Clinical Applications of Bee Venom Acupoint Injection
Abstract
:1. Introduction
2. Pharmacological Mechanisms of the Most Abundant Components of BV
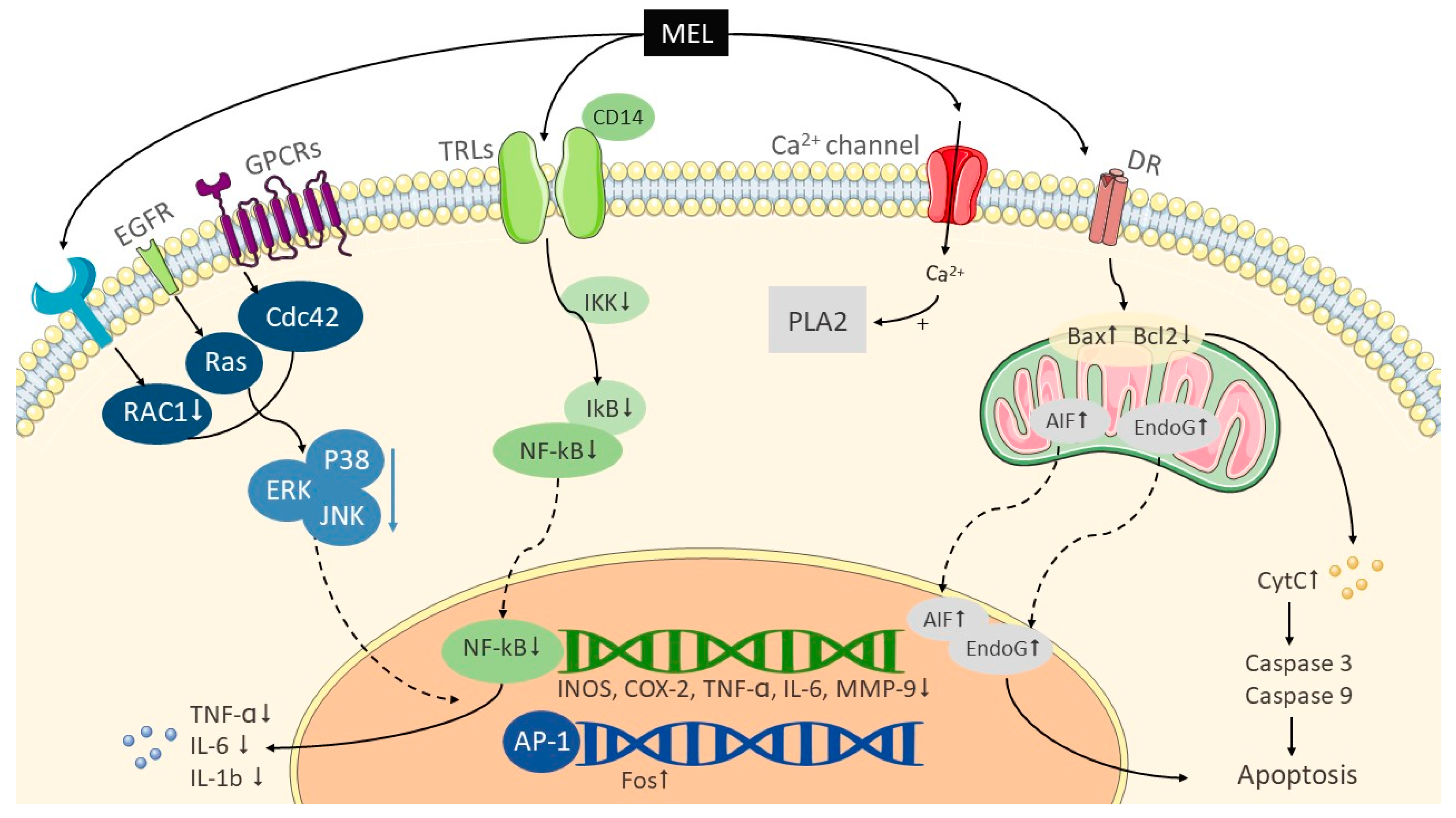
2.1. Melittin
2.2. PLA2
2.3. Apamin
3. Clinical Applications and Mechanism of BV Injection
3.1. Neural System Diseases
3.1.1. Parkinson Disease
Preclinical Studies
Clinical Studies
3.1.2. Neuropathic Pain
Preclinical Studies
3.1.3. Peripheral Neuropathy
Preclinical Studies
Clinical Studies
3.1.4. Alzheimer Disease
Preclinical Studies
3.1.5. Intervertebral Disc Disease
Preclinical Studies
3.1.6. Spinal Cord Injury
Preclinical Studies
3.1.7. Central Poststroke Pain
Clinical Studies
3.2. Muscle and Skeletal Disease
3.2.1. Musculoskeletal Pain
Clinical Studies
3.2.2. Osteoarthritis-Related Knee Pain
Clinical Studies
3.3. Autoimmune Disease
3.3.1. Rheumatoid Arthritis
Preclinical Studies
3.3.2. Multiple Sclerosis
Preclinical Studies
3.4. Skin Disease
3.4.1. Acne
Preclinical Studies
3.4.2. Atopic Dermatitis
Preclinical Studies
3.5. Cancer
Preclinical Studies
4. Safety
5. Conclusions
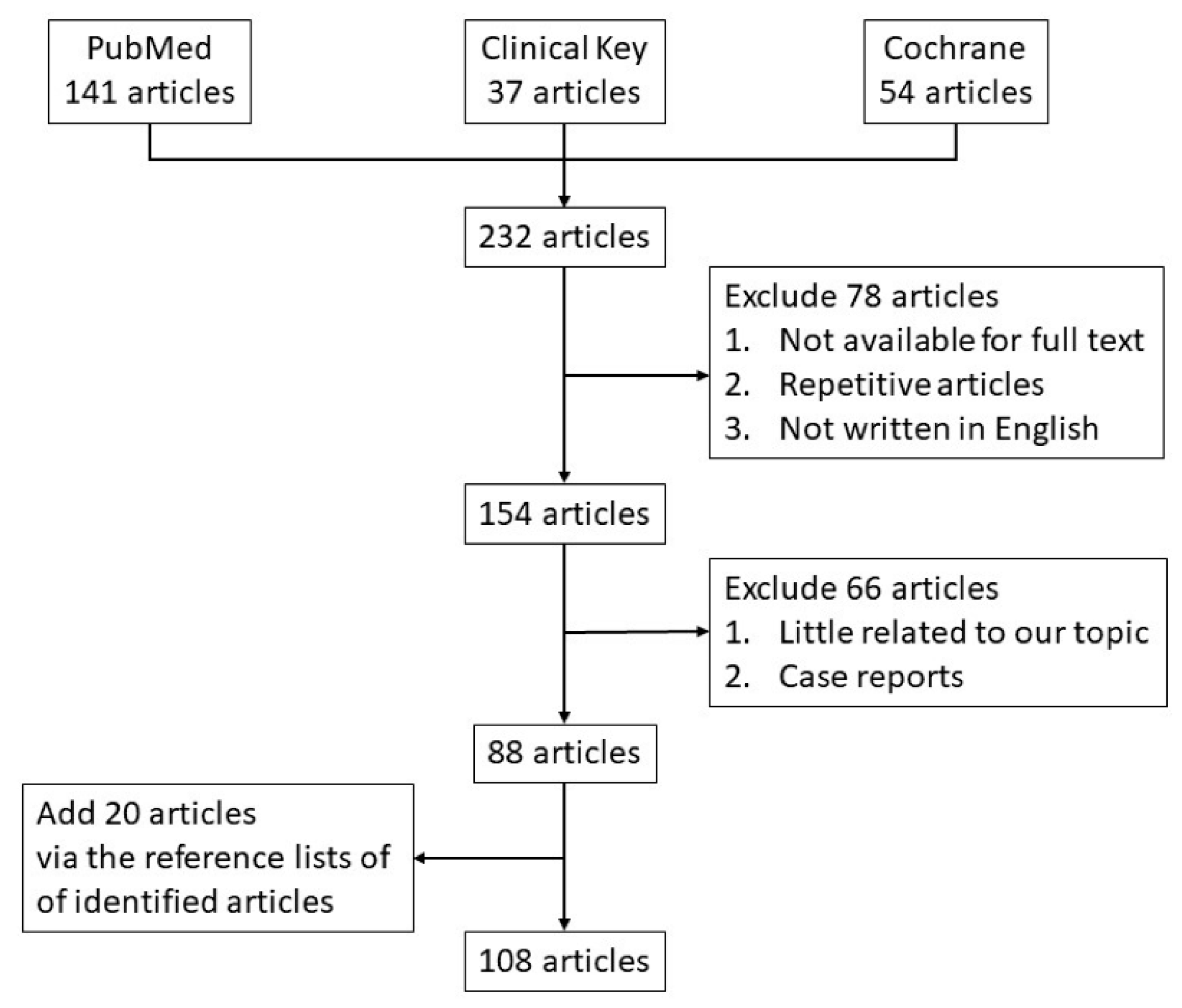
6. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| 5HT3 | 5hydroxytryptamine receptor; |
| A53T Tg | A53T α-Syn mutant transgenic mice; |
| AIFs | apoptosis-induced factors; |
| AKT | protein kinase B; |
| AP-1 | activator protein 1; |
| AT | classic or general acupuncture; |
| Bax | Bcl-2 associated X; |
| Bcl2 | B-cell lymphoma 2; |
| BV | Bee venom; |
| BVA | bee venom acupuncture; |
| bvPLA2 | bee venom phospholipase A2; |
| CCI | chronic constriction injury; |
| CD14 | cluster of differentiation 14; |
| CD206 | cluster of differentiation 206; |
| Cdc42 | cell division cycle protein 42; |
| CIA | collagen-induced arthritis; |
| CIPN | chemotherapy-induced peripheral neuropathy; |
| COX-2 | cyclooxygenase-2; |
| Cyt C | cytochrome c; |
| DBV | dilute bee venom; |
| DC | dendritic cells; |
| DR | death receptor; |
| EAE | experimental autoimmune encephalomyelitis; |
| EGFR | epidermal growth factor receptor; |
| EndoG | endonuclease G; |
| EP2 | prostaglandin E2 receptor 2; |
| ERK | extracellular regulated protein kinases; |
| ERK/p38 MAP | extracellular regulated protein kinases/p38 mitogen-activated protein kinases; |
| ERKs | extracellular regulated protein kinases; |
| Foxp3 | forkhead box P3; |
| GPCRs | G protein-coupled receptors; |
| ICR mice | introduction of C57BL6 mouse; |
| IENFs | intraepidermal nerve fibers; |
| IFN | interferon; |
| IgE | immunoglobulin E; |
| IKK | IkB kinase; |
| IL-10 | interleukin-10; |
| IL-17 | interleukin-17; |
| IL-17A | interleukin-17 A; |
| IL-1β | interleukin-1β; |
| IL-4 | interleukin-4; |
| IL-6 | interleukin-6; |
| IL-8 | interleukin-8; |
| iNOS | induced nitric oxide synthase; |
| iNOS | inducible nitric oxide synthase; |
| IVDD | intervertebral disc disease; |
| IκB | inhibitor of nuclear factor kappa B; |
| IκB | inhibitor of nuclear factor kappa B; |
| JNK | c-Jun N-terminal kinase; |
| M1 | M1 microglia produce toxic substances to neurons; |
| M2 | M2 microglia produce anti-inflammatory and tissue repair factors to promote survival and repair; |
| MAC-1 | macrophage-1 antigen; |
| MAPK | p38 mitogen-activated protein kinase; |
| MBP | myelin basic protein; |
| MCP-1 | monocyte chemotactic protein-1; |
| MEL | melittin; |
| MIP | macrophage inflammatory protein; |
| MMP-9 | matrix metalloproteinase-9; |
| MMP-9 | matrix metalloproteinase-9; |
| MPTP | 1-me+7:116thyl-4-phenyl-1,2,3,6-tetrahydropyridine; |
| MTX | methotrexate; |
| NA1 | nonacupoint 1; |
| NA2 | nonspecific acupoints near ST36; |
| NA3 and NA4 | nonspecific acupoints away from ST36; |
| NECK | the neck region; |
| NF-κB | nuclear factor kappa B; |
| NP | nucleus proprius; |
| P38 | p38 mitogen-activated protein kinases; |
| PDQL | Parkinson’s Disease Quality of Life Questionnaire; |
| PGE2 | prostaglandin E2; |
| PGE2 | prostaglandin E2; |
| PIGD | postural instability gait difficulty; |
| PKB | phosphoinositide 3-kinases/protein kinase B; |
| PLA2 | phospholipase A2; |
| PNQ | Patient Neurotoxicity Questionnaire; |
| RA | rheumatoid arthritis; |
| RAC1 | ras-related C3 botulinum toxin substrate 1; |
| ROS | reactive oxygen species; |
| SDH | superficial dorsal horn; |
| SNpc | substantia nigra pars compacta; |
| Th1 | T helper cell type 1; |
| TLR2 | Toll-like receptors; |
| TLRs | toll-like receptors; |
| TMA | trimellitic anhydride; |
| TNF-α | tumor necrosis factor-α; |
| Treg | regulatory T cells; |
| UPDRS | Unified Parkinson’s Disease Rating Scale; |
| VAS | visual analog scale; |
| WOMAC | Western Ontario and McMaster Universities Arthritis Index; |
| α-syn | α-synuclein. |
Appendix A
References
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.G.; Kim, I.S.; Lee, H.D. Standardization of the Manufacturing Process of Bee Venom Pharmacopuncture Containing Melittin as the Active Ingredient. Evid. Based Complement. Altern. Med. 2018, 2018, 2353280. [Google Scholar] [CrossRef]
