Botulinum Neurotoxins and Cancer—A Review of the Literature
Abstract
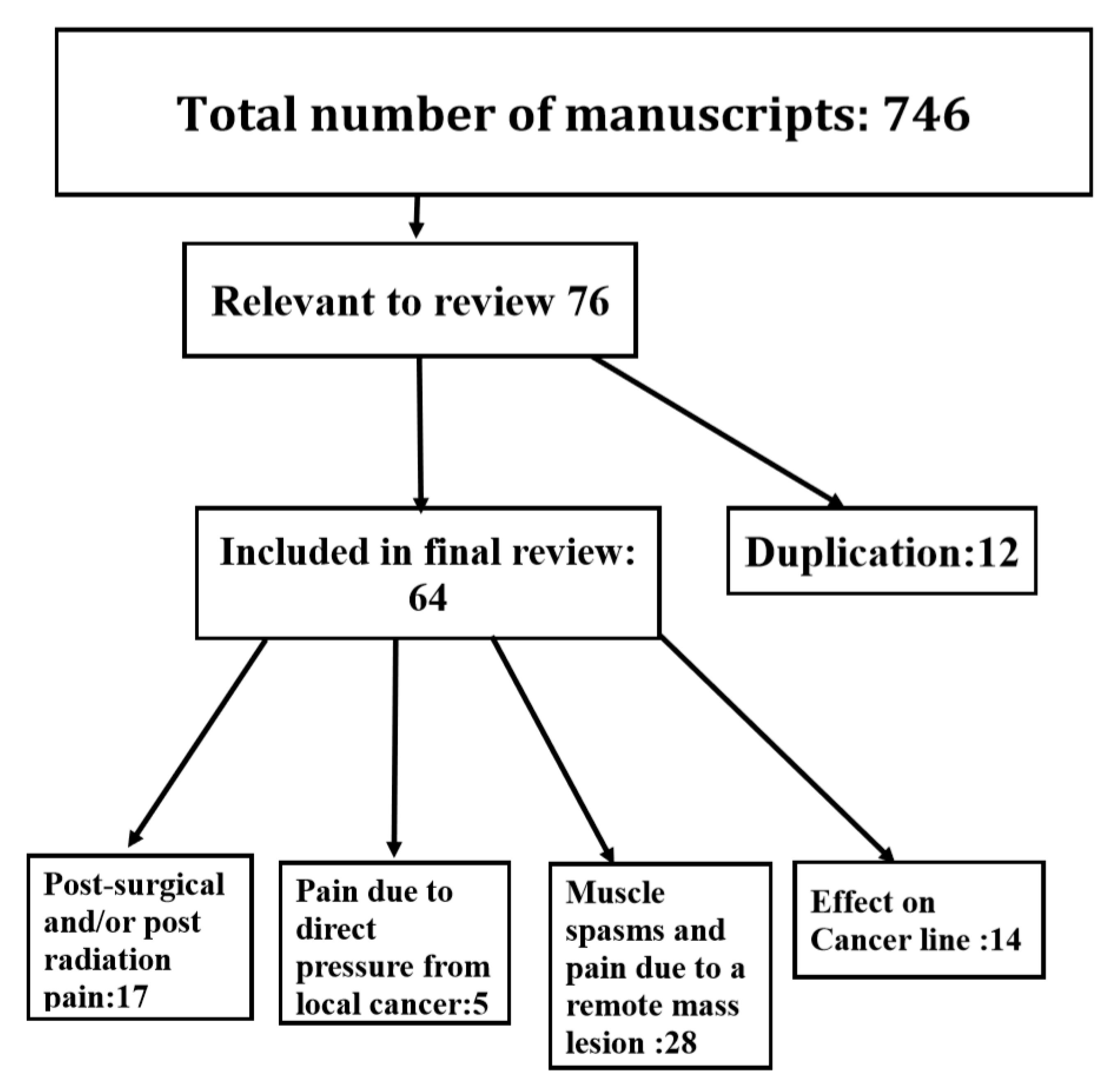
:1. Introduction
2. Results
2.1. Botulinum Neurotoxins Therapy for Post-Radiation And/Or Post-Surgical Cancer Pain
2.2. Botulinum Neurotoxins Therapy for Post-Radiation or Postsurgical Damage to Parotid Gland
2.3. The Effects of Botulinum Neurotoxins Injections on Malignant Tumors and Cancer Cell Line
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Jankovic, J. Botulinum toxin: State of the Art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef]
- Jabbari, B. (Ed.) A disease- oriented approach. In Botulinum Toxin Treatment in Clinical Medicine; Springer: New York, NY, USA, 2018. [Google Scholar]
- Aoki, K.R. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003, 43 (Suppl. S1), 9–15. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010, 50, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Chung, M.E. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins 2015, 7, 3127–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Park, H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safarpour, Y.; Jabbari, B. Botulinum toxin treatment of pain syndromes—An evidence based review. Toxicon 2018, 147, 120–128. [Google Scholar] [CrossRef]
- Shaw, L.; Bazzell, A.F.; Dains, J.E. Botulinum Toxin for Side-Effect Management and Prevention of Surgical Complications in Patients Treated for Head and Neck Cancers and Esophageal Cancer. J. Adv. Pract. Oncol. 2019, 10, 40–52. [Google Scholar] [PubMed]
- Melville, J.C.; Stackowicz, D.J.; Jundt, J.S.; Shum, J.W. Use of Botox (OnabotulinumtoxinA) for the Treatment of Parotid Sialocele and Fistula After Extirpation of Buccal Squamous Cell Carcinoma with Immediate Reconstruction Using Microvascular Free Flap: A Report of 3 Cases. J. Oral Maxillofac. Surg. 2016, 74, 1678–1686. [Google Scholar] [CrossRef]
- Xie, S.; Wang, K.; Xu, T.; Guo, X.S.; Shan, X.F.; Cai, Z.G. Efficacy and safety of botulinum toxin type A for treatment of Frey’s syndrome: Evidence from 22 published articles. Cancer Med. 2015, 4, 1639–1650. [Google Scholar] [CrossRef]
- Matak, I.; Lacković, Z. Botulinum neurotoxin type A: Actions beyond SNAP-25? Toxicology 2015, 335, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Van Daele, D.J.; Finnegan, E.M.; Rodnitzky, R.L.; Zhen, W.; McCulloch, T.M.; Hoffman, H.T. Head and neck muscle spasm after radiotherapy: Management with botulinum toxin A injection. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 956–959. [Google Scholar] [CrossRef] [Green Version]
- Layeeque, R.; Hochberg, J.; Siegel, E.; Kunkel, K.; Kepple, J.; Henry-Tillman, R.S.; Dunlap, M.; Seibert, J.; Klimberg, V.S. Botulinum toxin infiltration for pain control after mastectomy and expander reconstruction. Ann. Surg. 2004, 240, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Vasan, C.W.; Liu, W.C.; Klussmann, J.P.; Guntinas-Lichius, O. Botulinum toxin type A for the treatment of chronic neck pain after neck dissection. Head Neck 2004, 26, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, C.; Liu, W.C.; Preuss, S.F.; Guntinas-Lichius, O. Botulinum toxin A for neuropathic pain after neck dissection: A dose-finding study. Laryngoscope 2006, 116, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Cohen, M.; Juliéron, M.; Marandas, P.; Janot, F.; Bourhis, J. Botulinum toxin for radiation-induced facial pain and trismus. Otolaryngol. Head Neck Surg. 2008, 138, 459–463. [Google Scholar] [PubMed]
- Stubblefield, M.D.; Levine, A.; Custodio, C.M.; Fitzpatrick, T. The role of botulinum toxin type A in the radiation fibrosis syndrome: A preliminary report. Arch. Phys. Med. Rehabil. 2008, 89, 417–421. [Google Scholar] [CrossRef]
