.1 | 11 | 4095.71 | 4095.71 | 171.5 ± 51.0 (5) |
| D7A | 12.1 | 2.5 | 21 | 4011.73 | 4011.72 | 47.0 ± 40.9 (3) |
| S8A | 9.9 | 0.55 | 6 | 4039.72 | 4039.70 | 179.5 ± 118.4 (4) |
| K12A | 9.5 | 0.75 | 8 | 3998.66 | 3998.67 | 252.5 ± 13.6 (4) |
| K15A | 8.6 | 1.1 | 13 | 3998.66 | 3998.66 | 706.4 ± 87.8 (5) |
| W24A | 9.0 | 1.1 | 12 | 3940.68 | 3940.68 | > 10000 (4) |
| K25A | 9.2 | 2.0 | 22 | 3998.66 | 3998.67 | > 10000 (3) |
| Y26A | 11.1 | 2.5 | 23 | 3963.69 | 3963.68 | > 10000 (6) |
| Name * | Amino Acid Sequence | Identity | Target | IC50/hNaV1.7 (nM) | |
|---|---|---|---|---|---|
| PhlTx1 | (µ-TRTX-Pspp-1) | --A--CLGQWDSCDPKASKCCPNYACEWKYPWCRYKLF- | 100% | NaV | 254 |
| OAIP2 | --D--CLGQWASCEPKNSKCCPNYACTWKYPWCRYRAGK | 76% | ? | - | |
| TlTx1 | (κ-TRTX-Tb1a) | -AA--CLGMFESCDPNNDKCCPNRECNRKHKWCKYKLW- | 59% | KV | - |
| Osp1b | (µ-TRTX-Osp1b) | --E--CLGWMKGCEPKNNKCCSSYVCTYKYPWCRYDL-- | 58% | NaV | -1 |
| TlTx2 | (κ-TRTX-Tb1b) | -DD--CLGMFSSCDPKNDKCCPNRVCRSRDQWCKYKLW- | 56% | KV | - |
| Cd1a | (β-TRTX-Cd1a) | --D--CLGWFKSCDPKNDKCCKNYSCSRRDRWCKYDL-- | 55% | NaV | 16 [30] |
| CcoTx1 | (β-TRTX-Cm1a) | --D--CLGWFKSCDPKNDKCCKNYTCSRRDRWCKYDL-- | 55% | NaV | 2-130 [29,32] |
| CcoTx2 | (β-TRTX-Cm1b) | --D--CLGWFKSCDPKNDKCCKNYTCSRRDRWCKYYL-- | 55% | NaV | 95 [32] |
| TlTx3 | (κ-TRTX-Tb1c) | -DD--CLGMFSSCDPNNDKCCPNRVCRVRDQWCKYKLW- | 53% | KV | - |
| Pre1a | (β/δ-TRTX-Pre1a) | -ED--CLGWFSRCSPKNDKCCPNYKCSSKDLWCKYKIW- | 53% | NaV | 114 [33] |
| Hs1a | GND--CLGFWSACNPKNDKCCANLVCSSKHKWCKGKL- | 52% | NaV | - [34] | |
| HnTx-III | (µ-TRTX-Hhn2a) | ----GCKGFGDSCTPGKNECCPNYACSSKHKWCKVYLGK | 50% | NaV | 232 [28] |
| Ccy1b | (µ-TRTX-Ccy1b) | -DD--CLGFFKSCNPDNDKCCENYKCNRRDKWCKYVL-- | 48% | NaV | - [13] |
| Pmr1a | (µ-TRTX-Pmr1a) | -DD--CLGMFSSCDPDNDKCCEGRKCNRKDKWCKYVL-- | 48% | NaV | - [35] |
| HwTx-I | (µ/ω-TRTX-Hs1a) | ----ACKGVFDACTPGKNECCPNRVCSDKHKWCKWKL-- | 45% | NaV/CaV | <200 [36] |
| Channel Subtype | Drug Reference | ||||
|---|---|---|---|---|---|
| Name | Molecular Mass | Purity Rate | Provider | IC90–95 1 (µM) | |
| hNaV1.1–1.8 | Tetrodotoxin (citrate) | 319.27 | > 98% | Sigma-Aldrich | 1 |
| hCaV1.2 | Nifedipine | 346.33 | > 98% | Sigma-Aldrich | 10 |
| Cadmium (chloride) 1 | 183.32 | > 99% | Sigma-Aldrich | 200 | |
| hKV11.1 | BeKm-1 | 4091.70 | > 97% | Smartox Biotechnology | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, T.C.; Lesport, P.; Kuylle, S.; Stura, E.; Ciolek, J.; Mourier, G.; Servent, D.; Bourinet, E.; Benoit, E.; Gilles, N. Evaluation of the Spider (Phlogiellus genus) Phlotoxin 1 and Synthetic Variants as Antinociceptive Drug Candidates. Toxins 2019, 11, 484. https://doi.org/10.3390/toxins11090484
Gonçalves TC, Lesport P, Kuylle S, Stura E, Ciolek J, Mourier G, Servent D, Bourinet E, Benoit E, Gilles N. Evaluation of the Spider (Phlogiellus genus) Phlotoxin 1 and Synthetic Variants as Antinociceptive Drug Candidates. Toxins. 2019; 11(9):484. https://doi.org/10.3390/toxins11090484
Chicago/Turabian StyleGonçalves, Tânia C., Pierre Lesport, Sarah Kuylle, Enrico Stura, Justyna Ciolek, Gilles Mourier, Denis Servent, Emmanuel Bourinet, Evelyne Benoit, and Nicolas Gilles. 2019. "Evaluation of the Spider (Phlogiellus genus) Phlotoxin 1 and Synthetic Variants as Antinociceptive Drug Candidates" Toxins 11, no. 9: 484. https://doi.org/10.3390/toxins11090484
APA StyleGonçalves, T. C., Lesport, P., Kuylle, S., Stura, E., Ciolek, J., Mourier, G., Servent, D., Bourinet, E., Benoit, E., & Gilles, N. (2019). Evaluation of the Spider (Phlogiellus genus) Phlotoxin 1 and Synthetic Variants as Antinociceptive Drug Candidates. Toxins, 11(9), 484. https://doi.org/10.3390/toxins11090484