- Chen, H.S.; Qu, F.; He, X.; Liao, D.; Kang, S.M.; Lu, S.J. The anti-nociceptive effect and the possible mechanism of acupoint stimulation caused by chemical irritants in the bee venom pain model. Brain Res. 2010, 1355, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Roh, D.H.; Yoon, S.Y.; Moon, J.Y.; Kim, H.W.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Repetitive treatment with diluted bee venom reduces neuropathic pain via potentiation of locus coeruleus noradrenergic neuronal activity and modulation of spinal NR1 phosphorylation in rats. J. Pain 2012, 13, 155–166. [Google Scholar] [CrossRef]
- Kim, M.R.; Shin, J.S.; Lee, J.; Lee, Y.J.; Ahn, Y.J.; Park, K.B.; Lee, H.D.; Lee, Y.; Kim, S.G.; Ha, I.H. Safety of Acupuncture and Pharmacopuncture in 80,523 Musculoskeletal Disorder Patients: A Retrospective Review of Internal Safety Inspection and Electronic Medical Records. Medicine 2016, 95, e3635. [Google Scholar] [CrossRef]
- Chung, K.S.; An, H.J.; Cheon, S.Y.; Kwon, K.R.; Lee, K.H. Bee venom suppresses testosterone-induced benign prostatic hyperplasia by regulating the inflammatory response and apoptosis. Exp. Biol. Med. 2015, 240, 1656–1663. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.; Son, M.J.; Choi, J.; Jun, J.H.; Kim, J.I.; Lee, M.S. Bee venom acupuncture for rheumatoid arthritis: A systematic review of randomised clinical trials. BMJ Open 2014, 4, e006140. [Google Scholar] [CrossRef]
- Lee, K.H.; Yu, J.; Sun, S.; Kwon, K. Intravenous Single Dose Toxicity of Sweet Bee Venom in Sprague-Dawley Rats. J. Pharmacopunct. 2015, 18, 49–56. [Google Scholar] [CrossRef]
- Kang, H.; Lim, C.; Kwon, K.R.; Lee, K. Study of a 13-weeks, Repeated, Intramuscular Dose, Toxicity Test of Sweet Bee Venom in Sprague-Dawley Rats. J. Pharmacopunct. 2014, 17, 73–79. [Google Scholar] [CrossRef]
- Kang, H.; Lim, C.; Lee, S.; Kim, B.; Kwon, K.; Lee, K. Study on a 4-week recovery test of sweet bee venom after a 13-week, repeated, intramuscular dose toxicity test in sprague-dawley rats. J. Pharmacopunct. 2014, 17, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jung, D.J.; Choi, Y.M.; Kim, J.U.; Yook, T.H. Trend of pharmacopuncture therapy for treating cervical disease in Korea. J. Pharmacopunct. 2014, 17, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Park, S.; Sun, S.; Lee, K. Research on Korean Pharmacopuncture in South Korea since 2007. J. Pharmacopunct. 2014, 17, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Lee, Y.J.; Kim, M.R.; Cho, J.H.; Kim, K.W.; Kim, E.J.; Ha, I.H. Survey of Integrative Treatment Practices of Korean Medicine Doctors for Cervical Disc Herniation: Preliminary Data for Clinical Practice Guidelines. Evid. Based Complement. Altern. Med. 2019, 2019, 2345640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kang, D.I. A descriptive statistical approach to the Korean pharmacopuncture therapy. J. Acupunct. Meridian Stud. 2010, 3, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Kim, K.H.; Kang, S.; Lee, E.K.; Kim, J.-C.; Jang, B.-H.; Shin, Y.-C.; Ko, S.-G. Usage status and satisfaction with pharmacopuncture in Korea: A survey among Korean medicine doctors. Eur. J. Integr. Med. 2019, 27, 121–130. [Google Scholar] [CrossRef]
- Tsai, L.C.; Lin, Y.W.; Hsieh, C.L. Effects of Bee Venom Injections at Acupoints on Neurologic Dysfunction Induced by Thoracolumbar Intervertebral Disc Disorders in Canines: A Randomized, Controlled Prospective Study. Biomed Res. Int. 2015, 2015, 363801. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.S.; Lee, J.; Kim, M.R.; Jung, J.; Shin, B.C.; Lee, M.S.; Ha, I.H. The Short-Term Effect of Integrated Complementary and Alternative Medicine Treatment in Inpatients Diagnosed with Lumbar Intervertebral Disc Herniation: A Prospective Observational Study. J. Altern. Complement. Med. 2016, 22, 533–543. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Roh, D.H.; Kwon, Y.B.; Kim, H.W.; Seo, H.S.; Han, H.J.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Acupoint stimulation with diluted bee venom (apipuncture) potentiates the analgesic effect of intrathecal clonidine in the rodent formalin test and in a neuropathic pain model. J. Pain 2009, 10, 253–263. [Google Scholar] [CrossRef]
- Cherniack, E.P.; Govorushko, S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon 2018, 154, 74–78. [Google Scholar] [CrossRef]
- Kang, S.S.; Pak, S.C.; Choi, S.H. The effect of whole bee venom on arthritis. Am. J. Chin. Med. 2002, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, G.; Wang, Y.; Liu, J.P.; Smith, C.A.; Luo, H.; Liu, Y. Complementary therapies for acne vulgaris. Cochrane Database Syst. Rev. 2015, 1, Cd009436. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.Y.; Lee, G. Potential Therapeutic Applications of Bee Venom on Skin Disease and Its Mechanisms: A Literature Review. Toxins 2019, 11, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Choi, S.M.; Yang, E.J. The effects of bee venom acupuncture on the central nervous system and muscle in an animal hSOD1G93A mutant. Toxins 2015, 7, 846–858. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Pittler, M.H.; Shin, B.C.; Kong, J.C.; Ernst, E. Bee venom acupuncture for musculoskeletal pain: A review. J. Pain 2008, 9, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Lee, J.H.; Joo, J.C.; Park, S.J.; Song, Y.S. Bee Venom Acupuncture for Shoulder Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Pharmacopunct. 