- Mittal, S.; Machado, D.G.; Jabbari, B. OnabotulinumtoxinA for treatment of focal cancer pain after surgery and/or radiation. Pain Med. 2012, 13, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Bach, C.A.; Wagner, I.; Lachiver, X.; Baujat, B.; Chabolle, F. Botulinum toxin in the treatment of post-radiosurgical neck contracture in head and neck cancer: A novel approach. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Rostami, R.; Mittal, S.O.; Radmand, R.; Jabbari, B. Incobotulinum Toxin-A Improves Post-Surgical and Post-Radiation Pain in Cancer Patients. Toxins 2016, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- De Groef, A.; Devoogdt, N.; Van Kampen, M.; Nevelsteen, I.; Smeets, A.; Neven, P.; Geraerts, I.; Dams, L.; Van der Gucht, E.; Debeer, P. Effectiveness of Botulinum Toxin A for Persistent Upper Limb Pain After Breast Cancer Treatment: A Double-Blinded Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 1342–1351. [Google Scholar] [CrossRef]
- Mailly, M.; Benzakin, S.; Chauvin, A.; Brasnu, D.; Ayache, D. Douleurs post-radiques après radiothérapie pour cancer des voies aérodigestives superieures: Traitement par injections de toxine botulique A [Radiation-induced head and neck pain: Management with botulinum toxin a injections]. Cancer Radiother. 2019, 23, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.S.; Pleninger, P.; Kurlan, R. Botulinum toxin A is effective in treating trismus associated with postradiation myokymia and muscle spasm. Mov. Disord. 1995, 10, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, B.; Maher, N.; Difazio, M.P. Botulinum toxin a improved pain and allodynia in two patients with intracranial pathology. Pain Med. 2003, 4, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, K.E.; Kim, J.S.; Hong, B.Y. Botulinum Toxin Type A Injection for Neuropathic Pain in a Patient With a Brain Tumor: A Case Report. Ann. Rehabil. Med. 2017, 41, 1088. [Google Scholar] [CrossRef] [Green Version]
- Filippakis, A.; Ho, D.T.; Small, J.E.; Small, K.M.; Ensrud, E.R. Radiation-induced painful neurogenic hypertrophy Treated with Botulinum Toxin, A. J. Clin. Neuromuscul. Dis. 2018, 19, 135–137. [Google Scholar] [CrossRef]
- Boukovalas, S.; Mays, A.C.; Selber, J.C. Botulinum Toxin Injection for Lower Face and Oral Cavity Raynaud Phenomenon after Mandibulectomy, Free Fibula Reconstruction, and Radiation Therapy. Ann. Plast. Surg. 2019, 82, 53–54. [Google Scholar] [CrossRef]
- Schuler, A.; Veenstra, J.; Ozog, D. Battling Neuropathic Scar Pain with Botulinum Toxin. J. Drugs Dermatol. 2019, 18, 937–938. [Google Scholar]
- Laskawi, R.; Winterhoff, J.; Köhler, S.; Kottwitz, L.; Matthias, C. Botulinum toxin treatment of salivary fistulas following parotidectomy: Follow-up results. Oral Maxillofac. Surg. 2013, 17, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Marchese-Ragona, R.; Marioni, G.; Restivo, D.A.; Staffieri, A. The role of botulinum toxin in postparotidectomy fistula treatment. A technical note. Am. J. Otolaryngol. 2006, 27, 221–224. [Google Scholar] [CrossRef]
- Nolte, D.; Gollmitzer, I.; Loeffelbein, D.J.; Hölzle, F.; Wolff, K.D. Botulinumtoxin zur Behandlung des gustatorischen Schwitzens. Eine prospektive randomisierte Therapiestudie [Botulinum toxin for treatment of gustatory sweating. A prospective randomized study]. Mund Kiefer Gesichtschir. 2004, 8, 369–375. [Google Scholar] [CrossRef]
