Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells
Abstract
:1. Introduction
2. Results
2.1. Canine OSA Tissue Has High Expression of uPA and MMPs
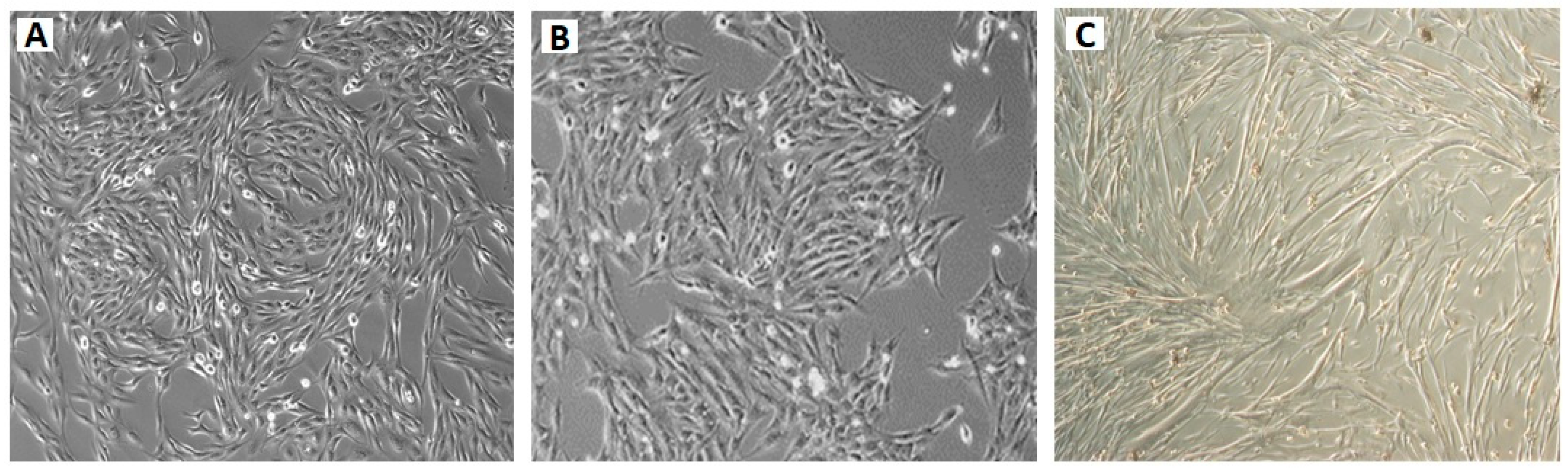
2.2. OSA Cells Express Tumour Markers
2.3. The Reengineered Anthrax Toxin Is More Cytotoxic to OSA Cells Than to COBS Cells
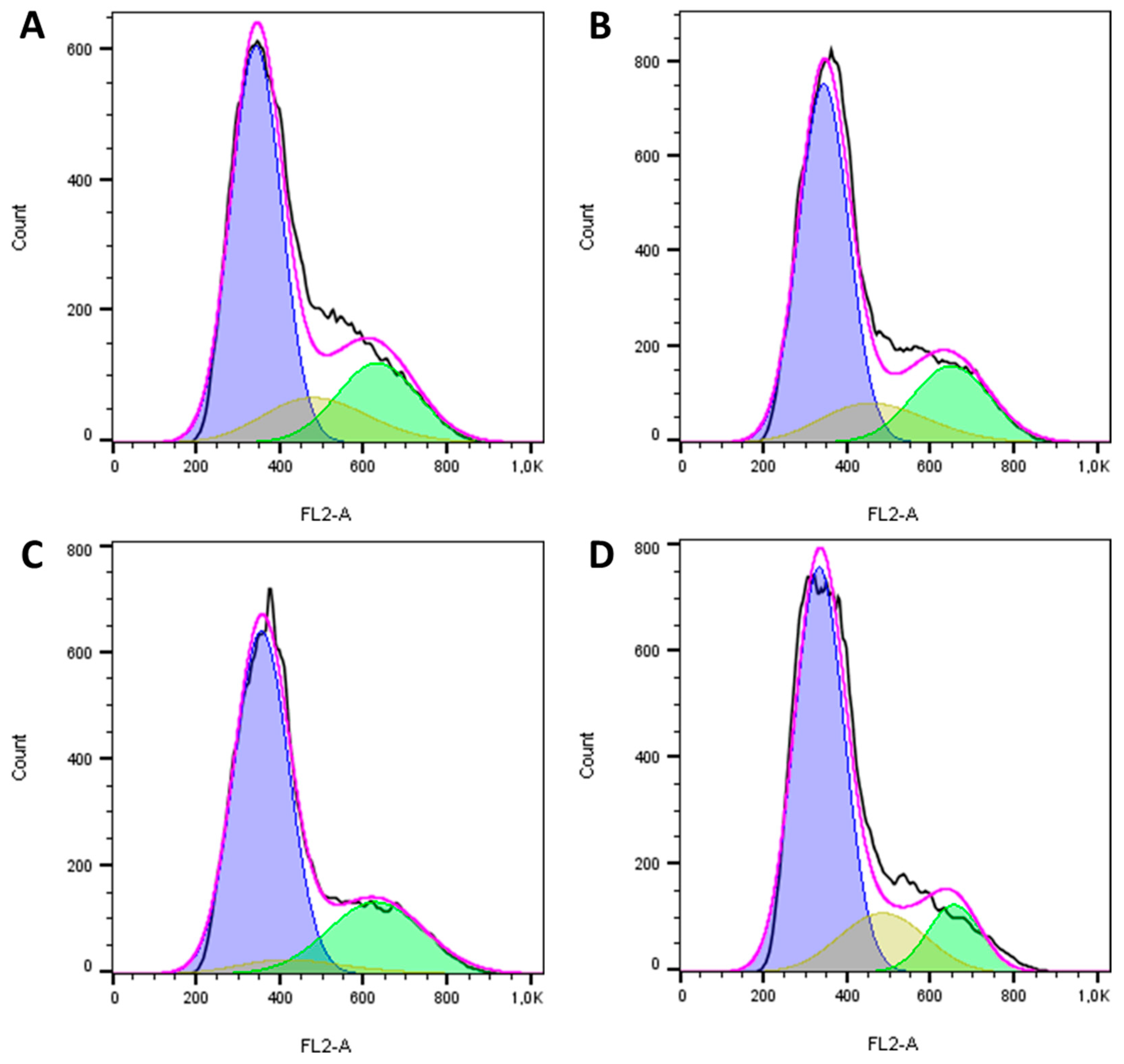
2.4. The Reengineered Anthrax Toxin Causes Cell Death in OSA Cells
2.5. The Reengineered Anthrax Toxin Decreased Migration Capacity in OSA Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Lines
5.2. Reengineered Anthrax Toxin
- The native protective antigen (PA);
- The reengineered protective antigen: PA-U2-R200A + PA-L1-I210A;
- The lethal factor (LF)
- The catalytic domain of Pseudomonas aeruginosa exotoxin A combined with the N-terminal domain of LF (FP59).