2020, 23, 44–53. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Invest. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ke, T.; He, C.; Cao, W.; Wei, M.; Zhang, L.; Zhang, J.X.; Wang, W.; Ma, J.; Wang, Z.R.; et al. The anti-arthritic effects of synthetic melittin on the complete Freund’s adjuvant-induced rheumatoid arthritis model in rats. Am. J. Chin. Med. 2010, 38, 1039–1049. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.H.; Yang, S.C.; Lee, S.M.; Choi, S.M. Melittin restores proteasome function in an animal model of ALS. J. Neuroinflammation 2011, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.J.; Cho, H.J.; Whang, K.; Lee, I.S.; Park, K.K.; Choe, J.Y.; Han, S.M.; Kim, C.H.; Chang, H.W.; Moon, S.K.; et al. Melittin has an inhibitory effect on TNF-α-induced migration of human aortic smooth muscle cells by blocking the MMP-9 expression. Food Chem. Toxicol. 2012, 50, 3996–4002. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V. Melittin-induced hyperactivation of phospholipase A2 activity and calcium influx in ras-transformed cells. Oncogene 1993, 8, 939–947. [Google Scholar] [PubMed]
- Liu, C.C.; Hao, D.J.; Zhang, Q.; An, J.; Zhao, J.J.; Chen, B.; Zhang, L.L.; Yang, H. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother. Pharmacol. 2016, 78, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.; Klementowicz, J.E.; Bluestone, J.A.; Tang, Q. Targeting Treg signaling for the treatment of autoimmune diseases. Curr. Opin. Immunol. 2015, 37, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Baek, H.; Park, S.Y.; Ku, S.J.; Ryu, K.; Kim, Y.; Bae, H.; Lee, Y.S. Bee Venom Phospholipase A2 Induces Regulatory T Cell Populations by Suppressing Apoptotic Signaling Pathway. Toxins 2020, 12, 198. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Keum, D.J.; Kwak, J.; Chung, H.S.; Bae, H. Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef]
- Ye, M.; Chung, H.S.; Lee, C.; Yoon, M.S.; Yu, A.R.; Kim, J.S.; Hwang, D.S.; Shim, I.; Bae, H. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J. Neuroinflammation 2016, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Fischer, D.; Noelker, C.; Vulinovic, F.; Grunewald, A.; Chevarin, C.; Klein, C.; Oertel, W.H.; Hirsch, E.C.; Michel, P.P.; Hartmann, A. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE 2013, 8, e61700. [Google Scholar] [CrossRef] [Green Version]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [Green Version]
- Korean Movement Disorders Society Red Tulip Survey Participants; Chung, S.J.; Koh, S.B.; Ju, Y.S.; Kim, J.W. Nationwide Survey of Patient Knowledge and Attitudes towards Human Experimentation Using Stem Cells or Bee Venom Acupuncture for Parkinson’s Disease. J. Mov. Disord. 2014, 7, 84–91. [Google Scholar] [CrossRef]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism. Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.; Abushouk, A.I.; AbdelKarim, A.H.; Mohammed, M.; Negida, A.; Shalash, A.S. Bee venom for the treatment of Parkinson’s disease: How far is it possible? Biomed Pharm. 2017, 91, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, Y.E.; Doo, K.H.; Lee, J.H.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Kim, H.; Rhee, H.Y.; et al. Efficacy of Combined Treatment with Acupuncture and Bee Venom Acupuncture as an Adjunctive Treatment for Parkinson’s Disease. J. Altern. Complement. Med. 2018, 24, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Doo, A.R.; Kim, S.T.; Kim, S.N.; Moon, W.; Yin, C.S.; Chae, Y.; Park, H.K.; Lee, H.; Park, H.J. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurol. Res. 2010, 32 (Suppl. 1), 88–91. [Google Scholar] [CrossRef]
- Doo, A.R.; Kim, S.N.; Kim, S.T.; Park, J.Y.; Chung, S.H.; Choe, B.Y.; Chae, Y.; Lee, H.; Yin, C.S.; Park, H.J. Bee venom protects SH-SY5Y human neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death. Brain Res. 2012, 1429, 106–115. [Google Scholar] [CrossRef]
- Kim, J.I.; Yang, E.J.; Lee, M.S.; Kim, Y.S.; Huh, Y.; Cho, I.H.; Kang, S.; Koh, H.K. Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. Int. J. Neurosci. 2011, 121, 209–217. [Google Scholar] [CrossRef]
- Khalil, W.K.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef]
- Ye, M.; Chung, H.S.; Lee, C.; Hyun Song, J.; Shim, I.; Kim, Y.S.; Bae, H. Bee venom phospholipase A2 ameliorates motor dysfunction and modulates microglia activation in Parkinson’s disease alpha-synuclein transgenic mice. Exp. Mol. Med. 2016, 48, e244. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H.; Kim, T.H.; Jung, W.S.; Moon, S.K.; Ko, C.N.; Cho, S.Y.; Jeon, C.Y.; Choi, T.Y.; Lee, M.S.; Lee, S.H.; et al. Pharmacoacupuncture for Idiopathic Parkinson’s Disease: A Systematic Review of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2018, 2018, 3671542. [Google Scholar] [CrossRef]