- Kuttner, C.; Tröger, M.; Dempf, R.; Eckardt, A. Effektivität von Botulinumtoxin A in der Behandlung des gustatorischen Schwitzens [Effectiveness of botulinum toxin A in the treatment of gustatory sweating]. Nervenarzt 2001, 72, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.; Galati, L.T.; Parnes, S.M. A pilot study evaluating the treatment of postparotidectomy sialoceles with botulinum toxin type A. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 421–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, A.; Hasselbacher, K.; Heinrichs, S.; Wollenberg, B. Botulinum toxin for salivary disorders in the treatment of head and neck cancer. Anticancer Res. 2014, 34, 6627–6632. [Google Scholar] [PubMed]
- Marchese, M.R.; Almadori, G.; Giorgio, A.; Paludetti, G. Post-surgical role of botulinum toxin-A injection in patients with head and neck cancer: Personal experience. Acta Otorhinolaryngol. Ital. 2008, 28, 13–16. [Google Scholar] [PubMed]
- Eckardt, A.; Kuettner, C. Treatment of gustatory sweating (Frey′s syndrome) with botulinum toxin A. Head Neck 2003, 25, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, G.; Berlusconi, A.; Mele, V.; Cogiamanian, F.; Barbieri, S. Treatment of Frey′s syndrome with botulinum toxin type B. Otolaryngol. Head Neck Surg. 2010, 143, 214–218. [Google Scholar] [CrossRef]
- Martos Díaz, P.; Bances del Castillo, R.; Mancha de la Plata, M.; Gías, L.; Nieto, C.M.; Lee, G.Y.; Guerra, M.M. Clinical results in the management of Frey′s syndrome with injections of Botulinum toxin. Med. Oral Patol. Oral Cir. Bucal 2008, 13, E248–E252. [Google Scholar]
- Hartl, D.M.; Julieron, M.; LeRidant, A.M.; Janot, F.; Marandas, P.; Travagli, J.P. Botulinum toxin A for quality of life improvement in post-parotidectomy gustatory sweating (Frey′s syndrome). J. Laryngol. Otol. 2008, 122, 1100–1104. [Google Scholar] [CrossRef] [Green Version]
- Pomprasit, M.; Chintrakarn, C. Treatment of Frey′s syndrome with botulinum toxin. J. Med. Assoc. Thail. 2007, 90, 2397–2402. [Google Scholar]
- Cavalot, A.L.; Palonta, F.; Preti, G.; Nazionale, G.; Ricci, E.; Staffieri, A.; Di Girolamo, S.; Cortesina, G. Sindrome di Frey post parotidectomia. Trattamento con tossina botulinica di tipo A [Post-parotidectomy Frey′s syndrome. Treatment with botulinum toxin type A]. Acta Otorhinolaryngol. Ital. 2000, 20, 187–191. [Google Scholar]
- Von Lindern, J.J.; Niederhagen, B.; Bergé, S.; Hägler, G.; Reich, R.H. Frey syndrome: Treatment with type A botulinum toxin. Cancer 2000, 89, 1659–1663. [Google Scholar] [CrossRef]
- Laccourreye, O.; Muscatelo, L.; Naude, C.; Bonan, B.; Brasnu, D. Botulinum toxin type A for Frey′s syndrome: A preliminary prospective study. Ann. Otol. Rhinol. Laryngol. 1998, 107, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Bjerkhoel, A.; Trobbe, O. Frey′s syndrome: Treatment with botulinum toxin. J. Laryngol. Otol. 1997, 111, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Ferron, C.; Cernea, S.S.; Almeida, A.R.T.; Cesar, D.V.G. Primary treatment of early fistula of parotid duct with botulinum toxin type A injection. An. Bras. Dermatol. 2017, 92, 864–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philouze, P.; Vertu, D.; Ceruse, P. Bilateral gustatory sweating in the submandibular region after bilateral neck dissection successfully treated with botulinum toxin. Br. J. Oral Maxillofac. Surg. 2014, 52, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Ihler, F.; Laskawi, R.; Matthias, C.; Rustenbeck, H.H.; Canis, M. Botulinumtoxin A gegen Hypersalivation. Einsatz bei Wundheilung nach Resektion eines Zungenkarzinoms [Botulinum toxin A after microvascular ALT flap in a patient with (corrected) squamous cell carcinoma of the tongue] [published correction appears in HNO. 2012 Oct; 60, 905]. HNO 2012, 60, 524–527. [Google Scholar] [CrossRef]
- Pantel, M.; Volk, G.F.; Guntinas-Lichius, O.; Wittekindt, C. Botulinum toxin type b for the treatment of a sialocele after parotidectomy. Head Neck 2013, 35, E11–E12. [Google Scholar] [CrossRef]