5.3. Immunofluorescence
5.4. In Vitro Cytotoxicity Assay
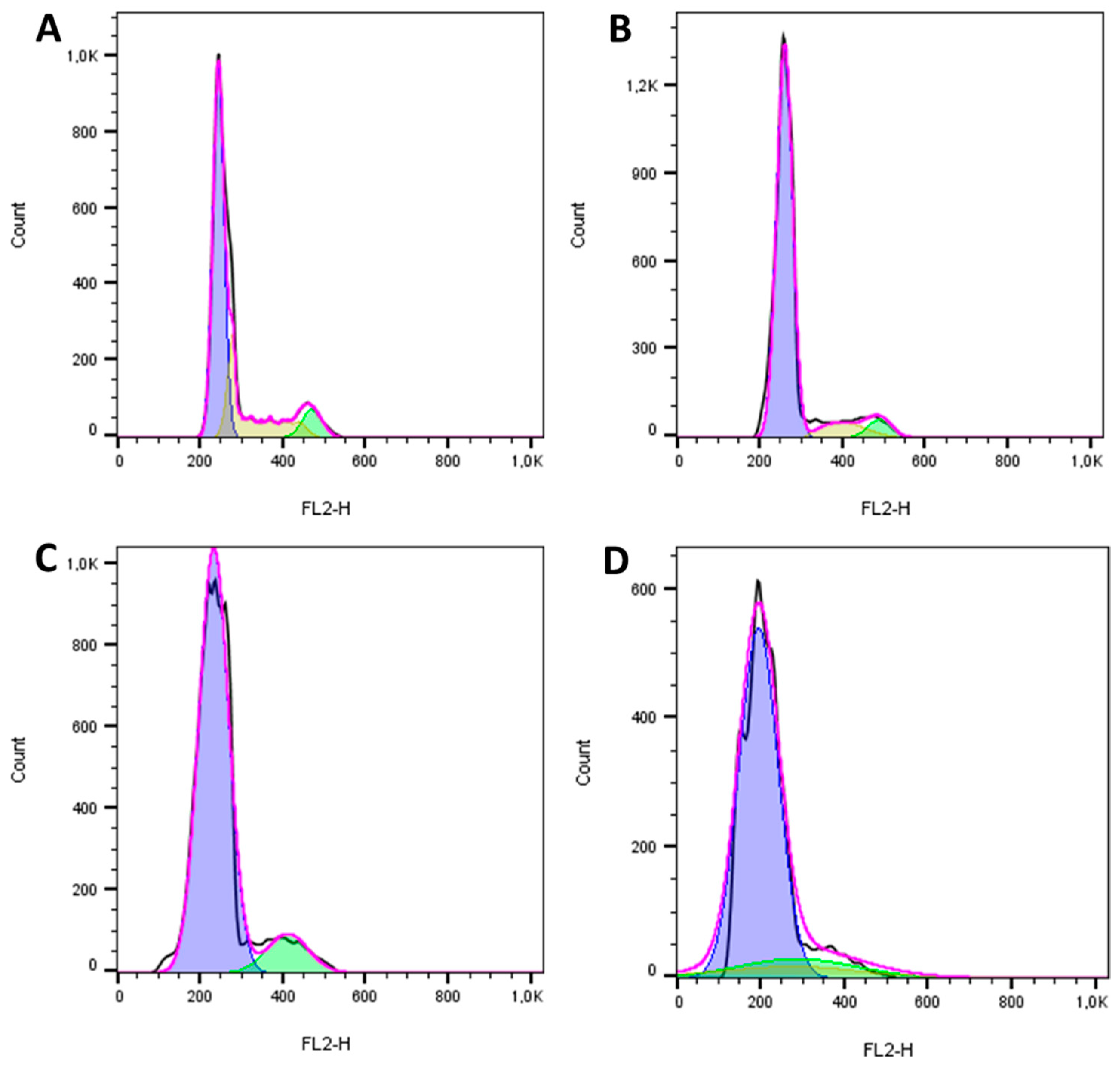
5.5. Cell Cycle Analysis
5.6. Apoptosis and Necrosis Assay
5.7. Wound-Healing Assay
5.8. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Withrow, S.J.; Powers, B.E.; Straw, R.C.; Wilkins, R.M. Comparative aspects of osteosarcoma. Dog versus man. Clin. Orthop. Relat. Res. 1991, 159–168. [Google Scholar]
- Misdorp, W.; Van der Heul, R.O. Tumours of bones and joints. Bull. World Health Organ. 1976, 53, 265–282. [Google Scholar] [PubMed]
- Covey, J.L.; Farese, J.P.; Bacon, N.J.; Schallberger, S.P.; Amsellem, P.; Cavanaugh, R.P.; Milner, R.J. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet. Surg. 2014, 43, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, G.T.; Bonestroo, F.A.; Kirpensteijn, J.; Kik, M.J.; van der Zee, R.; van Eden, W.; Timmermans-Sprang, E.P.; Slob, A.; Mol, J.A. Heat shock protein expression analysis in canine osteosarcoma reveals HSP60 as a potentially relevant therapeutic target. Cell Stress Chaperones 2013, 18, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Farese, J.P.; Kirpensteijn, J.; Kik, M.; Bacon, N.J.; Waltman, S.S.; Seguin, B.; Kent, M.; Liptak, J.; Straw, R.; Chang, M.N.; et al. Biologic behavior and clinical outcome of 25 dogs with canine appendicular chondrosarcoma treated by amputation: A Veterinary Society of Surgical Oncology retrospective study. Vet. Surg. 2009, 38, 914–919. [Google Scholar] [CrossRef]
- Casteleyn, C.; Sleeckx, N.; De Spiegelaere, W.; Heindryckx, F.; Coulon, S.; Van Steenkiste, C. New therapeutic targets in veterinary oncology: Man and dog definitely are best friends. Vet. J. 2013, 195, 6–7. [Google Scholar] [CrossRef]
- Castro, J.L.C.; Santalucia, S.; Nazareth, W.; Castro, V.S.P.; Pires, M.V.M.; Leme, P.T.O., Jr.; Paula, L.R.; Ururahy, K.C.B.; Corrêa, L.F.D.; Raiser, A.G. Axial osteosarcoma in dog—Case report. J. Vet. Adv. 2013, 3, 29–33. [Google Scholar]
- Kirpensteijn, J.; Kik, M.; Rutteman, G.R.; Teske, E. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet. Pathol. 2002, 39, 240–246. [Google Scholar] [CrossRef]