- Doo, K.H.; Lee, J.H.; Cho, S.Y.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Kim, H.; Park, H.J.; Park, S.U. A Prospective Open-Label Study of Combined Treatment for Idiopathic Parkinson’s Disease Using Acupuncture and Bee Venom Acupuncture as an Adjunctive Treatment. J. Altern. Complement. Med. 2015, 21, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Roh, D.H.; Park, J.H.; Lee, H.J.; Lee, J.H. Activation of Spinal alpha2-Adrenoceptors Using Diluted Bee Venom Stimulation Reduces Cold Allodynia in Neuropathic Pain Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 784713. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeon, C.; Lee, J.H.; Jang, J.U.; Quan, F.S.; Lee, K.; Kim, W.; Kim, S.K. Suppressive Effects of Bee Venom Acupuncture on Paclitaxel-Induced Neuropathic Pain in Rats: Mediation by Spinal alpha(2)-Adrenergic Receptor. Toxins 2017, 9, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, D.H.; Kwon, Y.B.; Kim, H.W.; Ham, T.W.; Yoon, S.Y.; Kang, S.Y.; Han, H.J.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: Involvement of spinal alpha 2-adrenoceptors. J. Pain 2004, 5, 297–303. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Kang, M.S.; Han, H.J.; Beitz, A.J.; Lee, J.H. Visceral antinociception produced by bee venom stimulation of the Zhongwan acupuncture point in mice: Role of alpha(2) adrenoceptors. Neurosci. Lett. 2001, 308, 133–137. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, C.Y.; Roh, D.H.; Yoon, S.Y.; Park, J.H.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Chemical stimulation of the ST36 acupoint reduces both formalin-induced nociceptive behaviors and spinal astrocyte activation via spinal alpha-2 adrenoceptors. Brain Res. Bull. 2011, 86, 412–421. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Lee, J.Y.; Roh, D.H.; Oh, S.B. Pharmacopuncture With Scolopendra subspinipes Suppresses Mechanical Allodynia in Oxaliplatin-Induced Neuropathic Mice and Potentiates Clonidine-induced Anti-allodynia Without Hypotension or Motor Impairment. J. Pain 2018, 19, 1157–1168. [Google Scholar] [CrossRef]
- Kang, S.Y.; Roh, D.H.; Kim, H.W.; Han, H.J.; Beitz, A.J.; Lee, J.H. Blockade of Adrenal Medulla-Derived Epinephrine Potentiates Bee Venom-Induced Antinociception in the Mouse Formalin Test: Involvement of Peripheral beta -Adrenoceptors. Evid. Based Complement. Altern. Med. 2013, 2013, 809062. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, M.J.; Yoon, I.; Li, D.X.; Bae, H.; Kim, S.K. Nicotinic Acetylcholine Receptors Mediate the Suppressive Effect of an Injection of Diluted Bee Venom into the GV3 Acupoint on Oxaliplatin-Induced Neuropathic Cold Allodynia in Rats. Biol. Pharm. Bull. 2015, 38, 710–714. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Kim, H.W.; Li, J.; Kwon, Y.B. Effect of bee venom acupuncture on methamphetamine-induced hyperactivity, hyperthermia and Fos expression in mice. Brain Res. Bull. 2011, 84, 61–68. [Google Scholar] [CrossRef]
- Roh, D.H.; Kim, H.W.; Yoon, S.Y.; Kang, S.Y.; Kwon, Y.B.; Cho, K.H.; Han, H.J.; Ryu, Y.H.; Choi, S.M.; Lee, H.J.; et al. Bee venom injection significantly reduces nociceptive behavior in the mouse formalin test via capsaicin-insensitive afferents. J. Pain 2006, 7, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Han, H.J.; Beitz, A.J.; Lee, J.H. Bee venom acupoint stimulation increases Fos expression in catecholaminergic neurons in the rat brain. Mol. Cells 2004, 17, 329–333. [Google Scholar] [PubMed]
- Al-Atiyyat, N.; Obaid, A. Management of peripheral neuropathy induced by chemotherapy in adults with cancer: A review. Int. J. Palliat. Nurs. 2017, 23, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Jeon, J.H.; Lee, Y.W.; Cho, C.K.; Kwon, K.R.; Shin, J.E.; Sagar, S.; Wong, R.; Yoo, H.S. Sweet bee venom pharmacopuncture for chemotherapy-induced peripheral neuropathy. J. Acupunct. Meridian Stud. 2012, 5, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.; Moon, H.J.; Li, D.X.; Gil, M.; Min, J.K.; Lee, G.; Bae, H.; Kim, S.K.; Min, B.I. Effect of bee venom acupuncture on oxaliplatin-induced cold allodynia in rats. Evid. Based Complement. Altern. Med. 2013, 2013, 369324. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Yeo, J.H.; Han, S.D.; Bong, D.J.; Oh, B.; Roh, D.H. Diluted bee venom injection reduces ipsilateral mechanical allodynia in oxaliplatin-induced neuropathic mice. Biol. Pharm. Bull. 2013, 36, 1787–1793. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.J.; Go, D.; Min, B.I.; Na, H.S.; Kim, S.K. Combined Effects of Bee Venom Acupuncture and Morphine on Oxaliplatin-Induced Neuropathic Pain in Mice. Toxins 2016, 8, 33. [Google Scholar] [CrossRef]
- Lee, J.H.; Li, D.X.; Yoon, H.; Go, D.; Quan, F.S.; Min, B.I.; Kim, S.K. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. BMC Complement. Altern. Med. 2014, 14, 471. [Google Scholar] [CrossRef] [Green Version]
- Baek, H.; Lee, C.J.; Choi, D.B.; Kim, N.S.; Kim, Y.S.; Ye, Y.J.; Kim, Y.S.; Kim, J.S.; Shim, I.; Bae, H. Bee venom phospholipase A2 ameliorates Alzheimer’s disease pathology in Abeta vaccination treatment without inducing neuro-inflammation in a 3xTg-AD mouse model. Sci. Rep. 2018, 8, 17369. [Google Scholar] [CrossRef]
- Lee, S.M.; Yune, T.Y.; Kim, S.J.; Park, D.W.; Lee, Y.K.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma 2003, 20, 1017–1027. [Google Scholar] [CrossRef]