- Steffen, A.; Wollenberg, B.; Schönweiler, R.; Brüggemann, N.; Meyners, T. Drooling nach Strahlentherapie. Botulinumtoxin als erfolgreiches Therapieverfahren [Drooling following radiation. Botulinum toxin as a successful treatment modality]. HNO 2011, 59, 115–117. [Google Scholar] [CrossRef]
- Hill, S.E.; Mortimer, N.J.; Hitchcock, B.; Salmon, P.J. Parotid fistula complicating surgical excision of a basal cell carcinoma: Successful treatment with botulinum toxin type A. Dermatol. Surg. 2007, 33, 1365–1367. [Google Scholar] [CrossRef]
- Kizilay, A.; Aladağ, I.; Ozturan, O. Parotidektomi sonrasi gelişen tükürük fistülünün botulinum toksini ile iyileşmesi [Successful use of botulinum toxin injection in the treatment of salivary fistula following parotidectomy]. Kulak Burun Bogaz Ihtis Derg. 2003, 10, 78–81. [Google Scholar]
- Guntinas-Lichius, O.; Sittel, C. Treatment of postparotidectomy salivary fistula with botulinum toxin. Ann. Otol. Rhinol. Laryngol. 2001, 110, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Marchese Ragona, R.; Blotta, P.; Pastore, A.; Tugnoli, V.; Eleopra, R.; De Grandis, D. Management of parotid sialocele with botulinum toxin. Laryngoscope 1999, 109, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Báez, A.; Paleari, J.; Durán, M.N.; Rudy, T.; Califano, I.; Barbosa, N.; Casas Parera, I. Síndrome de Frey por submaxilectomía y tratamiento con toxina botulínica [Frey syndrome secondary to submaxillectomy and botulinic treatment]. Medicina (B Aires) 2007, 67, 478–480. [Google Scholar]
- Hatzis, G.P.; Finn, R. Using botox to treat a mohs defect repair complicated by a parotid fistula. J. Oral Maxillofac. Surg. 2007, 65, 2357–2360. [Google Scholar] [CrossRef]
- Birch, J.F.; Varma, S.K.; Narula, A.A. Botulinum toxoid in the management of gustatory sweating (Frey′s syndrome) after superficial parotidectomy. Br. J. Plast. Surg. 1999, 52, 230–231. [Google Scholar] [CrossRef]
- Vezdrevanis, K. Prostatic carcinoma shrunk after intraprostatic injection of botulinum toxin. Urol. J. 2011, 8, 239–241. [Google Scholar]
- Ulloa, F.; Gonzàlez-Juncà, A.; Meffre, D.; Barrecheguren, P.J.; Martínez-Mármol, R.; Pazos, I.; Olivé, N.; Cotrufo, T.; Seoane, J.; Soriano, E. Blockade of the SNARE protein syntaxin 1 inhibits glioblastoma tumor growth. PLoS ONE 2015, 10, e0119707. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Manzoni, A.; Florentin, D.; Fisher, W.; Ding, Y.; Lee, M.; Ayala, G. Biologic effect of neurogenesis in pancreatic cancer. Hum. Pathol. 2016, 52, 182–189. [Google Scholar] [CrossRef]
- Karsenty, G.; Rocha, J.; Chevalier, S.; Scarlata, E.; Andrieu, C.; Zouanat, F.Z.; Rocchi, P.; Giusiano, S.; Elzayat, E.A.; Corcos, J. Botulinum toxin type A inhibits the growth of LNCaP human prostate cancer cells in vitro and in vivo. Prostate 2009, 69, 1143–1150. [Google Scholar] [CrossRef]
- Nam, H.J.; Kang, J.K.; Chang, J.S.; Lee, M.S.; Nam, S.T.; Jung, H.W.; Kim, S.K.; Ha, E.M.; Seok, H.; Son, S.W.; et al. Cells transformed by PLC-gamma 1 overexpression are highly sensitive to clostridium difficile toxin A-induced apoptosis and mitotic inhibition. J. Microbiol. Biotechnol. 2012, 22, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Proietti, S.; Nardicchi, V.; Porena, M.; Giannantoni, A. Attività della tossina botulinica A in linee cellulari di cancro prostatico [Botulinum toxin type-A toxin activity on prostate cancer cell lines]. Urologia 2012, 79, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bandala, C.; Perez-Santos, J.L.; Lara-Padilla, E.; Delgado Lopez, G.; Anaya-Ruiz, M. Effect of botulinum toxin A on proliferation and apoptosis in the T47D breast cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 891–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandala, C.; Cortés-Algara, A.L.; Mejía-Barradas, C.M.; Ilizaliturri-Flores, I.; Dominguez-Rubio, R.; Bazán-Méndez, C.I.; Floriano-Sánchez, E.; Luna-Arias, J.P.; Anaya-Ruiz, M.; Lara-Padilla, E.; et al. Botulinum neurotoxin type A inhibits synaptic vesicle 2 expression in breast cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 8411–8418. [Google Scholar] [PubMed]