- Selvarajah, G.T.; Kirpensteijn, J. Prognostic and predictive biomarkers of canine osteosarcoma. Vet. J. 2010, 185, 28–35. [Google Scholar] [CrossRef]
- Bachran, C.; Leppla, S.H. Tumor targeting and drug delivery by anthrax toxin. Toxins 2016, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Aaronson, H.; Mitola, D.J.; Leppla, S.H.; Bugge, T.H. Potent antitumor activity of a urokinase-activated engineered anthrax toxin. Proc. Natl. Acad. Sci. USA 2003, 100, 657–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Bugge, T.H.; Leppla, S.H. Targeting of tumor cells by cell surface urokinase plasminogen activator-dependent anthrax toxin. J. Biol. Chem. 2001, 276, 17976–17984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Bugge, T.H.; Frankel, A.E.; Leppla, S.H. Dissecting the urokinase activation pathway using urokinase-activated anthrax toxin. Methods Mol. Biol. 2009, 539, 175–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimpel, K.R.; Molloy, S.S.; Thomas, G.; Leppla, S.H. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. USA 1992, 89, 10277–10281. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Redeye, V.; Kuremsky, J.G.; Kuhnen, M.; Molinolo, A.; Bugge, T.H.; Leppla, S.H. Intermolecular complementation achieves high-specificity tumor targeting by anthrax toxin. Nat. Biotechnol. 2005, 23, 725–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rønø, B.; Rømer, J.; Liu, S.; Bugge, T.H.; Leppla, S.H.; Kristjansen, P.E. Antitumor efficacy of a urokinase activation-dependent anthrax toxin. Mol. Cancer Ther. 2006, 5, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Schafer, J.M.; Peters, D.E.; Morley, T.; Liu, S.; Molinolo, A.A.; Leppla, S.H.; Bugge, T.H. Efficient targeting of head and neck squamous cell carcinoma by systemic administration of a dual uPA and MMP-activated engineered anthrax toxin. PLoS ONE 2011, 6, e20532. [Google Scholar] [CrossRef] [Green Version]
- Nishiya, A.T.; Nagamine, M.K.; Fonseca, I.I.M.D.; Miraldo, A.C.; Scattone, N.V.; Guerra, J.L.; Xavier, J.G.; Santos, M.; Gomes, C.O.M.S.; Ward, J.M.; et al. Inhibitory Effects of a reengineered anthrax toxin on canine oral mucosal melanomas. Toxins 2020, 12, 157. [Google Scholar] [CrossRef]