- Yune, T.Y.; Lee, J.Y.; Jung, G.Y.; Kim, S.J.; Jiang, M.H.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J. Neurosci. 2007, 27, 7751–7761. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.C.; Lee, J.Y.; Moon, Y.J.; Kim, S.W.; Oh, T.H.; Yune, T.Y. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol. Dis. 2010, 39, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Houghtling, R.A.; MacArthur, L.; Bayer, B.M.; Bregman, B.S. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp. Neurol. 2003, 184, 313–325. [Google Scholar] [CrossRef]
- Nascimento de Souza, R.; Silva, F.K.; Alves de Medeiros, M. Bee Venom Acupuncture Reduces Interleukin-6, Increases Interleukin-10, and Induces Locomotor Recovery in a Model of Spinal Cord Compression. J. Acupunct. Meridian Stud. 2017, 10, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Roh, D.H.; Choi, J.W.; Ryu, Y.; Lee, J.H. Repetitive Treatment with Diluted Bee Venom Attenuates the Induction of Below-Level Neuropathic Pain Behaviors in a Rat Spinal Cord Injury Model. Toxins 2015, 7, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Sun, B.C. Apipuncture treatment for central post-stroke pain. J. Altern. Complement. Med. 2010, 16, 223–224. [Google Scholar] [CrossRef]
- Kim, M.; Han, C.-h. Pharmacopuncture for stroke survivors: A systematic review of randomized controlled trials in South Korea. Complementary Ther. Clin. Pract. 2020, 40. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, J.Y.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Park, S.U. Bee venom acupuncture point injection for central post stroke pain: A preliminary single-blind randomized controlled trial. Complement. Ther. Med. 2013, 21, 155–157. [Google Scholar] [CrossRef]
- Lim, S.M.; Lee, S.-H. Effectiveness of bee venom acupuncture in alleviating post-stroke shoulder pain: A systematic review and meta-analysis. J. Integr. Med. 2015, 13, 241–247. [Google Scholar] [CrossRef]
- Seo, B.K.; Lee, J.H.; Kim, P.K.; Baek, Y.H.; Jo, D.J.; Lee, S. Bee venom acupuncture, NSAIDs or combined treatment for chronic neck pain: Study protocol for a randomized, assessor-blind trial. Trials 2014, 15, 132. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Cho, Y.Y.; Kim, S.; Sun, S.H. History of Research on Pharmacopuncture in Korea. J. Pharmacopunct. 2016, 19, 101–108. [Google Scholar] [CrossRef]
- Lee, J.; Shin, J.S.; Lee, Y.J.; Kim, M.R.; Choi, A.; Lee, J.H.; Shin, K.M.; Shin, B.C.; Cho, J.H.; Ha, I.H. Long-Term Course of Failed Back Surgery Syndrome (FBSS) Patients Receiving Integrative Korean Medicine Treatment: A 1 Year Prospective Observational Multicenter Study. PLoS ONE 2017, 12, e0170972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.K.; Han, K.; Kwon, O.; Jo, D.J.; Lee, J.H. Efficacy of Bee Venom Acupuncture for Chronic Low Back Pain: A Randomized, Double-Blinded, Sham-Controlled Trial. Toxins 2017, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.K.; Lee, J.H.; Sung, W.S.; Song, E.M.; Jo, D.J. Bee venom acupuncture for the treatment of chronic low back pain: Study protocol for a randomized, double-blinded, sham-controlled trial. Trials 2013, 14, 16. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Choi, U.S.; Lee, S.D.; Kim, K.H.; Chung, K.H.; Chang, Y.C.; Park, K.K.; Lee, Y.C.; Kim, C.H. Effect of bee venom on aromatase expression and activity in leukaemic FLG 29.1 and primary osteoblastic cells. J. Ethnopharmacol. 2005, 99, 245–252. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Kim, J.H.; Yoon, J.H.; Lee, J.D.; Han, H.J.; Mar, W.C.; Beitz, A.J.; Lee, J.H. The analgesic efficacy of bee venom acupuncture for knee osteoarthritis: A comparative study with needle acupuncture. Am. J. Chin. Med. 2001, 29, 187–199. [Google Scholar] [CrossRef]
- Conrad, V.J.; Hazan, L.L.; Latorre, A.J.; Jakubowska, A.; Kim, C.M.H. Efficacy and Safety of Honey Bee Venom (Apis mellifera) Dermal Injections to Treat Osteoarthritis Knee Pain and Physical Disability: A Randomized Controlled Trial. J. Altern. Complement. Med. 2019, 25, 845–855. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, G.S.; Kang, M.S.; Yoon, H.M.; Kim, C.H. Comparative study on the effects of bee venom pharmacopuncture according to the treatment method for knee osteoarthritis. J. Pharmacopunct. 2012, 15, 7–14. [Google Scholar] [CrossRef]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (Apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by alpha2-Adrenoceptors. Brain Res. 2006, 1073-1074, 305–310. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Lee, J.D.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Beitz, A.J.; Lee, J.H. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain 2001, 90, 271–280. [Google Scholar] [CrossRef]
- Darwish, S.F.; El-Bakly, W.M.; Arafa, H.M.; El-Demerdash, E. Targeting TNF-alpha and NF-kappaB activation by bee venom: Role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS ONE 2013, 8, e79284. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Yoon, O.B.; Beitz, A.J.; Lee, J.H. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002, 71, 191–204. [Google Scholar] [CrossRef]