- Rust, A.; Leese, C.; Binz, T.; Davletov, B. Botulinum neurotoxin type C protease induces apoptosis in differentiated human neuroblastoma cells. Oncotarget 2016, 7, 33220–33228. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wheeler, M.B.; Kang, Y.H.; Sheu, L.; Lukacs, G.L.; Trimble, W.S.; Gaisano, H.Y. Truncated SNAP-25 (1-197), like botulinum neurotoxin A, can inhibit insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 1998, 12, 1060–1070. [Google Scholar] [CrossRef] [Green Version]
- Hajighasemlou, S.; Alebouyeh, M.; Rastegar, H.; Manzari, M.T.; Mirmoghtadaei, M.; Moayedi, B.; Ahmadzadeh, M.; Parvizpour, F.; Johari, B.; Naeini, M.M.; et al. Preparation of Immunotoxin Herceptin-Botulinum and Killing Effects on Two Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2015, 16, 5977–5981. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.T.; Chung, Y.H.; Kang, H.Y.; Tai, M.H.; Chancellor, M.B.; Chuang, Y.C. OnobotulinumtoxinA Has No Effects on Growth of LNCaP and PC3 Human Prostate Cancer Cells. Low. Urin. Tract Symptoms 2013, 5, 168–172. [Google Scholar] [CrossRef]
- Ansiaux, R.; Gallez, B. Use of botulinum toxins in cancer therapy. Expert Opin. Investig. Drugs 2007, 16, 209–218. [Google Scholar] [CrossRef]
- Coarfa, C.; Florentin, D.; Putluri, N.; Ding, Y.; Au, J.; He, D.; Ragheb, A.; Frolov, A.; Michailidis, G.; Lee, M.; et al. Influence of the neural microenvironment on prostate cancer. Prostate 2018, 78, 128–139. [Google Scholar] [CrossRef]
- Matak, I.; Bölcskei, K.; Bach-Rojecky, L.; Helyes, Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.O.; Safarpour, D.; Jabbari, B. Botulinum Toxin Treatment of Neuropathic Pain. Semin. Neurol. 2016, 36, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, Y.; Matsuka, Y.; Spigelman, I.; Ishihara, Y.; Yamamoto, Y.; Sonoyama, W.; Kamioka, H.; Yamashiro, T.; Kuboki, T.; Oguma, K. Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience 2009, 159, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Omoto, K.; Maruhama, K.; Terayama, R.; Yamamoto, Y.; Matsushita, O.; Sugimoto, T.; Oguma, K.; Matsuka, Y. Cross-Excitation in Peripheral Sensory Ganglia Associated with Pain Transmission. Toxins 2015, 7, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.J.; Terashima, T.; Steinauer, J.J.; Eddinger, K.A.; Yaksh, T.L.; Xu, Q. Botulinum toxin B in the sensory afferent: Transmitter release, spinal activation, and pain behavior. Pain 2014, 155, 674–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, B.; Yao, L.; Ni, L.; Wang, L.; Hu, X. Antinociceptive effect of botulinum toxin A involves alterations in AMPA receptor expression and glutamate release in spinal dorsal horn neurons. Neuroscience 2017, 357, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chu, X.; Wang, L.; Shi, H.; Li, T. Botulinum toxin type A reduces TRPV1 expression in the dorsal root ganglion in rats with adjuvant-arthritis pain. Toxicon 2017, 133, 116–122. [Google Scholar] [CrossRef]
- Restani, L.; Antonucci, F.; Gianfranceschi, L.; Rossi, C.; Rossetto, O.; Caleo, M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J. Neurosci. 2011, 31, 15650–15659. [Google Scholar] [CrossRef]