- Andreasen, P.A.; Egelund, R.; Petersen, H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell. Mol. Life Sci. 2000, 57, 25–40. [Google Scholar] [CrossRef]
- Fonseca, J.M. Effects of Bacillus anthracis Reengineered Toxin (PA-U2-R200A + PA-L1-I210A + LF) on Canine Osteosarcoma. Ph.D. Thesis, University of Sao Paulo, Sao Paulo, Brazil, 2019; 127p. [Google Scholar]
- Liu, S.; Liu, J.; Ma, Q.; Cao, L.; Fattah, R.J.; Yu, Z.; Bugge, T.H.; Finkel, T.; Leppla, S.H. Solid tumor therapy by selectively targeting stromal endothelial cells. Proc. Natl. Acad. Sci. USA 2016, 113, E4079–E4087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzell, J.W.; Ivins, B.E.; Leppla, S.H. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect. Immun. 1984, 45, 761–767. [Google Scholar] [PubMed]
- Wein, A.N.; Peters, D.E.; Valivullah, Z.; Hoover, B.J.; Tatineni, A.; Ma, Q.; Fattah, R.; Bugge, T.H.; Leppla, S.H.; Liu, S. An anthrax toxin variant with an improved activity in tumor targeting. Sci. Rep. 2015, 5, 16267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Wang, H.; Currie, B.M.; Molinolo, A.; Leung, H.J.; Moayeri, M.; Basile, J.R.; Alfano, R.W.; Gutkind, J.S.; Frankel, A.E.; et al. Matrix metalloproteinase-activated anthrax lethal toxin demonstrates high potency in targeting tumor vasculature. J. Biol. Chem. 2008, 283, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duesbery, N.S.; Webb, C.P.; Leppla, S.H.; Gordon, V.M.; Klimpel, K.R.; Copeland, T.D.; Ahn, N.G.; Oskarsson, M.K.; Fukasawa, K.; Paull, K.D.; et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 1998, 280, 734–737. [Google Scholar] [CrossRef]
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax Pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef]
- Abi-Habib, R.J.; Singh, R.; Leppla, S.H.; Greene, J.J.; Ding, Y.; Berghuis, B.; Duesbery, N.S.; Frankel, A.E. Systemic anthrax lethal toxin therapy produces regressions of subcutaneous human melanoma tumors in athymic nude mice. Clin. Cancer Res. 2006, 12, 7437–7443. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Ortiz, J.; Liu, S.; Bugge, T.H.; Singh, R.; Leppla, S.H.; Frankel, A.E. Systematic urokinase-activated anthrax toxin therapy produces regressions of subcutaneous human non-small cell lung tumor in athymic nude mice. Cancer Res. 2007, 67, 3329–3336. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Netzel-Arnett, S.; Birkedal-Hansen, H.; Leppla, S.H. Tumor cell-selective cytotoxicity of matrix metalloproteinase-activated anthrax toxin. Cancer Res. 2000, 60, 6061–6067. [Google Scholar]
- Santos, A.; Lopes, C.; Marques, R.M.; Amorim, I.; Ribeiro, J.; Frias, C.; Vicente, C.; Gartner, F.; de Matos, A. Immunohistochemical analysis of urokinase plasminogen activator and its prognostic value in canine mammary tumours. Vet. J. 2011, 189, 43–48. [Google Scholar]
- Anwar, S.; Yanai, T.; Sakai, H. Immunohistochemical Detection of Urokinase Plasminogen Activator and Urokinase Plasminogen Activator Receptor in Canine Vascular Endothelial Tumours. J. Comp. Pathol. 2015, 153, 278–282. [Google Scholar] [PubMed]
- Rossmeisl, J.H.; Hall-Manning, K.; Robertson, J.L.; King, J.N.; Davalos, R.V.; Debinski, W.; Elankumaran, S. Expression and activity of the urokinase plasminogen activator system in canine primary brain tumors. OncoTargets Ther. 2017, 10, 2077–2085. [Google Scholar]
- Ramos, S.C.; de Matos, A.J.; Ribeiro, J.N.; Leite-Martins, L.R.; Ferreira, R.R.F.; Viegas, I.; Santos, A.A. Serum levels of urokinase-type plasminogen activator in healthy dogs and oncologic canine patients. Vet. World 2017, 10, 918–923. [Google Scholar] [PubMed] [Green Version]
- McGill, L.D. Human and canine mammary tumors: A role for urokinase plasminogen activator? Vet. J. 2011, 189, 1–2. [Google Scholar]
- Hoekstra, R.; Eskens, F.A.; Verweij, J. Matrix metalloproteinase inhibitors: Current developments and future perspectives. Oncologist 2001, 6, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levinsohn, J.L.; Newman, Z.L.; Hellmich, K.A.; Fattah, R.; Getz, M.A.; Liu, S.; Sastalla, I.; Leppla, S.H.; Moayeri, M. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012, 8, e1002638. [Google Scholar] [CrossRef]
- Mueller, F.; Fuchs, B.; Kaser-Hotz, B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007, 27, 155–164. [Google Scholar]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef]