- Lee, J.D.; Kim, S.Y.; Kim, T.W.; Lee, S.H.; Yang, H.I.; Lee, D.I.; Lee, Y.H. Anti-inflammatory effect of bee venom on type II collagen-induced arthritis. Am. J. Chin. Med. 2004, 32, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.J.; Kim, K.S.; Kim, M.J.; Chang, Y.C.; Lee, S.D.; Kim, M.S.; Kwon, D.Y.; Kim, C.H. Effects of bee venom on protease activities and free radical damages in synovial fluid from type II collagen-induced rheumatoid arthritis rats. Toxicol. In Vitro 2006, 20, 1465–1471. [Google Scholar] [CrossRef]
- Yamasaki, S.C.; Mendes, M.T.; Alponti, R.F.; Silveira, P.F. Efficacy of parenteral administration of bee venom in experimental arthritis in the rat: A comparison with methotrexate. Toxicon 2015, 98, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jang, M.; Choi, J.; Lee, G.; Min, H.J.; Chung, W.S.; Kim, J.I.; Jee, Y.; Chae, Y.; Kim, S.H.; et al. Bee Venom Acupuncture Alleviates Experimental Autoimmune Encephalomyelitis by Upregulating Regulatory T Cells and Suppressing Th1 and Th17 Responses. Mol. Neurobiol. 2016, 53, 1419–1445. [Google Scholar] [CrossRef]
- Brañas, P.; Jordan, R.; Fry-Smith, A.; Burls, A.; Hyde, C. Treatments for fatigue in multiple sclerosis: A rapid and systematic review. Health Technol. Assess 2000, 4, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.R.; Pak, S.C.; Park, K.K. The protective effect of bee venom on fibrosis causing inflammatory diseases. Toxins 2015, 7, 4758–4772. [Google Scholar] [CrossRef] [Green Version]
- An, H.J.; Lee, W.R.; Kim, K.H.; Kim, J.Y.; Lee, S.J.; Han, S.M.; Lee, K.G.; Lee, C.K.; Park, K.K. Inhibitory effects of bee venom on Propionibacterium acnes-induced inflammatory skin disease in an animal model. Int. J. Mol. Med. 2014, 34, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Sur, B.; Lee, B.; Yeom, M.; Hong, J.H.; Kwon, S.; Kim, S.T.; Lee, H.S.; Park, H.J.; Lee, H.; Hahm, D.H. Bee venom acupuncture alleviates trimellitic anhydride-induced atopic dermatitis-like skin lesions in mice. BMC Complement. Altern. Med. 2016, 16, 38. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.O.; Park, S.Y.; Heo, M.S.; Kim, K.C.; Park, C.; Ko, W.S.; Choi, Y.H.; Kim, G.Y. Key regulators in bee venom-induced apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells through downregulation of ERK and Akt. Int. Immunopharmacol. 2006, 6, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H.; Ahn, Y.J.; Kim, J.Y.; Song, M.J.; Song, H.S.; et al. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Baek, Y.H.; Lee, M.H.; Choi, D.Y.; Park, D.S.; Lee, J.D. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett. 2010, 292, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.C.; Wu, C.C.; Hsieh, H.L.; Chen, C.Y.; Hsu, S.L. Honeybee venom induces calcium-dependent but caspase-independent apoptotic cell death in human melanoma A2058 cells. Toxicon 2008, 52, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.B.; Huh, J.E.; Lee, H.J.; Kim, D.; Lee, G.J.; Park, H.K.; Lee, J.D. Anti-cancer effect of bee venom on human MDA-MB-231 breast cancer cells using Raman spectroscopy. Biomed Opt. Express 2018, 9, 5703–5718. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Shin, J.S.; Lee, J.; Lee, Y.J.; Kim, M.R.; Shin, Y.S.; Park, K.B.; Kim, E.J.; Kim, M.J.; Lee, J.W.; et al. Safety of essential bee venom pharmacopuncture as assessed in a randomized controlled double-blind trial. J. Ethnopharmacol. 2016, 194, 774–780. [Google Scholar] [CrossRef]
- Yeo, J.H.; Yoon, S.Y.; Kwon, S.K.; Kim, S.J.; Lee, J.H.; Beitz, A.J.; Roh, D.H. Repetitive Acupuncture Point Treatment with Diluted Bee Venom Relieves Mechanical Allodynia and Restores Intraepidermal Nerve Fiber Loss in Oxaliplatin-Induced Neuropathic Mice. J. Pain 2016, 17, 298–309. [Google Scholar] [CrossRef]
- Yang, C.Y.; Park, S.A.; Oh, K.J.; Yang, Y.S. The assessment of bee venom responses in an experimental model of mono-arthritis using Tc-99m DPD bone scintigraphy. Ann. Nucl. Med. 2010, 24, 455–460. [Google Scholar] [CrossRef]

| Species | Size (N) | Venom/Compound | Acupoints | Dose | References/Results |
|---|---|---|---|---|---|
| Parkinson’s disease | |||||
| C57BL/6 mice | 18 | BV | Bilateral GB34 | 0.02 mL | Doo et al., 2010 [45] Prevented loss of tyrosine hydroxylase immunoreactivity and attenuated phospho-Jun immunoreactivity. |
| C57BL/6 mice | 48 | BV | Bilateral ST36 | 0.6 mg/kg | Kim et al., 2011 [47] BVA will decrease expression of the inflammation markers MAC-1 and iNOS in the SNpc. |
| C57BL/6 mice | BV/apamin | i.p. injections | Bee venom (low = 12 mg/kg; high = 120 mg/kg) Apamin (low: 0.5 mg/kg/BW; high 1.0 mg/kg/BW) | Alvarez-Fischer et al., 2013 [39] Apamin can partially reproduced the protective effects of the dopaminergic neurons. | |
| Swiss albino mice | 40 | BV | GB34 bilaterally | 0.02 ml | Khalil et al., 2015 [48] BVA lower the caspase-3 activation and apoptosis genes (Bax, Bcl2) expression in comparison to l-dopa in brain of rotenone treated mice. |
| A53T α-Syn transgenic mice | bvPLA2 | intraperitoneal treatment | 0.2 or 1 mg/kg | Ye et al., 2016 [49] After bvPLA2 injection, it was shown inhibit motor impairment, α-syn pathology, enhances microglial deactivation in the spinal cord and normalizes the ratio of M1/M2 microglial phenotypes. | |