- Matak, I.; Bach-Rojecky, L.; Filipović, B.; Lacković, Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 2011, 186, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Bach-Rojecky, L.; Salković-Petrisić, M.; Lacković, Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: Bilateral effect after unilateral injection. Eur. J. Pharmacol. 2010, 633, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Bach-Rojecky, L.; Lacković, Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol. Biochem. Behav. 2009, 94, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Arezzo, J.C. Possible mechanisms for the effects of botulinum toxin on pain. Clin. J. Pain. 2002, 18 (Suppl. S6), S125–S132. [Google Scholar] [CrossRef]
- Neufeld, N.J.; Elnahal, S.M.; Alvarez, R.H. Cancer pain: A review of epidemiology, clinical quality and value impact. Future Oncol. 2017, 13, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.L.; Kehlet, H.; Belfer, I.; Edwards, R.R. Predicting, preventing and managing persistent pain after breast cancer surgery: The importance of psychosocial factors. Pain Manag. 2014, 4, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Scott, C.; Stevens, R.; Marconi, B.; Champion, L.; Freedman, G.M.; Asrari, F.; Pilepich, M.V.; Gagnon, J.D.; Wong, G. Randomized phase III study comparing best supportive care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group [RTOG] 97–13. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1307–1310. [Google Scholar] [CrossRef]
- Chargari, C.; Fromantin, I.; Kirova, Y.M. Importance of local skin treatments during radiotherapy for prevention and treatment of radio-induced epithelitis. Cancer Radiother. 2009, 13, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kirova, Y.M.; Fromantin, I.; De Rycke, Y.; Fourquet, A.; Morvan, E.; Padiglione, S.; Falcou, M.C.; Campana, F.; Bollet, M.A. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy-results of phase III randomised trial. Radiother. Oncol. 2011, 100, 205–209. [Google Scholar] [CrossRef]
- Fleming, J.A.; O’Connor, B.D. Use of lidocaine patches for neuropathic pain in a comprehensive cancer center. Pain Res. Manag. 2009, 14, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Wiffen, P.J.; Derry, S.; Moore, R.A. Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain. Cochrane Database Syst. Rev. 2014, 5, CD011056. [Google Scholar]
- Lakraje, A.A.; Moghimi, N.; Jabbari, B. Sialorrhea, anatomy, physiology and treatment with emphasis on the role of botulinum toxins. Toxins 2013, 5, 1010–1031. [Google Scholar] [CrossRef] [Green Version]
- Schindel, J.; Markowicz, H.; Levie, B. Combined surgical-radiological treatment of parotid gland fistulae. J. Laryngol. Otol. 1968, 82, 867–870. [Google Scholar] [CrossRef]
- Lovato, A.; Restivo, D.A.; Ottaviano, G.; Marioni, G.; Marchese-Ragona, R. Botulinum toxin therapy: Functional silencing of salivary disorders. Terapia con tossina botulinica: Silenziamento funzionale dei disordini salivari. Acta Otorhinolaryngol. Ital. 2017, 37, 168–171. [Google Scholar] [CrossRef] [PubMed]

| Authors | Pts Study | Toxin | Dose Units | Treatment | Location of Cancer | Primary Outcome | Result |
|---|---|---|---|---|---|---|---|
| Van Daele et al., 2002 [12] | 6 Retro | OnaA | 20–25 | Radiation chemotherapy | Head and neck | Pain VAS | Complete pain relief in four of six patients. Significant improvement of quality of life using SF36, EQ-5D scales |
| Layeeque et al., 2004 [13] | 48 Pro | OnaA | 100 | Mastectomy, assessed for pain after expander placement | Breast | (1) Pain assessed by VAS (2) Narcotic use | Less pain in BoNT group (p < 0.00001) Less narcotic use in BoNT group (p < 0.0001) |
| Vasan et al., 2004 [14] | 16 Pro | AboA | 100 to 320 | Surgery | Head and neck | Pain (VAS- days 3 and 4 weeks), global. Quality of life | Significant pain reduction (p = 0.05); Quality of life improved (p = 0.7) |