- Gustafson, D.L.; Duval, D.L.; Regan, D.P.; Thamm, D.H. Canine sarcomas as a surrogate for the human disease. Pharmacol. Ther. 2018, 188, 80–96. [Google Scholar] [CrossRef]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine osteosarcoma: A naturally occurring disease to inform pediatric oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef] [Green Version]

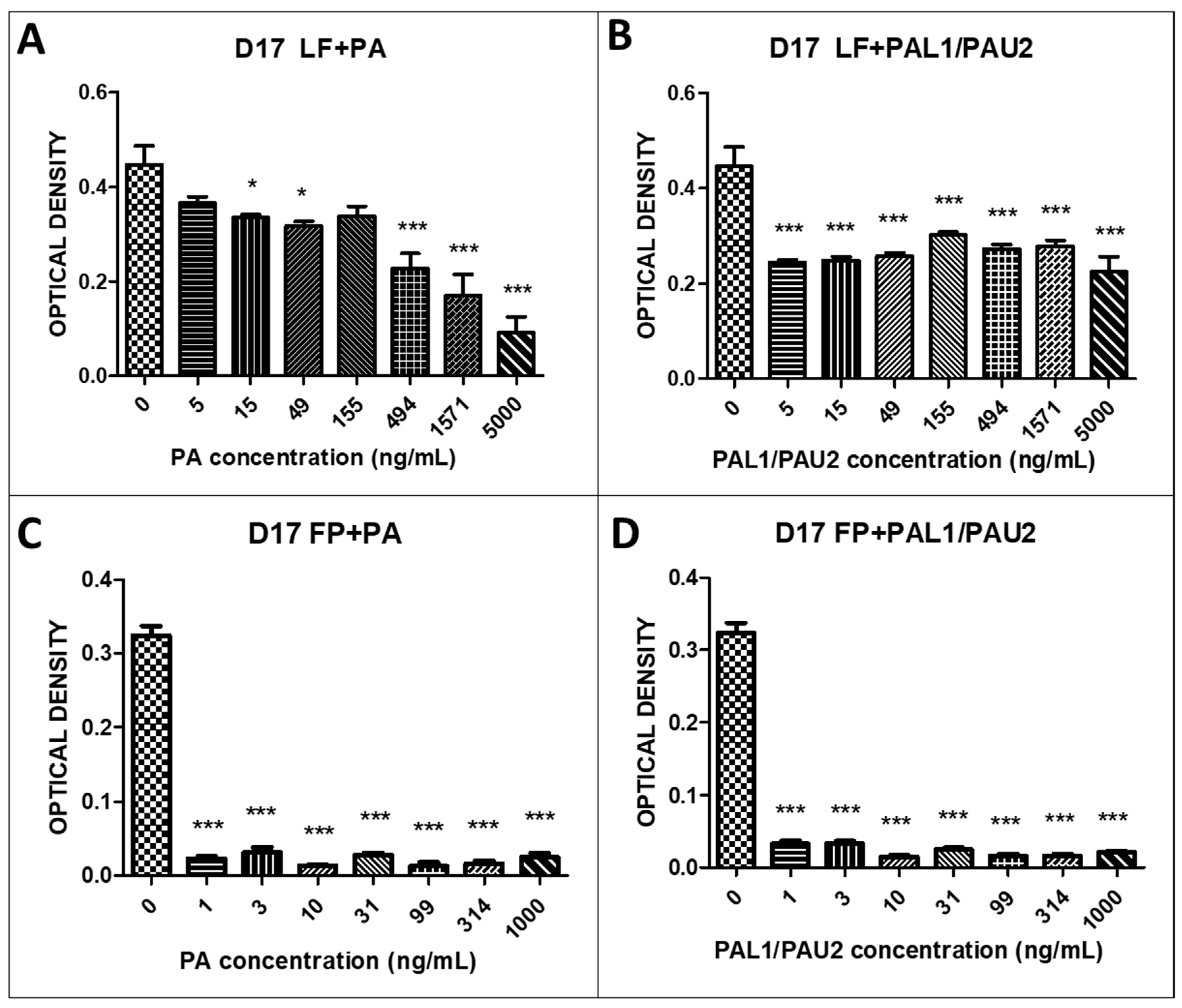




| IC50 | LF + PA (ng/mL) | LF + PAL1/PAU2 (ng/mL) | FP + PA (ng/mL) | FP + PAL1/PAU2 (ng/mL) |
|---|---|---|---|---|
| D17 | 511.2 | 9277 | 1.073 | 1.077 |
| MG63 | 20,439 | 2070 | 1.057 | 1.082 |
| COBS | 3418 | 79,655 | 37.76 | 42.12 |
| Antibody | Catalogue Number | Mono or Polyclonal | Mouse or Rabbit | Dilution | Subcellular Localisation |
|---|---|---|---|---|---|
| MMP-2–Abcam | Ab86607 | Monoclonal | Mouse | 1:200 | Membrane |
| uPA H140–Santa Cruz | Sc14019 | Polyclonal | Rabbit | 1:200 | Cytoplasm |
| β-catenin-Invitrogen | 138400 | Monoclonal | Mouse | 1:100 | Membrane, cytoplasm, and nucleus |
| p53-Serotec | MCA909 | Monoclonal | Mouse | 1:100 | Nucleus |
| MAPK-Cell Signaling | 2532 | Polyclonal | Rabbit | 1:100 | Cytoplasm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackowiak da Fonseca, J.; Mackowiak da Fonseca, I.I.; Nagamine, M.K.; Massoco, C.d.O.; Nishiya, A.T.; Ward, J.M.; Liu, S.; Leppla, S.H.; Bugge, T.H.; Dagli, M.L.Z. Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells. Toxins 2020, 12, 614. https://doi.org/10.3390/toxins12100614
Mackowiak da Fonseca J, Mackowiak da Fonseca II, Nagamine MK, Massoco CdO, Nishiya AT, Ward JM, Liu S, Leppla SH, Bugge TH, Dagli MLZ. Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells. Toxins. 2020; 12(10):614. https://doi.org/10.3390/toxins12100614
Chicago/Turabian StyleMackowiak da Fonseca, Jonathan, Ivone Izabel Mackowiak da Fonseca, Marcia Kazumi Nagamine, Cristina de Oliveira Massoco, Adriana Tomoko Nishiya, Jerrold Michael Ward, Shihui Liu, Stephen Howard Leppla, Thomas Henrik Bugge, and Maria Lucia Zaidan Dagli. 2020. "Inhibitory Effects of a Reengineered Anthrax Toxin on Canine and Human Osteosarcoma Cells" Toxins 12, no. 10: 614. https://doi.org/10.3390/toxins12100614