| Human | 43 | BV | Bilateral GB 20, LI 11, GB 34, ST36, and LR3 | 0.1 mL | Cho et al., 2012 [42] Improvement on the UPDRS, the Berg Balance Scale, and the 30 m walking time. |
| Human | 11 | BV | Bilateral GB20, LI4, GB34, ST36, and LR3 | 0.1 mL | Doo et al., 2015 [51] Combined treatment with BVA and acupuncture showed remarkable progress in gait speed, PDQL score, UPDRS scores. |
| Human | 63 | BV | Bilateral GB20, LI11, GB34, ST36, and LR3 | 0.1 mL | Cho et al., 2018 [44] A significant improvement was observed in UPDRS part II + III, part II, and part III scores, PIGD score. |
| Neuropathic Pain | |||||
| ICR mice | 63 | BV | CV12 | 0.25 mg/kg | Kwon et al., 2001 [55] BVA can produce antinociception via alpha 2-adrenoceptors, but not naloxone-sensitive opioid receptors. |
| Sprague-Dawley rats | 28/33/18 | BV | ST36 | 0.25 mg/kg | Roh et al., 2004 [54] BVA remarkably reduces the thermal hyperalgesia and this antihyperalgesic effect will activate alpha2-adrenoceptors. |
| Sprague-Dawley rats | 18 | BV | ST36 | 0.8 mg/kg | Kwon et al., 2004 [62] BV acupoint stimulation activates brainstem catecholaminergic neurons. |
| ICR mice | 53 | BV | ST36 | High: 10 mg/kg Middle: 0.1 mg/kg Low: 0.001 mg/kg | Roh et al., 2006 [61] Low dose of BV increase in Fos immunoreactive neurons in the ipsilateral; middle dose only in the SDH and NECK areas of the ipsilateral spinal dorsal horn; high dose throughout much of the ipsilateral dorsal horn consisting of the SDH, NP, and NECK areas. |
| ICR mice/Sprague-Dawley rats | 15 | BV | ST36 | Mice:0.25 mg/kg/20 µL Rats:0.01 mg/kg/50 µL | Yoon et al., 2009 [19] BVA produced a significant clonidine-induced analgesia. |
| ICR mice | 8/groups | BV | ST36 | 0.8 or 0.08 mg/kg | Kang et al., 2011 [56] BVA triggered activation of spinal astrocytes and this inhibition is associated with spinal alpha-2 adrenoceptors. |
| ICR mice | BV | ST36 or control points (SP9 or GB39 or tail base) | 1 mg/mL | Kim et al., 2011 [60] BVA administrated at ST36 may active the central α₂-adrenergic as well as the peripheral nerve and modulate METH-induced hyperthermia, hyperactivity and Fos expression. | |
| Sprague-Dawley rats | 24/16 | BV | ST36 | 0.25 or 2.5 mg/kg | Kang et al., 2012 [52] DBV could active spinal α2-adrenoceptor and alleviate CCI-induced cold allodynia. |
| ICR mice | BV | ST36 | 0.8 mg/kg | Kang et al., 2013 [58] Antinociceptive impact of BVA can be improved by inflection of adrenal medulla-derived epinephrine and this impact is moderated by peripheral β-adrenoceptors. | |
| Sprague-Dawley rats | 27 | BV | GV3 | 0.25 mg/kg | Yoon et al., 2015 [59] Spinal α4β2 receptors, nicotinic acetylcholine receptors, but not muscarinic receptors, moderate the suppressive impact of BVA. |
| Peripheral Neuropathy | |||||
| C57BL/6 mice | 17/18 | BV | Right ST36 | 0.1 mg/kg | Yoon et al., 2013 [66] BVA reduces ipsilateral mechanical allodynia depending on spinal cord alpha-2 adrenoceptors. |
| Sprague-Dawley rats | 24 | BV | LI11, ST36, GV3 | 1.0 mg/kg | Lim et al., 2013 [65] BVA reduces oxaliplatin-induced cold allodynia via alpha-2 adrenoceptors. |
| Sprague–Dawley rats | 25/34 | BV | GV3 | 0.25 mg/kg | Lee et al., 2014 [68] BVA reduces oxaliplatin-induced acute cold allodynia through the activation of serotonergic system and spinal 5-HT3 receptors. |
| C57BL/6 mice | 59 | BV | Right ST36 | 0.1 mg/kg | Yeo et al., 2016 [107] BVA decreased oxaliplatin-induced mechanical allodynia and recovered the loss of IENFs through an α-2 adrenoceptor mechanism. |
| C57BL/6 mice | 25 | BV | Right ST36 | 0.25, 1, and 2.5 mg/kg | Kim et al., 2016 [67] The combine treatment of BVA and morphine is moderated by spinal opioidergic and 5-HT3 receptors which could decrease oxaliplatin-induced neuropathic pain. |
| Sprague-Dawley rats | 14/11/28/36 | BV/melittin/phospholipase A2 | ST36, LI11 | BVA (1 mg/kg) melittin (0.5 mg/kg) phospholipase A2 (0.12 mg/kg) | Choi et al., 2017 [53] BVA could decrease paclitaxel-induced neuropathic pain with spinal α2-adrenergic receptor. |
| Human | 11 | melittin | Bilateral GB39, LV3: lower extremities neuropathy. Bilateral LI4, SJ5, GB39, and LV3: patients with both upper and lower extremities neuropathy. | 0.1 mL | Yoon et al., 2012 [64] After BVA, both PNQ scores and WHO CIPN grade decreased. VAS has also decrease. |
| Alzheimer Disease | |||||
| 3xTg AD mice | 27 | bvPLA2 | Intraperitoneal injection | 0.2 mg/kg 1 mg/kg | Ye et al., 2016 [38] PLA2 has neuroprotective effect via reduction in CD4+ T cell infiltration and microglial deactivation. |
| 3xTg-AD mice | 50 | bvPLA2 | Intraperitoneal injection | 0.5 mg/kg | Baek et al., 2018 [69] BvPLA2 could trigger the amelioration AD pathology and Tregs |
| Intervertebral Disk Disease | |||||
| canines | 40 | BV | Bilateral LI 04, SI 03, KI 03, ST 36, BL 23, BL 40, GB 30, GB 34, and LR 03, unilateral GV01, Baihui, and Ashipoints | 0.1 mL (20 μg) | Tsai et al., 2015 [17] BV injection exerted a strong effect on canines with moderate to severe IVDD and reduced clinical rehabilitation time. |
| Spinal Cord Injury | |||||