| Wittekindt et al., 2006 [15] | 23 Pro | OnaA | 60–120 160–240 | Radiation; surgery | Head and neck | Pain VAS: 28 weeks | Significant reduction of pain (<0.05) |
| Hartl et al., 2008 [16] | 19 Pro | OnaA AboA | 50 250 | Chemotherapy; radiation | Head and neck | Pain: (VAS) Function: At 4 weeks | Improved pain (p = 0.02) Function (p = 0.04) |
| Stubblefield et al., 2008 [17] | 23 Retro | OnaA | 25–200 | Radiation; surgery | Head, neck, breast | Pain (VAS) | Pain improved in Improved in 85% of patients |
| Mittal et al., 2012 [18] | 7 Retro | OnaA | 100 | Radiation; surgery | Head, neck, breast | Pain (VAS) PGIC At 4 weeks | VAS: Six of seven patients improved: p < 0.05 PGIC: Six of seven, very satisfied QoL: Six of seven improved (p < 0.05) |
| Bach et al., 2012 [19] | 9 Pro | OnaA | 100–400 | Radiation and surgery | Head and neck | Pain (VAS) and FDSNP at 4 weeks | Both pain and FDSNP improved (p < 0.01) |
| Rostami et al., 2014 [20] | 12 Pro | IncoA | 100 | Radiation and surgery | Head neck breast | Pain (VAS) and PGIC at week 6 | VAS improved (p < 0.05) PGIC: Very satisfied QoL improved in 38% of patients (p < 0.05) |
| De Groef et al. 2018 [21] | 50 DBPC | onaA | 100 | Surgery | Breast | Pain measured by VAS | Pain reduction 60% in the BoNT and 40% in saline group (statistically ns) |
| Mailly et al., 2019 [22] | 16 Retro | incoA aboA | 20 40 | Radiation and surgery | Head and neck | Pain (VAS) | VAS improved p < 0.01 |
| Authors | Design | Pts # | Clinical Problem | Injection Site | Toxin and Dose | Result |
|---|---|---|---|---|---|---|
| Laskawi et al., 2013 [29] | R | 10 | Post-parotidectomy fistula | Parotid gland | OnaA 30–50 units | Treated within 6 weeks of surgery: Fistulas healed in 9 of 10 patients |
| Marchese-Ragona et al., 2006 [30] | R | 3 | Post-parotidectomy fistula | Parotid gland | OnaA 15–20 units | Complete healing of fistula with follow ups 12,18, and 14 months |
| Nolte et al., 2004 [31] | P | 20 | Gustatory sweating after parotidectomy | Facial skin | OnaA 3 units/cm | Complete loss of sweating for 12 months |
| Kuttner et al., 2001 [32] | R | 8 | GH after parotidectomy | Face | BoNT-A 0.5 units/cm | Stopped facial sweating within one week |
| Vargas et al., 2000 [33] | P | 4 | Post-parotidectomy sialocele-pain | Parotid gland | OnaA 30–50 units | Total resolution in 4 weeks in all patients |
| Steffen et al., 2014 [34] | R | 25 | Head and neck cancer FHS: (19) Fistula (6) | Parotid gland | OnaA and incoA: Par: 30 U SM: 20 U | FHS: 11 of 19 improved. Fistula: 4 of 6 improved |
| Machese et al., 2008 [35] | R | 8 | Head and neck cancer sialorrhea: 6, fistula: 1, and sialocele: 1 | Parotid gland | AboA: 100 U | Fistulas healed. Sialorrhea stopped |
| Eckardt et al., 2003 [36] | R | 33 | GH after parotidectomy | Face | OnaA 16 to 80 units | Facial sweating disappeared within a week after injections |
| Cantarella and Barbieri [37] | R | 7 | GH after parotidectomy | Face | RimaB 2200 units | Cessation of sweating in 6 of 7 patients 4 weeks after injection |
| Matos Dias et al., 2008 [38] | R | 10 | GH after parotidectomy | Face | Ona-A 38 units | Sweating stopped |
| Hatrl et al., 2008 [39] | R | 7 | GH after parotidectomy | Face | BoNT-A | Sweating and quality of life improved |
| Pomprasit et al., 2007 [40] | P | 9 | GH after Parotidectomy | Face | Ona-A 10.6 units | Sweating stopped in 5 and reduced in 4 |
| Cavalot et al., 2000 [41] | P | 40 | GH after parotidectomy | Face | Ona-A, 2.5/cm2 | 100% response in severe group, 72% response in moderate group |
| Von Lindern et al., 2000 [42] | R | 7 | GH after parotidectomy | Face | Ona-A | Sweating stopped after BoNT injection |
| Laccourreye et al., 1998 [43] | P | 14 | GH after parotidectomy | Face | Ona-A | All showed total cessation of sweating |