| Wistar rats | 3-4 animals/group | BV | BVA: GV3 and ST36; nonacupoints AT: no treatment | 20 µL diluted in saline (0.08 mg/kg) | Raquel Nascimento de Souza et al., 2017 [74] BVA increased the expression of IL-10 at 6 h and reduced the expression of IL-6 at 24 h after SCI compared with the controls. |
| Sprague-Dawley rats | 16 | BV | ST36 | 0.25 mg/kg(50-µL) | Kang et al., 2015 [75] BVA assisted in motor function recuperation as suggested by the Basso-Beattie-Bresnahan score. |
| Central Post-Stroke Pain | |||||
| Human | 20 | BV | LI15, GB21, LI11, GB31, ST36 and GB39 of the affected side | 0.05 ml | Cho et al., 2013 [78] After BVA, there is significant decreases in visual analogue pain scores. |
| Musculoskeletal Pain | |||||
| Human (low back pain) | 54 | BV | BL23, BL24, BL25, GB30, GV3, GV4, GV5 | 0.2 mL for the first week, 0.4 mL for the second week, and 0.8 mL for the third week | Seo et al., 2017 [83] After BV injection, Beck’s Depression Inventory and Oswestry Disability Index, EuroQol 5-Dimensionshowed improved. |
| Osteoarthritis Knee Pain | |||||
| Human | 60 | BV | SP10, ST34, ST36, GB34, LR3, Ex-LE2, Ex-LE5 | 0.1 mL | Y-B Kwon et al., 2001 [86] After BV injection computerized infrared thermography (IRT) and pain relief scores showed significant improved. |
| Human | 69 | BV | ST35, GB34, EX32, ST36, SP9, Ashipoints | 0.1 mL | Lee et al., 2012 [88] BV injection exhibited significant improvement on VAS and KWOMAC effects when treating knee OA. |
| Human | 358 | BV | knee top, eye-1 medial, eye-2 lateral, ST 34, BL40, BL5, BL19, BL21, BL23, BL25, and BL27 | 100 μg | Conrad et al., 2019 [87] HBV biotherapy resulted in significant improvements in VAS of knee OA pain and physical function. |
| Rheumatoid Arthritis | |||||
| Sprague- Dawley rats | 60 | BV | ST36 | 1 mg/kg | Kwon et al., 2001 [90] BV treatment inhibit paw edema also decreased arthritis-induced nociceptive behaviors. |
| Sprague-Dawley rats | 60 | BV | ST36 | 0.9 mg/kg | Kwon et al., 2002 [92] BVA suppressed the increase of IL-6 caused by RA and decreased arthritis-induced nociceptive behaviors. |
| Sprague-Dawley rats | 90 | whole BV | Intraperitoneal injection | one bee/rat | Kang et al., 2018 [21] BVA showed erosions in inflammatory cell infiltrations and articular cartilage. |
| DBA/1 mice | 27 | BV | ST36 | 0.1 mL | Lee et al., 2004 [93] BV reduced the progression of arthritis and lead to the inhibition of the immune responses. |
| Sprague–Dawley rats | 80 | BV | ST36 | 0.25 mg/kg | Baek et al., 2006 [89] BVA can alleviate inflammatory pain via alpha2-adrenergic receptor. |
| Lewis rats | 12 | BV | bilateral Shinsu (B23) | 50 µL/kg | Suh et al., 2006 [94] BVA is an effective RA modulator, preventing protease activities and eliminating ROS. |
| Sprague–Dawley rats | 12 | BV | proximal tibialis anterior muscle around the right knee | 0.8 mg/kg | Yang et al., 2010 [108] After BVA there is more improved on the weight load test and revealed lower activity in bone scintigraphy. |
| Sprague-Dawley rats | 88 | BV/melittin | ST36 | BV (1 mg/kg/day) melittin (0.5 mg/kg/day) | Li et al., 2010 [29] BV and melittin therapies statistically decreasedarthritis-induced nociceptive behaviors. |
| Wister rats | 80 | BV | ST36 | 0.5 mg/kg | Darwish et al., 2013 [91] BVA ameliorated TNF-α and the over expression of NF-κB in liver induced by methotrexate. |
| Wistar rats | 47/39 | BV | subcutaneously | 0.25 mg | Yamasaki et al., 2015 [95] Treatment with MTX or BV alone will ameliorate edema. MTX is more effective in reducing hyperalgesia than BV. However, anti-arthritic effect of BV is better than MTX. |
| Multiple sclerosis | |||||
| Lewis rats/C57BL/6 mice | BV | ST36 placebo acupoints: SP9, GB39, NA1, NA2, NA3, NA4 | 0.25 and 0.8 mg/kg | Lee et al., 2016 [96] BVA with ST36 could attenuate the progression of EAE by increasing T cells and suppressing T-helper 1and T-helper 17 responses. | |
| Skin disease | |||||
| BALB/c mice | 50 | BV | BL40 | 0.3 mg/kg | Sur et al., 2016 [100] BVA inhibited the proliferation of T cells, the synthesis of Th1 and Th2 cytokines, and the production of immunoglobulin E and IL-4. |
| Cancer | |||||
| C57BL/6JmsSlc mice | BV | subcutaneously | 0.01, 0.1 or 1 mg/kg | Huh et al., 2010 [103] BV prevented MAPK and AKT phosphorylation and down modulated activation of vascular endothelial growth factor, which can suppress the vascular endothelial growth factor-induced proliferation and the viability of Lewis lung carcinoma | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-Y.; Hsieh, C.-L. Clinical Applications of Bee Venom Acupoint Injection. Toxins 2020, 12, 618. https://doi.org/10.3390/toxins12100618
Lin T-Y, Hsieh C-L. Clinical Applications of Bee Venom Acupoint Injection. Toxins. 2020; 12(10):618. https://doi.org/10.3390/toxins12100618
Chicago/Turabian StyleLin, Ting-Yen, and Ching-Liang Hsieh. 2020. "Clinical Applications of Bee Venom Acupoint Injection" Toxins 12, no. 10: 618. https://doi.org/10.3390/toxins12100618
APA StyleLin, T.-Y., & Hsieh, C.-L. (2020). Clinical Applications of Bee Venom Acupoint Injection. Toxins, 12(10), 618. https://doi.org/10.3390/toxins12100618