| Bjerkhoel et al., 1997 [44] | P | 15 | GH after parotidectomy | Face | Ona-A | Total cessation of facial sweating in 13 patients |
| Authors | Study Type | Type of Cells or Tissue | Study Design | Results |
|---|---|---|---|---|
| Vezdrevanis 2011 [57] | In vivo | Prostatic cancer | Injected BoNT into prostate | Tumor size reduction |
| Ulloa et al., 2015 [58] | In vivo | Glioblastoma cells | Cells with or without transfection by BoNT-C1 injected into mice striatum | By BoNT-C1 blocks the growth of Glioblastoma cells via blocking Syntaxin1 |
| He et al., 2016 [59] | In vivo | Mice with pancreatic tumor | Injected onaA or saline into tumor | Reduced tumor size; increased apoptosis |
| Karsenty et al., 2009 [60] | In vitro | Prostate LNCaP and PC-3 cell lines | LNCaP and PC-3 cell lines were exposed to onaA | OnaA inhibited LNCasP cell proliferation; had no effect on PC-3 cell |
| Nam et al., 2012 [61] | In vitro | Breast and colorectal cancer | PLC-γl-transformed cells were exposed to BoNT-A (difficile) | Caused apoptosis and mitotic inhibition |
| Proietti et al., 2012 [62] | In vitro | Prostate LNCaP and PC-3 cell lines | Prostate cancer cell lines were exposed to incoA | Tumor cell growth slowed down probably due to toxin effect on SV2 receptors |
| Bandala et al., 2013 [63] | In vitro | Breast T47D cancer cells | Breast T47D cancer cells were exposed to diverse dilutions of BoNT | BoNT via caspase 3, slow down the growth of T47d cells and caused apoptosis |
| Bandala et al., 2015 [64] | In vitro | Breast cancer cell line | Added BoNT-A to breast cancer cell line | BoNT-A diminished SV2 protein on the surface of breast cancer cells |
| Rust et al., 2016 [65] | In vitro | Human neuroblastoma cells | Added BoNT-C to human neuroblastoma cell culture | Apoptosis of neuroblastoma cells |
| Huang et al., 1998 [66] | Invitro | Insulin secreting HIT-T15 cells | Insulin secreting cells were transfected by BoNT-A | Marked reduction of insulin secretion- potential to treat insulinoma |
| Hajighasemlou et al., 2015 [67] | In vitro | Her2 positive breast cancer cell line | Assessed the effect of BoNT-A on Her2 positive cells responsive to Herceptin | Herceptin efficacy significantly improved |
| Cheng et al., 2013 [68] | In vitro and in vivo | Prostate cancer cell line in Mice | LNCaP and PC3 cancer cells were exposed to 1 to 10 units of onaA | No effect on tumor growth in LNCaP and PC3 cancer cells |
| Ansiaux et al., 2006 [69] | In vivo | Fibrosarcoma, hepatosarcoma | BoNT-A injected into the tumor | Increased oxygenation of the tumor and made it more susceptible to chemo and radiotherapy |
| Coarfa et al., 2017 [70] | In vivo | Prostate of 250 nude mice Four human cancerous prostates | Effect of onaA versus saline injection into cancer cells implanted into rodent’s prostate Assessed the effect of onaA versus saline injection in cancerous prostate before prostatectomy | Increased apoptosis; slowed cancer progression Increased apoptosis in ona-A injected side of prostate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, S.O.; Jabbari, B. Botulinum Neurotoxins and Cancer—A Review of the Literature. Toxins 2020, 12, 32. https://doi.org/10.3390/toxins12010032
Mittal SO, Jabbari B. Botulinum Neurotoxins and Cancer—A Review of the Literature. Toxins. 2020; 12(1):32. https://doi.org/10.3390/toxins12010032
Chicago/Turabian StyleMittal, Shivam O., and Bahman Jabbari. 2020. "Botulinum Neurotoxins and Cancer—A Review of the Literature" Toxins 12, no. 1: 32. https://doi.org/10.3390/toxins12010032
APA StyleMittal, S. O., & Jabbari, B. (2020). Botulinum Neurotoxins and Cancer—A Review of the Literature. Toxins, 12(1), 32. https://doi.org/10.3390/toxins12010032






